Abstract
Purpose
To investigate the ability of human lymphocytes labeled with DNA-incorporated 125I to exert an inhibitory (antiproliferative) bystander effect on co-cultured human colon adenocarcinoma LS174T cells in vitro.
Materials and methods
Human peripheral blood lymphocytes were stimulated to synthesize DNA in the presence of phytohemagglutinin (PHA) and labeled with 5-[125I]iodo-2′-deoxyuridine. Human colon adenocarcinoma LS174T cells were co-cultured with the 125I-labeled lymphocytes in various ratios for 5 days and the proliferation of the LS174T cells was assessed. Further, the supernatant media from these co-cultures were: (i) Transferred to LS174T cells and their proliferation measured after 5 days, (ii) used to assess the clonogenic survival of LS174T cells, and (iii) screened for factors that suppress growth.
Results
A significant reduction in the proliferation of LS174T cells was observed when co-cultured either with 125I-labeled lymphocytes (56 ± 3.5%) or the supernatant media (52.5 ± 1.3%) obtained from these co-cultures. Clonogenic survival of LS174T cells grown in the supernatant media corroborated the decrease in tumor cell growth.
Conclusion
The observed reduction in the proliferation of LS174T cells in presence of 125I-labeled lymphocytes or media obtained from such co-cultures can be attributed to an inhibitory (antiproliferative) bystander effect, probably mediated by factor(s) released from the dying 125I-labeled lymphocytes.
Keywords: Lymphocytes, bystander effect, Auger electron, tumor cells, iodine-125
Introduction
The bystander effect, a phenomenon elicited by irradiated cells, describes the responses and stimuli observed in neighboring cells that are not directly exposed to ionizing radiation (Azzam and Little 2004, Sgouros et al. 2007, Blyth and Sykes 2011). Although sporadic reports describing the damage inflicted at distant non-irradiated sites away from the primary sites of radiation exposure were documented as early as five decades ago (Parsons et al. 1954, Souto 1962), it was not until recently that this concept became one of the fundamental tenets of radiation biology (Blyth and Sykes 2011). The bystander responses and damage in unirradiated cells, which was first observed by Nagasawa and Little in a population of unirradiated cells adjacent to cells irradiated with alpha particles (Nagasawa and Little 1992), are characterized primarily by events leading to genomic instability (Ilnytskyy and Kovalchuk 2011) resulting in reduced cell survival (Lyng et al. 2002), elevated apoptosis (Lyng et al. 2000), DNA damage (Nagasawa and Little 1992, Deshpande et al. 1996, Zhang et al. 2008), increased sister chromatid exchange (Nagasawa and Little 1992), mutations (Huo et al. 2001), epigenetic changes (Ilnytskyy and Kovalchuk 2011), telomere shortening (Gorman et al. 2009, Belloni et al. 2011), alteration in the mitochondrial membrane potential (Nugent et al. 2007), micronucleus formation (Yang et al. 2011), increase in reactive oxygen species (ROS) (Lehnert and Goodwin 1997) and intracellular calcium flux (Lyng et al. 2006). The bystander effects have been observed not only with commonly used radiation sources such as X-rays, γ rays, α- and β-particles (Persaud et al. 2007, Blyth and Sykes 2011), but also with protons (Frankenberg et al. 2006), high energy β particles (Gow et al. 2010), low energy β particles (Bishayee et al. 1999, 2001, Persaud et al. 2005), Auger electrons (Howell and Bishayee 2002, Xue et al. 2002, Boyd et al. 2006, 208, Kishikawa et al. 2006), neutrons (Liu et al. 2006), and accelerated heavy ions (Fournier et al. 2007).
Many radionuclides exhibit an Auger effect and decay by electron capture and/or internal conversion (Auger 1925) resulting in a surge of low-energy electrons arising from Auger, Coster-Kronig and super Coster-Kronig transitions (Charlton et al. 1987, Kassis et al. 1987, Pomplun et al. 1987). Among prolific Auger electron emitters 125I has been the most well studied (Kassis 2004). The decay of 125I produces a cascade of extremely low-energy electrons, ∼21 on average: of these, ∼18 electrons have a range < 100 nm and ∼8 electrons travel < 2 nm (Charlton 1986). Due to their subcellular range and high dose deposition of these high LET (linear energy trasnsfer) particles in the radiolabeled cells, we (Xue et al. 2002, Kishikawa et al. 2006) and others (Howell and Bishayee 2002, Boyd et al. 2006, 2008) used this Auger electron emitter to investigate the bystander effect in neighboring cells. For example, studies from this laboratory (Xue et al. 2002) demonstrated that when mice are injected with a mixture of human colon LS174T adenocarcinoma cells and LS174T cells labeled with lethal doses (dose at which these cells stop proliferating) of DNA-incorporated Auger emitter 125I, a distinct inhibitory (antiproliferative) effect on the growth of s.c. tumor (derived from unlabeled cells) is observed. Similar findings were observed when the experiments were performed in vitro (Kishikawa et al. 2006). Such studies also showed that the bystander stimuli and signals are likely to be transmitted either through gap-junctions (Azzam et al. 2001) or are mediated by factors secreted by the irradiated cells (Mothersill and Seymour 1997, Kishikawa et al. 2006) into the surrounding media. Regardless of the underlying mechanisms, it is presumed that radiation-induced bystander effects would impact: (i) The assessment of carcinogenic risk from radiation exposures, and (ii) the estimation of risk associated with absorbed dose following administration of therapeutic and imaging radiopharmaceuticals.
To exploit the inhibitory (antiproliferative) bystander effect consequent to the decay of DNA incorporated 125I in scenarios where cell proliferation is undesirable, for example the inhibition of tumor cell growth or proliferation in tumors, we rationalized that a cancer patient's lymphocytes – labeled with lethal dose of DNA-incorporated 125I – may augment his/her therapy vis-à-vis an inhibitory (antiproliferative) bystander effect. The lymphocytes can be stimulated first to undergo cellular differentiation and proliferation (Nowell 1960, Stewart et al. 1975), then incubated in vitro with 5-[125I] iodo-2′-deoxyuridine (125IUdR), and finally administered to the patient. As these lymphocytes would be obtained from the patient being treated, they would not be rejected. In addition, since peripheral blood lymphocytes are quite radiosensitive (Belloni et al. 2005), the lethal 125IUdR dose (at which lymphocytes stop proliferating) is expected to be much smaller than the lethal 125IUdR dose (111 kBq/cell) required to stop proliferation of LS174T tumor cells (Xue et al. 2002, Kishikawa et al. 2006). In the present work, we investigated the ability of human lymphocytes – labeled with a lethal dose of 125IUdR – to exert an inhibitory (antiproliferative) bystander effect on the proliferation of unlabeled human adenocarcinoma colon cancer LS174T cells in vitro.
Materials and methods
Isolation of lymphocytes from human peripheral blood
Human blood was obtained from discarded apheresis collars from anonymous platelet donors from the Kraft Family Blood Donor Center, Dana-Farber Cancer Institute, Boston, MA, USA, in accordance with an Internal Review Board protocol (IRB #2005-P-001364/2). Each and every preparation of lymphocytes used in this study is from a single donor. 1 ml of heparinized blood in citrate buffer devoid of platelets was diluted to 4 ml with 1 × Hank's balanced salt solution (HBSS) (Cellgro Company, Manassas, VA, USA), overlaid on 3 ml of Ficoll-Paque™ 1.077 premium (GE Healthcare Co., Sweden) without mixing in a 15 ml falcon tube and centrifuged at 500 g for 40 min at 18°C. The upper layer containing plasma was discarded, the band containing mononuclear cells (∼2 ml) was transferred to a 15 ml falcon tube, washed with 6 ml of 1 × HBSS and centrifuged at 500 g at 18°C for 15 min. The cell pellet was washed once again, centrifuged and counted. Preparation contained 95% peripheral blood mononuclear cells (PBMC) with cell viability >95% as judged by trypan blue exclusion. PBMC were re-suspended in complete RPMI (Roswell Park Memorial Institute) 1640 medium (with L-Glutamine and 15% heat inactivated fetal bovine serum (FBS), ATCC, Manassas, VA, USA) and incubated in T-150 flasks for 1 h (37°C, 5% CO2) to allow the monocytes to adhere. The non-adherent lymphocytes were then transferred to another T-150 flask and incubated again for another 1 h. The nonadherent lymphocytes (>95% pure) were counted, diluted to 2 × 105 cells/ml, and cultured in the presence of PHA (0.12 μg/ml, Gibco, Grand Island, NY, USA) to stimulate cell division.
Labeling of dividing lymphocytes with 125IUdR
No-carrier added 125IUdR was synthesized from its trimethyl-stannyl derivative and Na125I as described previously (Foulon et al. 1996, Wang et al. 2008). 125IUdR was added to the proliferating PHA-stimulated lymphocytes at a concentration of ∼1.85 kBq/ml and the incubation continued for 48 h. This was chosen since it is the minimum concentration that leads to the incorporation of 125IUdR (often referred as lethal dose in the text) into nuclear DNA in amounts that are sufficient to arrest their proliferation, followed by cell death. The 125IUdR-labeled stimulated lymphocytes (125I-sL) were then spun down and washed three times with cold PBS (phosphate buffered saline) to remove non-DNA associated radioactivity. Viability of the cells was assessed by Trypan-blue staining followed by counting using hemocytometer. The uptake of 125I per cell was determined by counting known number of cells in γ-ray counter (Perkin Elmer 1480 Wizard Automatic; Calibrated and 80% efficiency). When required, dead 125IUdR-labeled lymphocytes were prepared by repeated cycles of freezing and thawing (three times).
LS174T cell culture and dead cell preparation
Human colon adenocarcinoma LS174T cells, purchased from American Type Culture Collection, were grown (37°C, 5% CO2) in complete Eagle Minimum Essential Medium (ATCC, Manassas, VA, USA) containing 10% FBS and penicillin/streptomycin. Dead LS174T cells used as spacers in experiments to maintain the same distance between the labeled cells were prepared by exposing live LS174T cells to three successive freezing (−135°C in Queue Cryostar) and thawing cycles at 37°C water bath. We have always found that the tumor cell growth is unaffected by the presence of dead spacer cells.
Suppression of cell proliferation – in vitro bystander effect
Co-cultures of LS174T cells(T)and 125I-lymphocytes(125I-sL). Logarithmically growing LS174T cells (4 × 105) were co-cultured with 125IUdR-labeled lymphocytes in various ratios (1:0, 1:0.25, 1:0.5, 1:1, 1:2.5, and 1:5) in six-well plates. Dead LS174T cells were used in the 1:0, 1:0.25, and 1:0.5 co-cultures as spacers such that the total number of cells in each well is the same (8 × 105). We did not add dead spacer cells to the co-cultures in ratios 1:2.5 and 1:5, as the total number of cells exceeded 8 × 105 in a well to start with. The summary of cell numbers in each co-culture experiment and control experiment carried out in parallel are shown in Tables I and II, respectively.
After five days of incubation, the adherent LS174T cells were washed (lymphocytes, which are non-adherent, are washed away), trypsinized, and counted to assess proliferation. As the lethally labeled lymphocytes do not proliferate, cell counts directly reflect the degree of cell proliferation of unlabeled live LS174T cells and any difference in the cell number relative to controls is attributed to suppression of the growth of live LS174T cells.
-
Incubations with supernatant media from co-cultures of LS174T cells and 125I-lymphocytes. Several co-cultures of LS174T tumor cells with 125IUdR labeled lymphocytes in the ratio 1:1 were set up in the tissue culture incubator. After 5 days, supernatant media were harvested from the individual co-cultures, pooled together, centrifuged (1750 g for 10 min at 4°C) to remove cell debris associated with residual radioactivity (∼27 Bq/ml) and stored at −135°C. These media were used in the following experiments to demonstrate whether the bystander effect exhibited by 125I-lymphocytes in the suppression of unlabeled LS174T tumor cell growth originates from the soluble factors secreted into the media either by the dying 125I-labeled lymphocytes or by the co-cultured tumor cells or by both.
Medium transfer experiment: The supernatant media was thawed, warmed to 37°C, and used as a culture medium (3 ml) for LS174T cells (4 × 105) seeded in triplicate in a six-well plate. Identical control experiment was also set up in which the supernatant media from the control incubations (unlabeled LS174T cells and dead LS174T cells in 1:1 ratio for 5 days, see Table II) was used as a culture medium for 4 × 105 LS174T cells. After 5 days of incubations, cells were trypsinized and counted to quantify the proliferation of the LS174T cells.
Clonogenic survival assay: Colony forming assay was performed using the supernatant media obtained from incubations of: (i) tumor cells + 125IUdR-labeled lymphocytes termed as ‘bystander-effect medium’, and (ii) tumor cells + dead tumor cells termed as ‘control medium’. Essentially, logarithmically growing LS174T cells (150) were seeded in T25 flasks in the presence of 8 ml either ‘control’ or ‘bystander-effect medium’ and flasks incubated at 37°C in 5% CO2. After 10 days of incubation, medium was decanted and the colonies washed with 1 × PBS, fixed with 8 ml of Bouin's solution (Sigma, St Louis, MO, USA) for 10 minutes, followed by an ethanol wash before they were stained with 8 ml 0.1% trypan-blue (Sigma, St Louis, MO, USA) for 30 min, rinsed in distilled water, air dried, and counted.
Screening for anti-proliferation factors: Supernatant media was also screened for factors associated with angiogenesis/cell proliferation pathways using an ELISA (Enzyme-linked immunosorbent assay) based antibody array (Raybiotech, Norcross, GA, USA) following vendor's protocol. RayBio Human Angiogenesis Antibody Array G Series 1000, allows simultaneous detection of 43 human angiogenic factors. After incubation with supernatant media, arrays were processed and shipped to Raybiotech (Norcross, GA, USA) for scanning and quantification of signals. Fluorescence signals were imaged at excitation wavelength of 532 nm in an Axon GenePix laser scanner. Signal intensities were used to quantify the expression levels of various relevant anti-proliferation factors.
Table I.
Number of cells† (× 105) used in co-cultures to demonstrate inhibitory (antiproliferative) bystander effect.
| Ratio (T:125l-sL) | T | T[K] | 125l-sL | Total number of cells per well |
|---|---|---|---|---|
| 1:0 | 4 | 4 | 0 | 8 |
| 1:0.25 | 4 | 3 | 1 | 8 |
| 1:0.5 | 4 | 2 | 2 | 8 |
| 1:1 | 4 | 0 | 4 | 8 |
| 1:2.5 | 4 | 0 | 10 | 14 |
| 1:5 | 4 | 0 | 20 | 24 |
T - Live LS174T cells; T[K] – dead LS174T cells; 125l-sL – 125IUdR-labeled stimulated lymphocytes.
Table II.
Number of cells† (× 105) used in control co-cultures.
| Co-culture (ratio 1:1) | T | T[K] | 125l_sL[K] | L | sL | Total number of cells per well |
|---|---|---|---|---|---|---|
| T:T[K] | 4 | 4 | 0 | 0 | 0 | 8 |
| T:125l-sL[K] | 4 | 0 | 4 | 0 | 0 | 8 |
| T:L | 4 | 0 | 0 | 4 | 0 | 8 |
| T:sL | 4 | 0 | 0 | 0 | 4 | 8 |
T - Live LS174T cells; T[K] - Dead LS174T cells; 125l-sL[K] - Dead 125IUdR-labeled stimulated lymphocytes; L - Unstimulated lymphocytes; sL - Stimulated lymphocytes.
Statistical analysis
Statistically significant differences between various experimental and control groups were plotted and analyzed using Origin (OriginLab Corp., Northampton, MA, USA) and Microsoft Excel. The adherence of two sets of data to the null hypothesis was tested by paired Student's t-test and represented by a P value. If P > 0.05, null hypothesis cannot be rejected and it is concluded that the mean values are not statistically different from each other.
Results
125IUdR labeling of human peripheral blood lymphocytes
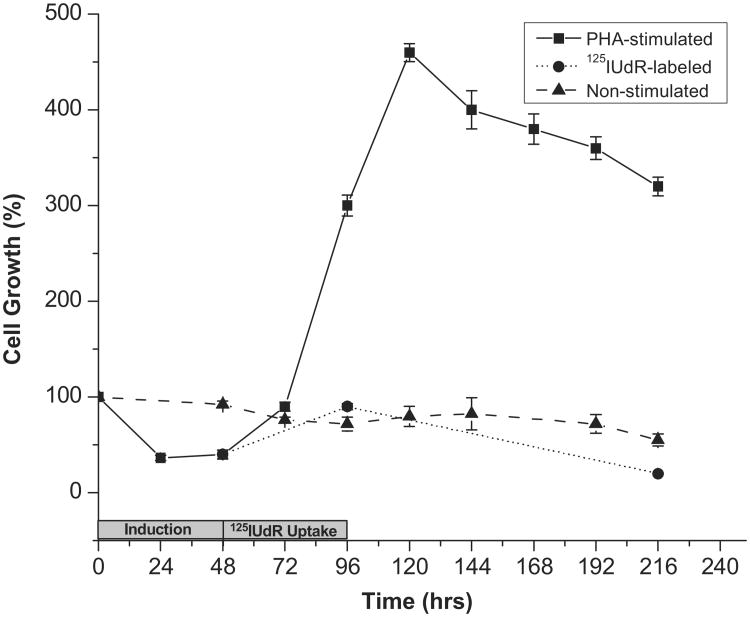
When lymphocytes were cultured in the presence of PHA, a mitogen that stimulates cell division as described previously (Stewart et al. 1975), the cells began to divide after 48 h of incubation with PHA and continued to divide until 120 h, after which the cell numbers decreased. As expected, lymphocytes that were not incubated with PHA do not divide (Figure 1).
Figure 1.
Growth of cultured human peripheral blood lymphocytes as a function of time when stimulated to proliferate with PHA (---■---), in the absence of PHA (---▲---), and after the addition of 125IUdR (1.85 kBq/ml) in the presence of PHA (---●---). Note that at 96 hours, 125IUdR labeled cells were washed and cultured in fresh medium containing no PHA or 125IUdR. Error bars indicate the ± standard deviation (SD) of the mean (n = 3) from three independent experiments.
To radiolabel the lymphocytes, 125IUdR was added to the lymphocyte culture 48 h after PHA induction (final concentration of 1.85 kBq/ml). The incubation was continued for another 48 h to enable the dividing cells to incorporate 125IUdR into their DNA. Under these experimental conditions, the mean cellular uptake of 125IUdR per lymphocyte was found to be 0.16 ± 0.03 mBq/cell (mean of 30 repeats). To determine whether this low cellular uptake is lethal (enough to stop the lymphocytes from proliferating), 125IUdR labeled lymphocytes were washed with PBS to remove unincorporated 125IUdR and cultured. Cell proliferation assessed by counting the viable cells after 5 days showed a marked decrease (∼ 80%) in cell number (Figure 1). Non-stimulated lymphocytes showed no change in their numbers during the same time course.
Bystander effect induced by 125IUdR-labeled human lymphocytes
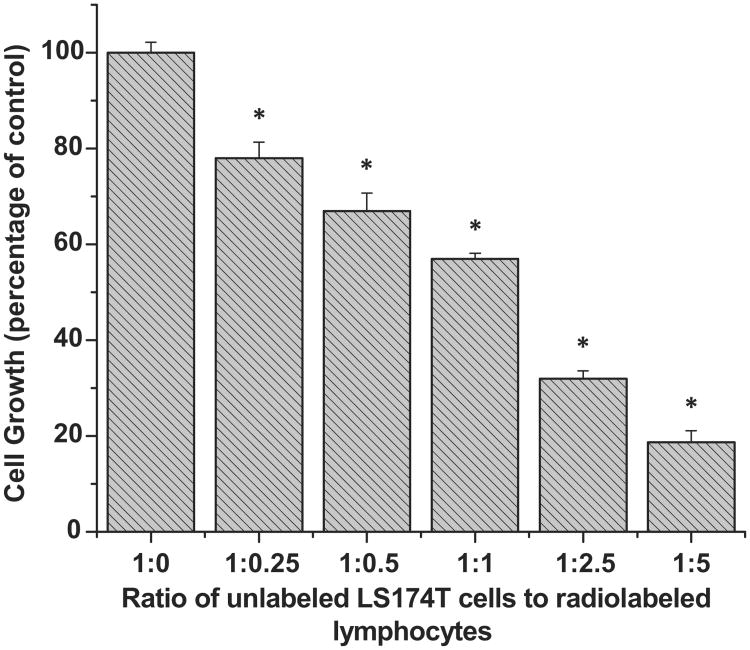
When logarithmically growing unlabeled LS174T cells (4 × 105) were mixed with 125IUdR labeled lymphocytes in increasing ratios (Table I) and the mixtures incubated for 5 days, the proliferation of unlabeled LS174T cells decreased proportionally (Figure 2). With regard to the proliferation of live LS174T cells containing no 125IUdR labeled tumor cells (taken as 100%), the proliferation of tumor cells was found to be 78 ± 3.3%, 67 ± 3.8%, 57 ± 1.1%, 32 ± 1.6%, and 18.7 ± 2.4% when mixed with 125IUdR labeled lymphocytes in the ratios 1:0.25, 1:0.5, 1:1, 1:2.5 and 1:5, respectively.
Figure 2.
Bystander effect induced in human colon adenocarcinoma LS174T cells when co-cultured with 125IUdR-labeled human lymphocytes. The ratio 1:0 represents the control. The total number of cells in each well is kept constant (8 × 105) by the addition of dead spacer cells (except at 1:1, 1:2.5, and 1:5 where no spacer cells were used). Co-cultures at each ratio were done in triplicate and error bars indicate ± SD of the mean (n = 3). *Indicates when p values are < 0.005 by paired Student's t-test.
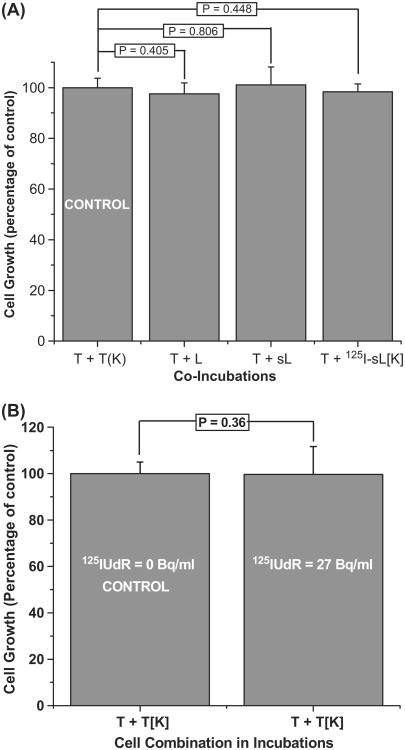
In order to rule out the possibility that reduced proliferation of LS174T cells was due to the either direct irradiation by the 125I-labeled cells in proximity or any 125I that leached out of dying radiolabeled cells, killed (by repeated freeze/ thaw cycles) 125IUdR labeled lymphocytes (125I-sL[K]) were co-cultured (Table II) with viable LS174T cells (1:1 ratio for 5 days). Under these experimental conditions, we did not observe any decrease in the growth of tumor cells (T + 125I-sL[K], Figure 3A). Similarly, no growth suppression of LS174T cells was seen when incubations contained equal number (Table II) of either naïve (not stimulated with PHA) or PHA-stimulated unlabeled lymphocytes (T + L and T + sL, Figure 3A, Table II). Finally, when LS174T cells were incubated in media containing 27 Bq/ml of 125IUdR, i.e., the concentration of 125I found in the supernatant media harvested from the 1:5 incubation of LS174T cells and 125IUdR-labeled lymphocytes for 5 days, no significant change in cell growth was observed (Figure 3B). Taken together, these data indicate that the reduction seen in the proliferation of live tumor cells in presence of 125I-lymphocytes can only be attributed to bystander effects exerted by the 125I-labeled human lymphocytes.
Figure 3.
(A) Growth of LS174T cells (T) when co-cultured with equal number (4 × 105) of un-stimulated lymphocytes (T + L), stimulated lymphocytes (T + sL), and dead (killed by freeze/thaw cycles) labeled lymphocytes (T + 125I-sL[K]) relative to the control LS174T cells (4 × 105) that were cultured with equal number of dead LS174T cells (T + T[K]). P values indicate that the growth of LS174T cells are unaffected by the presence of un-stimulated and stimulated lymphocytes as well as killed 125IUdR-labeled lymphocytes. Error bars indicate the ± SD of the mean (n = 3) from three independent experiments. (B) Growth of unlabeled LS174T cells following incubation for 5 days in the presence of 125IUdR at the extracellular concentration found in the supernatant media of co-cultures containing unlabeled tumor cells and 125I-lymphocytes incubated for 5-days (1:5 ratio, see Figure 2). P values demonstrate that the growth of LS174T cells is unaffected at these low extracellular concentrations (27 Bq/ml) of 125IUdR. Error bars indicate the ± SD of the mean (n = 3) from three independent experiments.
Bystander effect induced by supernatant medium in which 125IUdR labeled lymphocytes are co-incubated with LS174T cells
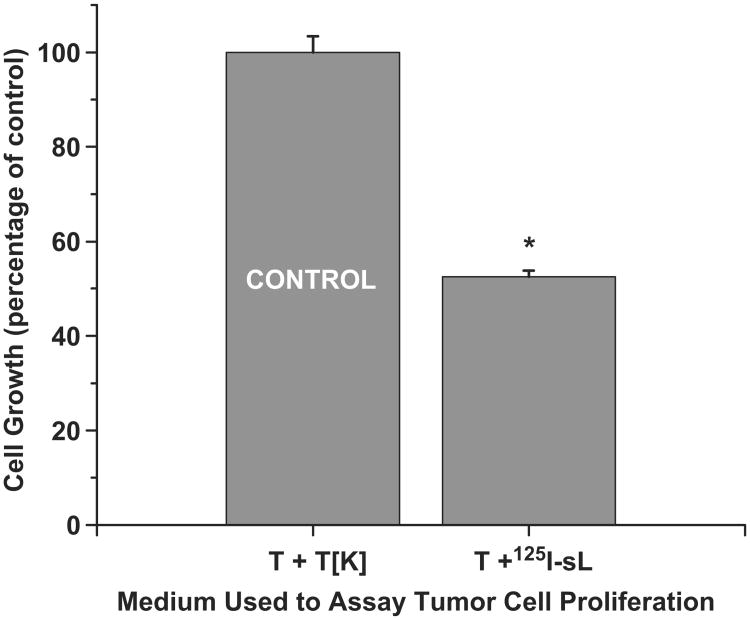
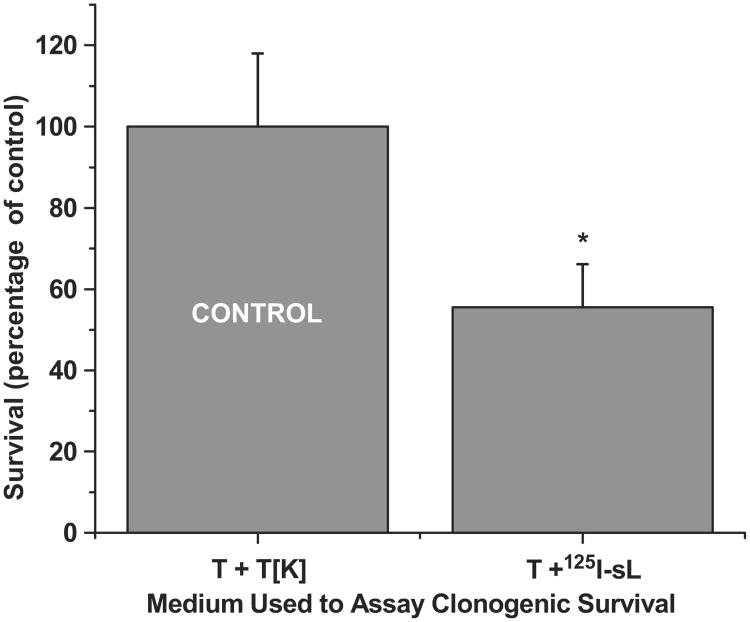
The supernatant media harvested following 5 day incubations of 125IUdR labeled lymphocytes with LS174T tumor cells was tested to assess its ability to induce the bystander effect in LS174T tumor cells. The harvested ‘bystander media’ contained extremely low levels of 125I activity (∼ 27 Bq/ml). Compared to the growth of tumor cells (4 × 105) incubated in ‘control media’ (tumor cells + dead tumor cells), tumor cells growth was suppressed (52.5 ± 1.3%) in the presence of ‘bystander-effect media’ (tumor cells + 125IUdR labeled lymphocytes, Figure 4) suggesting that factors secreted by the dying labeled lymphocytes and/or tumor cells are responsible for the observed bystander effect. Prompted by these observations, we carried out clonogenic survival assay for LS174T cells using the same supernatant ‘bystander-effect media’ and ‘control media’ . When the same numbers of LS174T cells (150) were seeded in these media and colonies were counted after 10 days, the results (Figure 5) showed that significantly fewer colonies (55.5 ± 10.6%) formed in the presence of the ‘bystander-effect media’ as compared to those that formed when the cells were grown in the ‘control media’.
Figure 4.
Media transfer experiment demonstrating that the bystander effect is observed in LS174T cells when these are cultured in media obtained from 5-day co-cultures of 125I-labeled lymphocytes and LS174T cells. Cultures was done in triplicate and error bars indicate ± SD of the mean (n = 3) from three independent experiments. *Indicates when p values are < 0.005 by paired Student's t-test.
Figure 5.
Clonogenic survival of LS174T cells when these are incubated in media obtained from 5-day co-cultures of 125I-labeled lymphocytes and LS174T cells. Each incubation was done in triplicate and error bars indicate ± SD of the mean (n = 3) from three independent experiments. *Indicates when p values are < 0.005 by paired Student's t-test.
Screening of supernatant bystander media for secreted factors TIMP-1 (Tissue inhibitor of metalloproteinase-1), TIMP-2 (Tissue inhibitor of metalloproteinase-2) IL-1α (Interleukin-1α), IL-1β (Interleukin-1β)
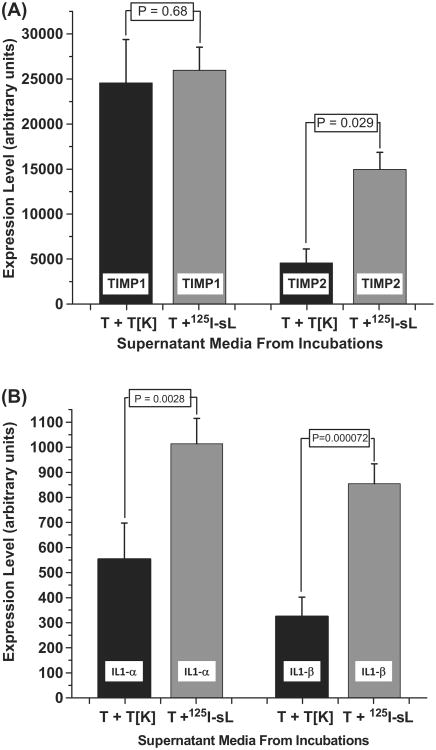
Earlier studies from our laboratory demonstrated that TIMP-1 and TIMP-2 – anti-angiogenic factors known to inhibit cell proliferation – were secreted into the media following the incubations of 125IUdR-labeled LS174T cells and unlabeled LS174T cells (Kishikawa et al. 2006). A simple ELISA-based antibody array revealed that the concentration of these proteins were several fold higher in bystander effect media compared to the control media. The supernatant media from the present incubations were also screened for anti-angiogenic factors and cytokines known to inhibit tumor growth and cell proliferation. Strikingly, bystander effect media contained ∼3.5-fold times elevated level of TIMP-2 than the control media while the level of TIMP-1 was about the same (Figure 6A). In addition, IL1-α and β levels were also found to be elevated by ∼ 2 times in the bystander effect media compared to the control media (Figure 6B).
Figure 6.
Expression levels of (A) TIMP1 and TIMP2 (B) IL1-α and IL1-β as determined by an ELISA-based antibody array, in the supernatant media harvested from the 1:1 co-cultures of tumor cells and 125I-labeled lymphocytes (T + 125I-sL) in which an inhibitory (antiproliferative) bystander effect was observed. Tumor cells and dead tumor cells (T + T[K]) were used as controls. Assays were repeated four times and the mean values ± SD of the mean (n = 2) from two independent experiments are shown.
Discussion
In previous studies, we had shown that when mice are injected with a mixture of the viable human colon LS174T adenocarcinoma cells and LS174T cells labeled with lethal doses of DNA-incorporated Auger emitter 125I, the growth of these s.c. tumors was inhibited by ∼ 50% (Xue et al. 2002, Kishikawa et al. 2006). The proliferation of these tumor cells was similarly retarded when the tumor cells were co-incubated with 125I-labeled tumor cells in vitro (Kishikawa et al. 2006). Our studies also showed that the antiproliferative growth inhibitory effects were observed even when the LS174T cells were cultured in the presence of media obtained from these co-cultures. While these in vitro and in vivo studies were definitive, they are basically phenomenological and do not have translational value. However, because of our longtime continued interest in the therapeutic potential of Auger electron emitters (Kassis et al. 1996, Kassis 2003), we wondered whether the inhibitory (antiproliferative) bystander effect could play an adjuvant role in cancer therapy. To these ends, we assessed the ability of human lymphocytes labeled with DNA-incorporated 125I to exert an (antiproliferative) bystander effect on co-cultured human colon adenocarcinoma LS174T cells in vitro. Our choice for the use of blood lymphocytes was guided by the following: (i) These cells can be easily isolated from a patient's blood and can be re-injected into the same patient after radiolabeling (no risk of being rejected); (ii) using the mitogen PHA, lymphocytes can be stimulated to synthesize DNA (Nowell 1960, Stewart et al. 1975) and can therefore be radiolabeled with 125IUdR; (iii) lymphocytes are highly radiosensitive. Thus, the DNA-incorporated 125I activities necessary to kill these cells and induce an inhibitory (antiproliferative) bystander effect should be very low; (iv) the targeted tumor cells used in our in vitro experiments are also of human origin; and (v) risks associated with using such lymphocytes labeled with very low 125I activity, in human should be significantly lower. In this context, it is worth noting that the 125I activity incorporated per lymphocyte (0.16 ± 0.03 mBq) used in our study to demonstrate bystander effect is much (∼ 350-fold to 13,000-fold) lower compared to the 111In (Thakur and McAfee 1984) and 99mTc (Merz et al. 1986) activities in radio-labeled lymphocytes, required to induce severe chromosomal aberrations, sufficient to onset lymphoid malignancy.
Our studies confirmed our expectations. First, the incubation of PHA-stimulated human lymphocytes with 125IUdR led to the efficient incorporation of 125I into the DNA of these cells. Second, the PHA-induced proliferation of these cells was inhibited following 125I-DNA-incorpora-tion (Figure 1). As expected, the DNA-incorporated activity necessary to inhibit the growth of these radiosensitive cells (∼ 0.16 mBq/cell) was much lower (∼ 1%) than that needed to inhibit the proliferation of LS174T tumor cells (Kishikawa et al. 2006). Finally, the co-culture of PHA-stimulated 125I-DNA-incorporated human lymphocytes (125I-sL) with unlabeled human colon LS174T adenocarcinoma cells inhibited the proliferation of the unlabeled LS174T cells and that greater inhibition was found when the ratio of the 125I-sL to LS174T is increased (Figure 2). On the contrary, 3H-labeled rat liver epithelial cells (WB-F344) exhibited an exactly opposite trend - a proliferative bystander effect - increase in the ratio of labeled to unlabeled bystander cells in co-cultures led to increase in the proliferation of bystander cells (Gerashchenko and Howell 2005). The growth inhibitions observed for unlabeled LS174T cells co-cultured with 125I-labeled lymphocytes, were also seen when the LS174T cells were cultured in supernatant media obtained from these co-cultures highlighting the role of soluble factors secreted either by the dying 125I-lymphocytes or/and the bystander tumor cells as a response (Figure 4). Intriguingly, the degree of reduction in the clonogenic survival of LS174T cells (55.5 ± 10.6%, see Figure 5) in the presence of bystander effect media is consistent with the extent of inhibition of cell growth observed in the presence of either 125IUdR labeled lymphocytes (57 ± 1.1%, see Figure 2) or the media from such incubations (52.5 ± 1.3%, see Figure 4), further validating that this bystander effect is mediated by the soluble factors secreted into the media.
The experimental protocol used in our studies necessitated that PHA-stimulated lymphocytes – radiolabeled with 125IUdR at a concentration that inhibits further cell division (Figure 1) – are co-cultured with LS174T cells. While various studies had shown that the addition of such mitogen-stimulated lymphocytes to tumor cells leads to pronounced growth suppression (Nelson et al. 1976, Huges-Law et al. 1978), we believe that the tumor-cell growth retardation seen in our studies can only be ascribed to a bystander effect since: (i) The ratios used in our studies (≤ 1:1 ratios) are much lower than the ≥ 5:1 ratios used by other investigators to induce cytotoxicity, and (ii) the growth rate of the LS174T tumor cells was not altered when they were co-cultured with PHA-stimulated unlabeled lymphocytes (Figure 3A). Finally, that these observed cell growth retardations are only a consequence of a bystander effect and not due to a radiation dose-mediated effect is demonstrated by the absence of any cell growth inhibition following the incubation of the tumor cells in radioiodinated stimulated lymphocytes (1:5 ratio) that had been killed (repeated freeze/thaw cycles) prior to their addition to viable LS174T cells (Figure 3A) or the culture of LS174T cells in the presence of 125IUdR at an extracellular concentration that is equivalent to that present in the media of 125I-sL – LS174T of 5-day co-cultures (Figure 3B).
The molecular repertoire involved in the signaling mechanisms behind bystander stimuli from irradiated lymphocytes and responses by the un-irradiated bystander tumor cells are yet to be understood clearly. Various pathways, mechanisms and molecules have been suggested so far. Cytokines released systemically in response to ionizing radiation have been implicated in antitumor activity at a distant site, termed as abscopal effect (Camphausen et al. 2003). For example, the rise in p53 levels during this event was thought to have a role in secretion of stress induced growth inhibitors (Komarova et al. 1998). Antioxidants, which have been shown to inhibit bystander effects, indicated a possible role for reactive oxygen species (Azzam et al. 2002). Such elevations of ROS have been accompanied by over expression of signaling molecules such as IL8, IL1, transforming growth factor-beta 1 (Sawant et al. 2001, Osterreicher et al. 2003). In medium transfer experiments, recipient cells showed a rapid calcium pulse followed by changes in mitochondrial membrane permeability and increases in ROS levels (Freeman et al. 1995). While the exact signaling cascade remains elusive, we too have found elevated levels of cytokines IL1-α and β, which are known to be upregulated in response to oxidative stress in bystander cells (Osterreicher et al. 2003) in the media from tumor cell- 125I-lymphocytes co-cultures (Figure 6B).
In previous studies (Kishikawa et al. 2006), we reported elevated levels of TIMP1 and TIMP2 in the media derived from the co-incubation of 125I-labeled LS174T cells and unlabeled tumor cells. TIMP2 is clearly known to be involved in anti-cell proliferation pathways (Hojilla et al. 2003, Seo et al. 2003, Stetler-Stevenson and Seo 2005). However the role of TIMP1 is less clear. This factor has been shown to either inhibit or promote proliferation depending on its extracellular concentration (Hayakawa et al. 1992, Narayanan et al. 1999). In our present studies, 125I-labeled lymphocytes were used instead of 125I-labeled LS174T cells. Our data demonstrate an increase in the levels of TIMP2 only in the supernatant bystander media while the level of TIMP-1 was found to be the same as that of control media (Figure 6A). Taken together with our previously published work, these results suggest that the mechanisms of the inhibitory (antiproliferative) bystander effect may depend on 125I-labeled cell type.
To ensure that the elevated TIMP2 secretions are a response to the exposure of lymphocytes to 125I decays and not due to the cytotoxic properties of PHA stimulated lymphocytes, PHA-stimulated lymphocytes (unlabeled with 125IUdR) were incubated with tumor cells for five days and the supernatant media was screened for TIMP2. The results of these studies (not shown) demonstrated that TIMP2 is not upregulated in this situation. This finding implies that the over expression (∼ 3.5-fold) of TIMP2 only in co-cultures containing PHA-stimulated 125I-labeled lymphocytes and tumor cells may be partly responsible for the observed tumor cell proliferation bystander effect by triggering hitherto unknown signaling pathways.
In conclusion, we report a reduction in the proliferation of LS174T cells in presence of 125I-labeled lymphocytes or media obtained from such co-cultures. These observations can be attributed to an inhibitory (antiproliferative) bystander effect, probably mediated by factor(s) released by the dying 125I-labeled lymphocytes.
Acknowledgments
This work was supported in part by NIH 5 R01 CA15523 (Amin I. Kassis).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Auger P. Sur les rayons b secondaires produits dans un gaz par des rayons X. Comptes Rendus Hebdomadaires des Seances de l Academie des Sciences. 1925;180:65–68. [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proceedings of the National Academy of Sciences of the USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Research. 2002;62:5436–5442. [PubMed] [Google Scholar]
- Azzam EI, Little JB. The radiation-induced bystander effect: Evidence and significance. Human and Experimental Toxicology. 2004;23:61–65. doi: 10.1191/0960327104ht418oa. [DOI] [PubMed] [Google Scholar]
- Belloni P, Latini P, Palitti F. Radiation-induced bystander effect in healthy G(o) human lymphocytes: Biological and clinical significance. Mutation Research. 2011;713:32–38. doi: 10.1016/j.mrfmmm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Belloni P, Meschini R, Czene S, Harms-Ringdahl M, Palitti F. Studies on radiation-induced apoptosis in G0 human lymphocytes. International Journal of Radiation Biology. 2005;81:587–599. doi: 10.1080/09553000500303690. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Hill HZ, Stein D, Rao DV, Howell RW. Free radical-initiated and gap junction-mediated bystander effect due to nonuniform distribution of incorporated radioactivity in a three-dimensional tissue culture model. Radiation Research. 2001;155:335–344. doi: 10.1667/0033-7587(2001)155[0335:friagj]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee A, Rao DV, Howell RW. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiation Research. 1999;152:88–97. [PMC free article] [PubMed] [Google Scholar]
- Blyth BJ, Sykes PJ. Radiation-induced bystander effects: What are they, and how relevant are they to human radiation exposures? Radiation Research. 2011;176:139–157. doi: 10.1667/rr2548.1. [DOI] [PubMed] [Google Scholar]
- Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, Mairs RJ. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. Journal of Nuclear Medicine. 2006;47:1007–1015. [PubMed] [Google Scholar]
- Boyd M, Sorensen A, McCluskey AG, Mairs RJ. Radiation quality-dependent bystander effects elicited by targeted radionuclides. Journal of Pharmacy and Pharmacology. 2008;60:951–958. doi: 10.1211/jpp.60.8.0002. [DOI] [PubMed] [Google Scholar]
- Camphausen K, Moses MA, Menard C, Sproull M, Beecken WD, Folkman J, O'Reilly MS. Radiation abscopal antitumor effect is mediated through p53. Cancer Research. 2003;63:1990–1993. [PubMed] [Google Scholar]
- Charlton DE. The range of high LET effects from 125I decays. Radiation Research. 1986;107:163–171. [PubMed] [Google Scholar]
- Charlton DE, Pomplun E, Booz J. Some consequences of the Auger effect: Fluorescence yield, charge potential, and energy imparted. Radiation Research. 1987;111:553–564. [PubMed] [Google Scholar]
- Deshpande A, Goodwin EH, Bailey SM, Marrone BL, Lehnert BE. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: Evidence for an extranuclear target. Radiation Research. 1996;145:260–267. [PubMed] [Google Scholar]
- Foulon CF, Adelstein SJ, Kassis AI. Kit formulation for the preparation of radiolabeled iododeoxyuridine by demetallation. Journal of Nuclear Medicine. 1996;37(4, suppl.):1S–3S. [PubMed] [Google Scholar]
- Fournier C, Becker D, Winter M, Barberet P, Heiss M, Fischer B, Topsch J, Taucher-Scholz G. Cell cycle-related bystander responses are not increased with LET after heavy-ion irradiation. Radiation Research. 2007;167:194–206. doi: 10.1667/rr0760.1. [DOI] [PubMed] [Google Scholar]
- Frankenberg D, Greif KD, Giesen U. Radiation response of primary human skin fibroblasts and their bystander cells after exposure to counted particles at low and high LET. International Journal of Radiation Biology. 2006;82:59–67. doi: 10.1080/09553000600582979. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Ramesh R, Shastri M, Munshi A, Jensen AK, Marrogi AJ. The role of cytokines in mediating the bystander effect using HSV-TK xenogeneic cells. Cancer Letters. 1995;92:167–174. doi: 10.1016/0304-3835(95)03771-n. [DOI] [PubMed] [Google Scholar]
- Gerashchenko BI, Howell RW. Bystander cell proliferation is modulated by the number of adjacent cells that were exposed to ionizing radiation. Cytometry A. 2005;66:62–70. doi: 10.1002/cyto.a.20150. [DOI] [PubMed] [Google Scholar]
- Gorman S, Tosetto M, Lyng F, Howe O, Sheahan K, O'Donoghue D, Hyland J, Mulcahy H, O'Sullivan J. Radiation and chemotherapy bystander effects induce early genomic instability events: Telomere shortening and bridge formation coupled with mitochondrial dysfunction. Mutation Research. 2009;669:131–138. doi: 10.1016/j.mrfmmm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Gow MD, Seymour CB, Ryan LA, Mothersill CE. Induction of bystander response in human glioma cells using high-energy electrons: A role for TGF-beta1. Radiation Research. 2010;173:769–778. doi: 10.1667/RR1895.1. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Letters. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. British Journal of Cancer. 2003;89:1817–1821. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell RW, Bishayee A. Bystander effects caused by nonuniform distributions of DNA-incorporated (125)I. Micron. 2002;33:127–132. doi: 10.1016/s0968-4328(01)00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huges-Law G, de Gast GC, The TH. PHA-induced cytotoxicity of human lymphocytes against adherent hela cells. Journal of Immunological Methods. 1978;19:29–39. doi: 10.1016/0022-1759(78)90004-2. [DOI] [PubMed] [Google Scholar]
- Huo L, Nagasawa H, Little JB. HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations. Radiation Research. 2001;156:521–525. doi: 10.1667/0033-7587(2001)156[0521:hmiibc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ilnytskyy Y, Kovalchuk O. Non-targeted radiation effects – an epigenetic connection. Mutation Research. 2011;714:113–125. doi: 10.1016/j.mrfmmm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Kassis AI. Cancer therapy with Auger electrons: Are we almost there? Journal of Nuclear Medicine. 2003;44:1479–1481. [PubMed] [Google Scholar]
- Kassis AI. The amazing world of auger electrons. International Journal of Radiation Biology. 2004;80:789–803. doi: 10.1080/09553000400017663. [DOI] [PubMed] [Google Scholar]
- Kassis AI, Adelstein SJ, Mariani G. Radiolabeled nucleoside analogs in cancer diagnosis and therapy. Quarterly Journal of Nuclear Medicine. 1996;40:301–319. [PubMed] [Google Scholar]
- Kassis AI, Sastry KS, Adelstein SJ. Kinetics of uptake, retention, and radiotoxicity of 125IUdR in mammalian cells: Implications of localized energy deposition by Auger processes. Radiation Research. 1987;109:78–89. [PubMed] [Google Scholar]
- Kishikawa H, Wang K, Adelstein SJ, Kassis AI. Inhibitory and stimulatory bystander effects are differentially induced by Iodine-125 and Iodine-123. Radiation Research. 2006;165:688–694. doi: 10.1667/RR3567.1. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Diatchenko L, Rokhlin OW, Hill JE, Wang ZJ, Krivokry-senko VI, Feinstein E, Gudkov AV. Stress-induced secretion of growth inhibitors: A novel tumor suppressor function of p53. Oncogene. 1998;17:1089–1096. doi: 10.1038/sj.onc.1202303. [DOI] [PubMed] [Google Scholar]
- Lehnert BE, Goodwin EH. Extracellular factor(s) following exposure to a particles can cause sister chromatid exchanges in normal human cells. Cancer Research. 1997;57:2164–2171. [PubMed] [Google Scholar]
- Liu Z, Mothersill CE, McNeill FE, Lyng FM, Byun SH, Seymour CB, Prestwich WV. A dose threshold for a medium transfer bystander effect for a human skin cell line. Radiation Research. 2006;166:19–23. doi: 10.1667/RR3580.1. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiation Research. 2006;165:400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. British Journal of Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: A possible mechanism for bystander-induced genomic instability? Radiation Research. 2002;157:365–370. doi: 10.1667/0033-7587(2002)157[0365:ioaice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Merz T, Tatum J, Hirsch J. Technetium-99m-labeled lymphocytes: A radiotoxicity study. Journal of Nuclear Medicine. 1986;27:105–110. [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. International Journal of Radiation Biology. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Research. 1992;52:6394–6396. [PubMed] [Google Scholar]
- Narayanan PK, LaRue KE, Goodwin EH, Lehnert BE. Alpha particles induce the production of interleukin-8 by human cells. Radiation Research. 1999;152:57–63. [PubMed] [Google Scholar]
- Nelson DL, Bundy BM, Pitchon HE, Blaese RM, Strober W. The effector cells in human peripheral blood mediating mitogen-induced cellular cytotoxicity and antibody-dependent cellular cytotoxicity. Journal of Immunology. 1976;117:1472–1481. [PubMed] [Google Scholar]
- Nowell PC. Phytohemagglutinin: An initiator of mitosis in cultures of normal human leukocytes. Cancer Research. 1960;20:462–466. [PubMed] [Google Scholar]
- Nugent SM, Mothersill CE, Seymour C, McClean B, Lyng FM, Murphy JE. Increased mitochondrial mass in cells with functionally compromised mitochondria after exposure to both direct gamma radiation and bystander factors. Radiation Research. 2007;168:134–142. doi: 10.1667/RR0769.1. [DOI] [PubMed] [Google Scholar]
- Osterreicher J, Skopek J, Jahns J, Hildebrandt G, Psutka J, Vilasova Z, Tanner JM, Vogt J, Butz T. Beta1-integrin and IL-1alpha expression as bystander effect of medium from irradiated cells: the pilot study. Acta Histochemistry. 2003;105:223–230. doi: 10.1078/0065-1281-00710. [DOI] [PubMed] [Google Scholar]
- Parsons WB, Jr, Watkins CH, Pease GL, Childs DS., Jr Changes in sternal marrow following roentgen-ray therapy to the spleen in chronic granulocytic leukemia. Cancer. 1954;7:179–189. doi: 10.1002/1097-0142(195401)7:1<179::aid-cncr2820070120>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Persaud R, Zhou H, Baker SE, Hei TK, Hall EJ. Assessment of low linear energy transfer radiation-induced bystander mutagenesis in a three-dimensional culture model. Cancer Research. 2005;65:9876–9882. doi: 10.1158/0008-5472.CAN-04-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud R, Zhou H, Hei TK, Hall EJ. Demonstration of a radiation-induced bystander effect for low dose low LET beta-particles. Radiation and Environmental Biophysics. 2007;46:395–400. doi: 10.1007/s00411-007-0116-1. [DOI] [PubMed] [Google Scholar]
- Pomplun E, Booz J, Charlton DE. A Monte Carlo simulation of Auger cascades. Radiation Research. 1987;111:533–552. [PubMed] [Google Scholar]
- Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiation Research. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, Wingfeld PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: An MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Sgouros G, Knox SJ, Joiner MC, Morgan WF, Kassis AI. MIRD continuing education: Bystander and low dose-rate effects: Are these relevant to radionuclide therapy? Journal of Nuclear Medicine. 2007;48:1683–1691. doi: 10.2967/jnumed.105.028183. [DOI] [PubMed] [Google Scholar]
- Souto J. Tumour development in the rat induced by blood of irradiated animals. Nature. 1962;195:1317–1318. doi: 10.1038/1951317a0. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Seo DW. TIMP-2: An endogenous inhibitor of angiogenesis. Trends in Molecular Medicine. 2005;11:97–103. doi: 10.1016/j.molmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Stewart CC, Cramer SF, Steward PG. The response of human peripheral blood lymphocytes to phytohemagglutinin: Determination of cell numbers. Cellular Immunology. 1975;16:237–250. doi: 10.1016/0008-8749(75)90115-x. [DOI] [PubMed] [Google Scholar]
- Takur ML, McAfee JG. The significance of chromosomal aberrations in indium-111-labeled lymphocytes. Journal of Nuclear Medicine. 1984;25:922–927. [PubMed] [Google Scholar]
- Wang K, Adelstein SJ, Kassis AI. DMSO increases radioiodination yield of radiopharmaceuticals. Applied Radiation and Isotopes. 2008;66:50–59. doi: 10.1016/j.apradiso.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LY, Butler NJ, Makrigiorgos GM, Adelstein SJ, Kassis AI. Bystander effect produced by radiolabeled tumor cells in vivo. Proceedings of the National Academy of Sciences of the USA. 2002;99:13765–13770. doi: 10.1073/pnas.182209699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Magpayo N, Held KD. Targeted and non-targeted effects from combinations of low doses of energetic protons and iron ions in human fibroblasts. International Journal of Radiation Biology. 2011;87:311–319. doi: 10.3109/09553002.2010.537431. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhou J, Held KD, Redmond RW, Prise KM, Liber HL. Deficiencies of double-strand break repair factors and effects on mutagenesis in directly gamma-irradiated and medium-mediated bystander human lymphoblastoid cells. Radiation Research. 2008;169:197–206. doi: 10.1667/RR1189.1. [DOI] [PubMed] [Google Scholar]