Abstract
Objectives
To examine the relationship between a belief about Food and Drug Administration (FDA) safety evaluation of cigarettes and smoking risk perceptions.
Methods
A nationally representative, random-digit-dialed telephone survey of 1046 adult current cigarette smokers.
Results
Smokers reporting that the FDA does not evaluate cigarettes for safety (46.1%), exhibited greater comprehension of the health risks of smoking and were more likely (48.5%) than other participants (33.6%) to report quit intentions. Risk perceptions partially mediated the relationship between FDA evaluation belief and quit intentions.
Conclusions
These findings highlight the need for proactive, effective communication to the public about the aims of new tobacco product regulations.
Keywords: Food and Drug Administration, evaluation, regulation, smoking, risk
Despite the well-documented negative health effects of tobacco, most countries lack a comprehensive system for regulation of manufacturing, marketing, and distribution of tobacco products aimed at protecting public health.1,2 Historically, cigarettes and other tobacco products have been exempt from health and safety standards governing contents and designs that are typically applied to other consumer products, including foods, beverages, and drugs.3 However, in recent years there have been substantial international efforts to develop principles and guidelines for tobacco product regulations.4 Articles 9 and 10 of the Framework Convention on Tobacco Control (FCTC) require participating countries to implement measures for testing and regulating the contents and emissions of tobacco products and for mandating the disclosure of product information by manufacturers.5 On June 22, 2009, US President Obama signed legislation granting the Food and Drug Administration (FDA) broad regulatory authority over many aspects of the manufacture, sale, labeling, advertising, and promotion of tobacco products.6 Tobacco product regulatory systems include a wide variety of measures important for public health, such as restrictions on sales and marketing of tobacco products and stronger warning labels. However, a central component is the introduction of product testing and performance standards.
Concerns have been raised about the potential unintended consequences of government-mandated testing of tobacco products on consumer perception. In particular, some commentators have suggested that consumers may develop a false sense of security from information that a government agency is establishing product standards or conducting product testing, leading them to believe that newly evaluated tobacco products are “safer” or “less harmful” compared to products available previously.7,8 Although product testing protocols and performance standards are not necessarily aimed at protecting health and safety, consumers may misinterpret such activities as protecting them against risk. Even if information about regulatory actions is presented with appropriate caveats, consumers may be misled as a result of inadequate communication by the media or by the promotional activities of tobacco manufacturers. For example, communications about tobacco product testing may “be reduced to a simple and misleading message by the media that the newly regulated tobacco products are now ‘safer’.”7 As a result, if consumers do interpret mandated product testing or performance standards as implying reduced risks, some individuals may be more likely to initiate smoking, increase their tobacco use, or be less motivated to quit.8 Past experience with “light” and low-tar cigarettes demonstrates how data from standardized testing protocols can be misrepresented or misunderstood by the public. The promotion of low-yield cigarettes by the tobacco industry likely played a significant role in promoting initiation and impeding cessation, the most important determinants of smoking-related diseases.9 Standardized labeling of tar and nicotine content in cigarette advertising based on a laboratory test sanctioned by the Federal Trade Commission (FTC) may have contributed to consumers’ confidence in the significance of these values for health. Recent evidence shows that smokers of low-yield cigarettes continue to erroneously believe that they are substantially reducing their risk in choosing lower yield products.10–12 The Family Smoking Prevention and Tobacco Control Act of 2009 prohibits the use of terms like “light,” “low,” and “mild” on cigarette labels and advertising,6 but data suggest that consumers may continue to perceive differences in risk across brands based on packaging color and other features.13
Lay beliefs about cigarette smoking and health are varied and multifaceted. In many cases, these beliefs indicate the degree to which an individual understands the risks of smoking. People can be said to understand the risks of smoking if they can correctly identify: (1) the difficulty of avoiding harm (ie, intentions of smoking in the future), (2) factors that might increase or decrease an individual’s susceptibility to harm (ie, beliefs about “safer” cigarettes), (3) the absolute and relative probability of harm, and (4) the nature of potential harm (ie, the name and severity of illnesses that can result from smoking).14,15 Unfortunately, many smokers do not fully comprehend the risks of experiencing severe negative health consequences as a result of smoking.15–17 For example, a 2003 US nationally representative survey found that 51.7% of current smokers incorrectly reported that they could reduce their risk of experiencing tobacco-related adverse health outcomes by exercising and 13.4% believed there is no risk of cancer from smoking for only a few years.18
Adding to the complexity of the situation is the observation that many people lack an understanding of what it means for FDA to regulate a product. For example, prior experimental research in the context of dietary supplements has shown that individuals are poorly informed about the FDA’s role in regulating dietary supplements, and many believe that regulated supplements are safer and more effective than nonregulated products.19 One integral component of FDA’s public health approach to tobacco regulation includes public education about regulation, particularly about tobacco constituents and exposure consequences.20 It is possible that perceptions of safety may exist for regulated tobacco products; however, little is known about consumers’ beliefs about government evaluation of tobacco products or how such beliefs may be related to their perceptions about the risks of smoking.
Study Objective
The objective of the current study is to examine, via analysis of an existing data set, how smokers’ beliefs about whether or not FDA evaluates cigarettes for safety are related to their comprehension of the risks of smoking. Although it is important to note that at the time of the study (2001) there was no regulation of tobacco, the purpose of this study was not to understand evaluation knowledge, but rather to examine the relationship between the belief that there is (or is not) FDA evaluation of tobacco products for safety and perceptions of risk. Based on Weinstein’s empirically derived framework for comprehending risk, variables were categorized accordingly: (1) difficulty of avoiding the harmful consequences of smoking, (2) factors that influence individual susceptibility to harm, (3) estimates of the probability of harm, and (4) identification of the nature of potential harm.14 Conceptualizing variables into elements within this framework allows us to better understand the relationships under study. Belief about government evaluation of tobacco products was assessed by one question in the survey (“Do you think cigarettes are evaluated for safety by the US Food and Drug Administration before they are sold to consumers?”). It is predicted that smokers who report that the FDA does not evaluate cigarettes for safety would have more realistic perceptions of the smoking-associated risks than smokers who reported that the FDA does evaluate cigarettes. That is, they will be more likely to intend to stop smoking in the future, endorse fewer myths about the factors that influence individual susceptibility to harm, provide more accurate estimates of the absolute probability of smoking-related mortality, be less likely to believe that their risk of developing a smoking-related illness is less than the average person’s, and will know more about the illnesses caused by smoking. In this study, risk comprehension is assessed by measuring the accuracy of a series of tobacco-related risk perceptions held by the respondents.
Moreover, some health behavior theories posit that behavioral intentions are predicted by risk beliefs (eg, theory of planned behavior).21,22 One concern about tobacco product regulatory proposals that involve routine product testing and performance standards is that consumers may erroneously infer that newly regulated products are less harmful than those available before the onset of regulation and, in turn, that these consumers will be less likely to quit. In other words, as shown in Figure 1, risk perceptions may mediate a relationship between beliefs about government evaluation of tobacco products and intentions to quit smoking in the next year. We conducted a mediational analysis to assess whether there is evidence for such a relationship.
Figure 1.
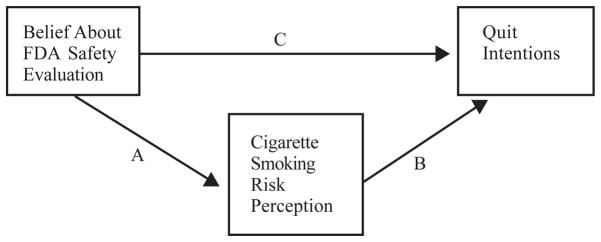
Risk Perception May Mediate the Relationship Between FDA Safety Evaluation Belief and Quit Intentions
Note.
Path A represents the relationship between belief about FDA evaluation and risk perception; Path B represents the relationship between risk perception and quit intentions; Path C represents the change in the relationship between FDA evaluation belief and quit intentions after controlling for each risk perception item.
METHOD
The Beliefs About Alternative Nicotine Delivery Devices (BAND) survey is a nationally representative, random-digit-dialed telephone survey of adult current cigarette smokers that was conducted between May and September 2001. Its primary objective was to assess participants’ beliefs about nicotine products and medications. A total of 49,593 households were screened, resulting in a survey group of 1046 current smoking adults (ie, people who report smoking at least 100 cigarettes in their lifetime and currently smoke cigarettes every day or some days). The response rate was 77%, computed as the proportion of households originally designated for the sample that provided information for the research.23 The data were weighted to adjust for the probability of selection and the age, race, and gender distribution of US adult smokers, using estimates from the 1998–1999 Tobacco Use Supplement to the current Population Survey (TUS-CPS). The original sample proportions were comparable in age, race, and education level to the national estimates of adult smokers, with a slightly higher percentage of women than men in the sample. All statistics were run using proportions obtained by this weighting procedure, with weighted N values normalized to the original sample size (1046).24
Approximately 52% of participants were male and the majority were white (78.5%). About 8% of participants were black, 8% were Hispanic, and 6% were categorized as Other (Asian/Pacific Islander, American Indian/Alaska Native, other race). Approximately 12% of participants had not completed high school, 41% completed high school, 31% had schooling beyond high school, and 16% completed 16 years of education or more. Approximately 19% were 18–24 years old, 18% were 25–34, 26% were 35–44, 22% were 45–54, and 15% were 55 or older.
Measures
Respondents were asked a limited number of questions pertaining to their perceptions about the hazards of smoking. To provide a conceptual framework, items were organized according to Weinstein’s 4 criteria for comprehending risk (see Table 1 for organization and full list of items).
Table 1.
Bivariate Analyses Examining the Relationship Between Cigarette Smoking Risk Perceptions and FDA Safety Evaluation Beliefs
| FDA Evaluation (n unweighted) | No FDA Evaluation (n unweighted) | Total | |||
|---|---|---|---|---|---|
| Difficulty of Avoiding Harm | |||||
| Do you think you will quit smoking before you experience a serious health problem caused by smoking? | Yes | 78.6% (350) | P=.011 | 73.9% (284) | 76.4% (634) |
| If we called you back in a year, will you be smoking? | No | 33.6% (160) | P<.001 | 48.5% (182) | 40.5% (342) |
| Factors That Influence Individual’s Susceptibility | |||||
| High-tar cigarettes are at least twice as likely to cause illness as ones that are low in tar. | Disagree | 27.8% (156) | P=.004 | 38.4% (185) | 32.7% (341) |
| If a person smokes only 5 cigarettes per day, their chances of getting cancer from smoking are about the same as someone who never smokes. | Disagree | 54.5% (303) | P=.008 | 64.4% (296) | 59.0% (599) |
| If you don’t inhale, smoking is not really dangerous. | Disagree | 73.1% (414) | P=.346 | 76.7% (383) | 74.7% (797) |
| Cigarettes with additives are more harmful than the ones that don’t have additives. | Disagree | 23.5% (133) | P=.018 | 31.7% (154) | 27.3% (287) |
| The milder the smoke, the less dangerous the cigarette. | Disagree | 57.9% (324) | P<.001 | 76.3% (352) | 66.4% (676) |
| Probability of Harm | |||||
| Only about 1 out of 10 smokers die because of smoking. | Disagree | 42.5% (227) | P<.001 | 58.9% (294) | 50.0% (521) |
| Do you think your risk of having a HEART ATTACK is higher, lower, or about the same as other (men/women) your age? | Higher | 54.1% (299) | P=.867 | 53.5% (274) | 53.8% (573) |
| Do you think your risk of CANCER is higher, lower, or about the same as other (men/women) your age? | Higher | 55.7% (313) | P=.262 | 60.1% (293) | 57.8% (606) |
| Do you think your risk of LUNG CANCER is higher, lower, or about the same as other (men/women) your age? | Higher | 58.8% (327) | P=.043 | 66.4% (315) | 62.3% (642) |
| Nature of Potential Harm | |||||
| Cigarettes still haven’t been proven to cause cancer. | Disagree | 66.9% (367) | P=.943 | 66.6% (321) | 66.8% (688) |
| Nicotine is a cause of cancer. | Disagree | 35.6% (202) | P=.223 | 31.1% (145) | 33.5% (347) |
Difficulty of avoiding harmful consequences
Perceived difficulty of avoiding harm was assessed with 2 items: (1) “Do you think you will quit smoking before you experience a serious health problem caused by smoking?” and (2) “If we called you back in a year, will you be smoking?” Response options were coded “yes,” “no,” and “do not know.” For the mediational analyses, the second item (quit intentions) was used as the dependent variable, and response options were recoded “yes/I don’t know” and “no” because of the interest in predicting intentions for cessation among smokers. Those individuals who indicated that they intended not to be smoking in a year were thought to differ psychologically from those who answered that they would still be smoking or reported uncertainty about smoking.
Factors that influence individual susceptibility
Many smokers believe that engaging in certain behaviors or using certain tobacco products may reduce the risks of smoking. Five statements addressed this issue (eg, “High-tar cigarettes are at least twice as likely to cause illness as ones that are low in tar”). Response options were coded as “disagree,” “agree,” and “no opinion/do not know.” For all questions, the accurate answer was “disagree.”
Probability of harm
Perceptions about absolute probability of harm was assessed with the question, “Only about 1 out of 10 smokers die because of smoking.” Response options were coded as “disagree,” “agree,” and “no opinion/do not know.” The accurate answer was “disagree.” Three relative-probability-of-harm items asked, “Do you think your risk of <having a heart attack/cancer/lung cancer> is higher, lower, or about the same as other (men/women) your age?” Responses were coded “higher” versus “lower/about the same/do not know.”
Nature of potential harm
Perceptions about the nature of potential harm were assessed with 2 questions: (1) “Cigarettes still haven’t been proven to cause cancer,” and (2) “Nicotine is a cause of cancer.” Response options were coded as “disagree,” “agree,” and “no opinion/do not know.” For both questions, the accurate answer was “disagree.”
FDA evaluation belief
Participants were asked, “Do you think cigarettes are evaluated for safety by the US Food and Drug Administration before they are sold to consumers?” Response options were “yes,” “no,” and “I don’t know.” Responses were recoded and are defined as No FDA Evaluation and FDA Evaluation. After preliminary analyses, smokers who reported that they did not know if there was FDA evaluation of cigarettes were not statistically significantly different from those who reported there was FDA evaluation of cigarettes. Thus, the do-not-know group was reported with FDA Evaluation.
Demographic variables
Sex, ethnicity, age, and educational attainment were examined as possible confounders.
Data Analysis
SPSS 14.0 complex sample analyses were used in order to correct for design effects, to correct for unequal probability of selection, and to ensure that the results were nationally representative with unbiased estimates. Chi-square analyses were used to assess the bivariate relationships between FDA evaluation belief and risk comprehension items.
Mediational analyses using ANOVA were conducted to examine whether risk perceptions explain the relationship between FDA evaluation belief and smokers’ intentions to quit smoking in one year. Mediation helps delineate the process by which an independent variable produces an outcome, identifying factors that facilitate or inhibit that outcome. As shown in Figure 1, a mediator serves as a dependent variable because it is affected by the independent variable (path a) and also serves as an independent variable because it affects the dependent variable (path b). According to Baron and Kenny, in order to determine mediation, 4 criteria must be met.25 In this study, ANOVA was used to assess each of these 4 criteria. Each risk perception item was tested independently as a mediator. In step 1, we assessed the relationship between FDA evaluation belief and quit intentions (path c). Step 2 assessed the relationship between FDA evaluation belief and each risk perception item (path a). Step 3 assessed the relationship between each risk perception item (that had a significant relationship with FDA safety evaluation at step 2) and quit intentions while controlling for the belief about FDA evaluation to check for significance. Finally, step 4 assessed whether the relationship between FDA evaluation belief and quit intentions was weaker after controlling for each risk perception item.
RESULTS
Demographics
Overall, 46.1% of respondents reported that cigarettes are not evaluated for safety by the FDA before they are sold to consumers (No FDA Evaluation) whereas other respondents either responded that cigarettes are evaluated for safety by the FDA (43.6%) or did not know (10.2%) (FDA Evaluation). Age was significantly associated with FDA evaluation belief (P=.03). Belief that the FDA did not evaluate cigarettes was greatest among participants aged 35 to 44 (56%) and was lowest among participants aged 18 to 24 (37.2% correct). About 49% of women reported that the FDA does not evaluate cigarette safety versus 43.4% of men. Additionally, more than half (53.4%) of participants with some college education responded that the FDA does not evaluate cigarettes for safety, compared with only 40% of those in other education groups. These differences by gender and education were not statistically significant. Additionally, no statistically significant difference was observed in FDA evaluation belief by ethnicity. The relationships between demographic variables and risk perceptions have previously been reported.24
Comprehension of Smoking Risks
Results of the bivariate analyses of FDA evaluation belief and perceptions about the risks of cigarette smoking are shown in Table 1. Controlling for age did not change the relationship between FDA evaluation belief and risk perceptions. Consequently, age was not controlled for in the following analyses.
Difficulty of avoiding harm
Approximately 76% of smokers reported that they would quit smoking before they experienced a serious health problem, and approximately 41% reported that they would no longer be smoking if called back in one year. A greater proportion of FDA Evaluation respondents (78.6%) believed that they would quit smoking cigarettes before experiencing a serious health problem than No FDA Evaluation respondents (73.9%). Intending to quit smoking within the year was more common among No FDA Evaluation (48.5%) than among FDA Evaluation (33.6%) respondents.
Factors that influence individual susceptibility
As shown in Table 1, smokers endorsed several myths about the factors that influence risk of smoking-related harm. Endorsing these myths was less common among No FDA Evaluation than among FDA Evaluation respondents. Compared to FDA Evaluation, No FDA Evaluation respondents were significantly less likely to believe that (1) smoking high-tar cigarettes is twice as risky than smoking low-tar cigarettes, (2) smoking 5 cigarettes confers the same degree of cancer risk as being a nonsmoker, (3) smoking cigarettes with additives is more dangerous than smoking cigarettes without additives, (4) smoking milder cigarettes is less dangerous than smoking full-strength cigarettes. FDA evaluation belief was not significantly associated with the perception that smoking is not dangerous if one does not inhale.
Probability of harm
Only 50% to 62% of smokers correctly endorsed items regarding probability of harm from smoking. The belief that “only 1 in 10 smokers dies because of smoking” was less common among No FDA Evaluation (58.9%) than among FDA Evaluation (42.5%) respondents. No FDA Evaluation respondents were also more likely (66.4%) to rate their risk of lung cancer as higher than that of other people their age, compared with FDA Evaluation (58.8%) respondents. However, no such statistically significant difference was observed for the risk of having a heart attack or developing cancer in general.
Nature of potential harm
Only 66.8% of all respondents rejected the notion that cigarettes still have not been proven to cause cancer, and 33.5% disagreed that nicotine is a cause of cancer. Belief about FDA safety evaluation was not significantly associated with recognizing that cigarettes are a cause of cancer or that nicotine is not a cause of cancer.
Mediational Analyses
Intention to quit smoking in the next year was assessed with the variable “If we called you back in a year, will you be smoking?” In order to assess whether risk perceptions explain the relationship between FDA safety evaluation belief and quit intentions, mediational analyses using ANOVA were conducted.25 The main effects analysis (step 1) indicated that No FDA Evaluation participants were more likely to intend to quit smoking within the year compared to FDA Evaluation participants, F(1,829)=12.353, P<.001. As shown in the bivariate analyses in Table 1, 8 smoking-related risk perceptions measured in this study were significantly related to the belief about FDA safety evaluation (step 2). In addition, these 8 risk items were significantly associated with participants’ intentions to still be smoking in one year, controlling for FDA evaluation belief (step 3). For example, smokers who disagreed that high-tar cigarettes are more dangerous than low-tar cigarettes were more likely to intend to quit smoking within the year. According to step 4 of the criteria for mediation, controlling for each risk-perception item should reduce the level of statistical significance of the relationship between FDA safety evaluation belief and quit intention. This would demonstrate that the perception of risk explains the relationship between FDA safety evaluation belief and quit intention. Three of the 8 risk perceptions met the criteria for partial mediation, and these are shown in Table 2. Partial mediation, for example, is shown by the significance level dropping from P<.0001 in step 1 to P<.01 in step 4. These analyses were repeated testing FDA evaluation belief as the mediator of the relationship between risk perception and quit intentions (changing the direction of the mediation pathway); however, the relationship did not hold.
Table 2.
Mediational Analyses Exploring if Risk Perceptions Mediate the Relationship Between FDA Evaluation Belief and Quit Intentions
| Risk Perception | Step 1 Estimate (SE) | Step 2 Estimate (SE) | Step 3 Estimate (SE) | Step 4 Estimate (SE) |
|---|---|---|---|---|
| Factors That Influence Individual’s Susceptibility | ||||
| High-Tar cigarettes are at least twice as likely to cause illness as ones that are low in tar. | −.148 (.042) **** | −.106 (.037) ** | −.150 (.046) *** | −.127 (.042) ** |
| The milder the smoke, the less dangerous the cigarette. | −.148 (.042) **** | −.185 (.033) **** | −.161 (.039) **** | −.125 (.041) ** |
| Probability of Harm | ||||
| Only about 1 out of 10 smokers die because of smoking. | −.148 (.042) **** | −.164 (.038) **** | −.107 (.042) * | −.117 (.043) ** |
Note.
FDA evaluation belief was coded as: 0= FDA Evaluation or 1= No FDA Evaluation. Quit intention was coded as: 1= yes, will be smoking/do not know and 2= no, will not be smoking. Risk perception was coded as: 0=agree/do not know/no opinion and 1= disagree.
Step 1: Regress quit intentions onto FDA evaluation belief. Step 2: Regress risk perceptions onto FDA evaluation belief. Step 3: Regress quit intentions onto risk perceptions when controlling for FDA evaluation belief. Step 4: Regress quit intentions onto FDA evaluation belief while controlling for risk perceptions and observe a reduction in statistical significance.25
P<.05,
P<.01,
P<.001,
P<.0001
DISCUSSION
The results of this study suggest that smokers are poorly informed about some important aspects of the risks of smoking and FDA evaluation of tobacco. Central to this study, the results suggest that there is an association between a belief about FDA evaluation of cigarettes for safety and perceptions about the risks of smoking. Smokers who responded that the FDA does not evaluate cigarettes for safety had greater perceptions of the risks associated with smoking. The mediational analysis further suggests that an association between belief about FDA evaluation and intentions to quit smoking may be partially explained by smoking-related risk perceptions. This is the first study to provide data on the relationship between consumer beliefs about government safety evaluation of cigarettes and tobacco-related risk perceptions.
This study demonstrated a complex relationship between FDA safety evaluation and items assessing the difficulty of avoiding harm. Approximately 41% of respondents in this sample intended to quit smoking in the next year, and 76% stated that they intend to quit smoking before experiencing a serious health problem caused by smoking. This is consistent with other studies reporting that smokers are overly optimistic about their ability to avoid harm by quitting smoking.15,26 One of this study’s unique contributions is in its finding that smokers who indicated that the FDA does not evaluate cigarettes for safety were more likely (48.5%) than other participants (33.6%) to report intentions to not be smoking in one year. At the same time, smokers who reported no FDA evaluation responded they were less likely (73.9%) than others (78.6%) to quit smoking before suffering harm. Conversely, smokers reporting FDA evaluation expressed lower intentions to quit and, at the same time, were more positive about their ability to avoid harm.
Moreover, the mediational analyses suggest that some risk perceptions may partially explain the relationship between belief about FDA evaluation and intentions of future smoking. When FDA evaluation belief and risk perceptions were swapped in the mediational pathway, the models did not hold, providing support for the hypothesized pathway. Thus, FDA evaluation belief influences risk perceptions rather than risk perceptions influencing a belief about FDA evaluation. In particular, smokers who believed that the FDA evaluates cigarettes for safety were more likely to hold misconceptions about the beneficial effects of smoking low-tar or mild cigarettes and about the number of smokers who die as a result of their smoking. These misconceptions, in turn, were associated with reduced intentions of quitting within the next year. These results are compelling because they illustrate how perceptions of federal oversight of tobacco products may be related to health behaviors. However, the mechanism and direction of this relationship are unclear. One possibility is that the relationship is causal. For example, a misconception that the FDA evaluates cigarettes may provide smokers with a false sense of security and protection from risk. Support for this hypothesis was shown through the mediational pathway tested in the current study; however, experimental studies are needed. An alternative possibility is that there is an unmeasured third variable underlying both variables. For instance, it may be that individuals who are less inclined to seek information about the health risks of smoking might also be poorly informed about both federal tobacco regulatory policies and about the health risks of smoking. Similarly, it may be that smokers who are better informed overall about the risks of smoking and about tobacco control policies may also be more likely to report intentions to quit; for example, smokers who are better educated may be better informed and more motivated to quit.
The results of this study have relevance for public understanding of tobacco regulation, including FDA authority over tobacco product regulation. There is broad support for comprehensive federal regulation of tobacco products within the US public health community, and the new law includes a range of provisions important for protecting public health, including restricting sales and marketing of tobacco to youth, granting FDA authority to restrict tobacco advertising, and requiring stronger, more effective health warnings.6,27 The findings from this study highlight the need for effective communication to the public about the aims of tobacco product regulation (ie, what such regulations are or are not set out to achieve) as well as the need for ongoing education about the health risks associated with tobacco product use. Additionally, it is essential to conduct research and surveillance on tobacco-related consumer behavior and risk perception and to identify the most effective means of communicating information about tobacco-product-related policies. For example, the new law requires consumer perception research related to reporting of information about tobacco product contents.6 At the same time, marketing and use of media have been shown to influence public risk perceptions of tobacco, both positively and negatively28; thus, it is important to consider how a variety of sources of information about tobacco-related health risks and tobacco regulatory policies may influence public risk perceptions and behaviors.
This study has several limitations that are important to acknowledge. Given that this study utilized an existing data set, the items in this survey were not specifically designed to examine the relationships of interest, and a limited range of items was available to assess risk perceptions. Belief about FDA evaluation of tobacco products was assessed with a single item in this study. Future studies should assess FDA evaluation with a greater number of items. Moreover, the concept of government evaluation of cigarettes is complex and has not been previously assessed. Thus, it is unclear how respondents’ conceptualization of FDA evaluation is related to the forms of product testing and regulatory mechanisms that have been proposed. This study was limited to current smokers, so it was not possible to assess beliefs among non-smokers or former smokers. Additionally, the cross-sectional nature of the survey made it impossible to determine whether a causal relationship exists between FDA safety evaluation beliefs, risk perceptions, and behavior; however, this primary analysis can be used to inform and drive future studies in this area. Although the data were collected in 2001, this study is relevant and informative as it demonstrates that a belief about FDA evaluation (regardless of whether or not tobacco is truly regulated) is associated with risk perceptions about smoking.
Further research is needed to better understand the complex relationship between beliefs about tobacco product regulatory policies, health risk perceptions, and related behaviors. Carefully designed experimental studies need to be conducted to clarify the mechanism and direction of the relationships and to assess whether providing individuals with information about tobacco product regulation will influence risk perceptions. One such experimental study showed that telling young-adult nonsmokers that cigarettes were or were not regulated (vs no information about regulation) increased their perceived health risks of smoking.29 This lends support that the relationship between regulation and risk perceptions about smoking has held over the last 10 years despite changes in policies and the social environment. Future studies should examine a wide range of risk beliefs and questions assessing knowledge of tobacco control policies and regulation of tobacco products. Additionally, future research should examine other populations, such as youth, nonsmokers, and former smokers. This study provides support that a belief about FDA evaluation of cigarette safety is significantly associated with numerous risk perceptions about smoking and may impact smoking behavior among smokers.
Acknowledgments
Partially funded by the Robert Wood Johnson Foundation: Substance Abuse Prevention Research Program, the Roswell Park Cancer Institute: NCI-funded Cancer Center Support Grant (CA 16056-26), and the Biomathematics/Biostatistics Core Resource. The views expressed here are those of the authors only and do not represent any official position of the National Cancer Institute or National Institutes of Health.
Contributor Information
Annette R. Kaufman, Behavioral Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD.
Erika A. Waters, Washington University School of Medicine, Division of Public Health Sciences, St. Louis, MO.
Mark Parascandola, Tobacco Control Research Branch/DCCPS, National Cancer Institute, Bethesda, MD.
Erik M. Augustson, Tobacco Control Research Branch/DCCPS, National Cancer Institute, Bethesda, MD.
Maansi Bansal-Travers, Department of Health Behavior, Roswell Park Cancer Institute.
Andrew Hyland, Department of Health Behavior, Roswell Park Cancer Institute.
K. Michael Cummings, Department of Health Behavior, Roswell Park Cancer Institute.
References
- 1.World Health Organization. [Accessed February 26, 2008.];WHO Report on the Global Tobacco Epidemic. 2008 Available at http://www.who.int/tobacco/mpower/mpower_report_full_2008.pdf.
- 2.U.S. Department of Health and Human Services. The health consequences of smoking: A report of the Surgeon General. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2004. [Google Scholar]
- 3.World Health Organization. [Accessed September 18, 2009.];The Scientific Basis of Tobacco Product Regulation. 2007 Available at http://www.who.int/tobacco/global_interaction/tobreg/9789241209458.pdf.
- 4.World Health Organization. [Accessed September 18, 2009.];Monograph: Advancing Knowledge on Regulating Tobacco Products. 2001 Available at http://www.who.int/tobacco/media/en/OsloMonograph.pdf.
- 5. [Accessed February 3, 2009.];World Health Organization Framework Convention on Tobacco Control (WHO FCTC) 2009 Available at http://www.who.int/fctc/en/index.html.
- 6. [Accessed September 10, 2009.];Family Smoking Prevention and Tobacco Control and Federal Retirement Reform. 2009 111th Congress, Public Law No: 111-31. Available at http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_public_laws&docid=f:publ031.111.
- 7.Chapman S. Benefits and risks in ending regulatory exceptionalism for tobacco. Tob Control. 2008;17:73–74. doi: 10.1136/tc.2008.025106. [DOI] [PubMed] [Google Scholar]
- 8.Kozlowski LT. The proposed tobacco regulation: the triumph of hope over experience? Tob Control. 2008;17:74–75. doi: 10.1136/tc.2008.025155. [DOI] [PubMed] [Google Scholar]
- 9.Burns DM, Benowitz NL. Smoking and Tobacco Control Monograph No. 13: Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine U.S. Department of Health and Human Services. Public Health Service. National Institutes of Health, National Cancer Institute; 2001. Public health implications of changes in cigarette design and marketing; pp. 1–12. [Google Scholar]
- 10.Hughes JR. Do “light” cigarettes undermine cessation? Tob Control. 2001;10(Suppl I):i41–i42. doi: 10.1136/tc.10.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor RJ, Ashare RL, Fix BV, et al. College students’ expectancies for light cigarettes and potential reduced exposure products. Am J Health Behav. 2007;31(4):402–410. doi: 10.5555/ajhb.2007.31.4.402. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton WL, Norton G, Ouellette TK, et al. Smokers’ responses to advertisements for regular and light cigarettes and potential reduced –exposure tobacco products. Nicotine Tob Res. 2004;6(Suppl 3):S353–S362. doi: 10.1080/14622200412331320752. [DOI] [PubMed] [Google Scholar]
- 13.Hammond D, Parkinson C. The impact of cigarette package design on perceptions of risk. J Public Health. 2009;31:345–353. doi: 10.1093/pubmed/fdp066. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein ND. What does it mean to understand a risk? Evaluating risk comprehension. J Natl Cancer Inst Monogr. 1999;25:15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024192. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein ND, Slovic P, Gibson G. Accuracy and optimism in smokers’ beliefs about quitting. Nicotine Tob Res. 1999;6 (Suppl 3):S375–S380. doi: 10.1080/14622200412331320789. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein ND. Accuracy of smokers’ risk perceptions. Ann Behav Med. 1998;20:135–140. doi: 10.1007/BF02884459. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein ND, Slovic P, Waters E, et al. Public understanding of illnesses caused by cigarette smoking. Nicotine Tob Res. 2004;6:349–355. doi: 10.1080/14622200410001676459. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein ND, Marcus SE, Moser RP. Smokers’ unrealistic optimism about their risk. Tob Control. 2005;14:55–59. doi: 10.1136/tc.2004.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodge T, Kaufman A. What makes consumers think dietary supplements are safe and effective? The Role of Disclaimers and FDA Approval. Health Psychol. 2007;26:513–517. doi: 10.1037/0278-6133.26.4.513. [DOI] [PubMed] [Google Scholar]
- 20.Deyton L, Sharfstein J, Hamburg M. Tobacco Product Regulation- A Public Health Approach. New Engl J Med. 2010;362:1753–1756. doi: 10.1056/NEJMp1004152. [DOI] [PubMed] [Google Scholar]
- 21.Ajzen I. From intentions to actions: a theory of planned behavior. In: Beckman JKJ, editor. Action-control: From Cognition to Behavior. Heidelberg: Springer; 1985. pp. 11–39. [Google Scholar]
- 22.Conner M, Sparks P. The theory of planned behaviour and health behaviours. In: Conner M, Norman P, editors. Predicting Health Behaviour. Buckingham: Open University Press; 1995. pp. 121–162. [Google Scholar]
- 23.American Association for Public Opinion Research. Standard definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 2. Ann Arbor, MI: AAPOR; 2000. [Google Scholar]
- 24.Cummings KM, Hyland A, Giovino GA, et al. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res. 2004;6:1–8. doi: 10.1080/14622200412331320734. [DOI] [PubMed] [Google Scholar]
- 25.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 26.Arnett JJ. Optimistic bias in adolescent and adult smokers and nonsmokers. Addict Behav. 2000;25:625–632. doi: 10.1016/s0306-4603(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 27.Campaign for Tobacco Free Kids. [Accessed October 9, 2008.];Organization supporting the FDA Tobacco Legislation. 2008 Available at http://www.tobaccofreekids.org/reports/fda/organizations.pdf.
- 28.National Cancer Institute. The Role of the Media in Promoting and Reducing Tobacco Use. Tobacco Control Monograph No. 19. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Jun, 2008. NIH Pub. No. 07-6242. [Google Scholar]
- 29.Kaufman A. The Influence of cigarette regulatory information on belief expectancy and value. Diss Abstr Int B Sci Eng. 2009 #1382. [Google Scholar]


