Abstract
Resilin, an insect structural protein, exhibits rubber-like elasticity characterized by low stiffness, high extensibility, efficient energy storage, and exceptional resilience and fatigue lifetime. The outstanding mechanical properties of natural resilin have motivated recent research in the engineering of resilin-like polypeptide-based biomaterials, with a wide range of applications including use as bio-rubbers, nanosprings, elements in biosensors, and tissue engineering scaffolds.
Biosynthetic approaches have been employed widely over the past few decades to produce precisely controlled protein polymers with tailored materials functions.1-3 The diversity of the types of protein and polypeptide domains employed in these materials has undergone a rapid expansion with the development, growth and maturation of recombinant DNA technologies;4 materials have been engineered to contain not only consensus repeats borrowed from naturally occurring proteins, but also many sequences derived from components of the native extracellular matrix (ECM).5-8 A wide variety of other naturally occurring proteins with specific mechanical and cell signaling functions have also been recently employed in materials design, including immunoglobulin- and peptide-binding domains and fluorescent proteins.9-12 The diverse library of de novo and naturally occurring sequences has supported the development of these materials in applications ranging from regenerative medicine and drug delivery to diagnostic biosensors and high-efficiency industrial elastomers. In particular, tremendous effort has been devoted to the biosynthesis of elastin-like polypeptides (ELPs), comprising the consensus repeats of native elastin, to yield materials that mimic the inherent elasticity of natural elastin,13-20 and more recently, polypeptides based on the insect structural protein resilin have emerged as an additional candidate to generate bio-elastomers.21,22 This viewpoint describes the early discovery of natural resilin, the evolution of resilin-like polypeptides (RLP), and the potential biomedical applications of RLP-based materials.
Resilin is a natural extracellular matrix protein that was discovered half a century ago by Weis-Fogh during the investigation of the wing-hinge ligament of locusts and the elastic tendon of dragonflies.23 The composition of resilin was elucidated shortly after its discovery and was shown to comprise solely amino acids, with a significantly higher percentage of acidic residues and lower percentage of non-polar residues when compared to other elastic proteins; this composition gives resilin a low isoelectric point and high hydrophilic capacity.24 The existence of resilin in a wide range of organs in other types of arthropods (such as the sound-producing tymbal in the cicada shown Figure 1) was quickly established.25,26
Figure 1.
The sound-producing tymbal of the Australian cicada Cyclochila australasiae. (Top) Diagram showing the position of the tymbal (red arrow) at the anterior end of the insect's abdomen. (A) An unstained tymbal photographed in natural light (original). (B) A different tymbal stained for 24 hours in very dilute methylene blue solution. (C) The tymbal presented in A shows fluorescence when illuminated by an ultraviolet light at 365 nm. Reprinted with permission from Bennet-Clark, H. J. Exp. Biol. 2007, 210, 3879. Copyright (2007) The Company of Biologists.
The cross-linked protein performs like an almost ideal isotropic rubber and exhibits long-range reversible elasticity and superior resilience, all of which are critical for supporting the rapid deformation in these insect organs without significant hysteresis even at high frequencies of up to 13kHz.23,27,28 These characteristics also contribute to the remarkable fatigue lifetime of resilin, which supports these high-frequency, repeated contraction/extension cycles in diverse functions (e.g. sound, wing motion) over the 20 million cycles that occur during the lifetime of the insect.25,29 The high content of glycine and proline residues confers to resilin a high degree of conformational flexibility which contributes to these outstanding mechanical properties; it has been proposed the presence of β-turn and polyproline II conformations derived from PG dyads contribute to the flexible, dynamic chain movements in elastomeric structural proteins.27,28,30-34 Given that recombinant routes to the production of resilin have now been developed, there is increasing information about the relationship between structure and function of resilin and an emergence of RLPs with engineered compositions and functions.
In 2001, Ardell and Anderson identified the tentative resilin gene sequences in the Drosophila melanogaster genome, thus opening routes to the engineering of resilin-like polypeptides with physicochemical and structural properties comparable to natural resilin.35 The first reported example of a recombinantly synthesized resilin-like polypeptide was reported by Elvin and co-workers in 2005; the authors cloned, expressed and purified the first exon of the D. melanoganster CG15920 gene with 17 copies of the putative elastic motif (Exon I) GGRPSDSYGAPGGGN.36 The resulting protein (Rec1-resilin) could be cast into a rubber-like biomaterial with Ru(II)-mediated photo-crosslinking, yielding a dityrosine-crosslinked material with similar mechanical properties as those of natural resilin, even though Rec1-resilin contains only Exon I from the natural resilin sequence.36
Further studies have revealed that Rec1-resilin is highly pH- and temperature-responsive, exhibiting dual phase-transition behavior characterized by both lower and upper critical solution temperatures (Figure 2); the thermal properties of resilin have allowed simple and inexpensive purification methods that are likely to be useful in the scale-up of resilin-related materials.37-39 This stimuli-responsive behavior of Rec1-resilin has also been exploited in the functionalization of surfaces for uses in biosensors or diagnostic tools. Troung et al demonstrated that discrete changes in pH elicited rapid, reversible absorption, to a gold substrate, of Rec1-resilin with different orientations and conformations; the resilin underwent transitions between compact and brush-like conformations on the surface, based upon the differences in electrostatic interactions between the resilin and gold surface under various solution conditions.40 Rec1-resilin-modified gold nanoparticles have also been investigated as a fluorescent reporter system for diagnostic application.41 Altering the assembly, structure, and morphology of modified nanoparticle surfaces by changing the physical properties of the Rec1-resilin could be exploited for the controlled immobilization of drugs, nanoparticles, enzymes, and catalysts for delivery and diagnostic applications.42
Figure 2.
Photographs of a series of Rec1-resilin solutions as a function of temperature showing a transparent sol at 6-10 °C and an opaque gel below the UCST (~6 °C). Above the LCST, Rec1-resilin solution does not form an opaque gel instantaneously. Reprinted with permission from Dutta, N. K.; Truong, M. Y.; Mayavan, S.; et al. Angew Chem. Int. Ed. 2011, 50, 4428. Copyright (2011) Wiley-VCH.
In order to determine whether different repetitive consensus motifs derived from various insect sources are similarly able to generate highly resilient, elastomeric resilin-like materials, two alternative RLP sequences, An16 (adopted from the Anopheles gambiae resilin sequence which contains 16 repeats of the sequence AQTPSSQYGAP) and Dros16 (from the D. melanogaster CG15920 and containing 16 repeats of GGRPSDSYGAPGGGN), have been constructed and compared.34 Ru(II)-mediated photo-crosslinking was employed to generate An16- and Dros16-based hydrogels; mechanical analysis of the dityrosine-crosslinked hydrogels confirmed that they demonstrated negligible hysteresis, excellent resilience (>90%) and Young's moduli that compared favorably to both those of Rec1-resilin hydrogels and natural resilin.34 The fact that the An16 constructs generally exhibit a higher protein yield than the other sequences has motivated the Liu group to incorporate mosquito-based resilin motifs into a modular protein with RGD cell-binding domains, for cartilage tissue engineering applications. This modular resilin-like polypeptide displayed an unconfined compressive modulus on the order of that of human cartilage and supported the adhesion and spreading of hMSCs (human mesenchymal stem cells), indicating potential for these materials in directing chondrogenesis of MSCs for cartilage tissue therapies.43
The excellent reversible elasticity, high-frequency responsiveness and hydrophilic capacity of resilin and resilin-based materials have also motivated investigations toward its use as a new class of bio-elastomers in the engineering of mechanically active tissues. In first steps toward producing such scaffolds, we employed a modular resilin-like polypeptide (containing 12 repeats of the resilin consensus motif from exon 1 of the D. melanogaster CG15920 gene) that was strategically combined with domains to impart cell adhesion (RGDSP),44 proteolytic degradation (GPQGIWGQ),45-47 and/or heparin immobilization (KAAKRPKAAKDKQTK)48-50 (Figure 3).51 Spectroscopic investigations (CD and FTIR) indicated that the RLP12 adopted a highly unordered conformation with minor contributions from type-II β-turn, consistent with structures observed for other recombinant RLPs lacking biological domains.32,52,53 The RLP12 could be rapidly cross-linked via a Mannich-type reaction of lysine residues with the cross-linker THPP (β-[tris(hydroxymethyl)phosphino] propionic acid)51 to yield materials with useful mechanical behavior. Dynamic oscillatory rheology, uniaxial tensile testing and high-frequency torsional wave analysis revealed storage shear moduli (500Pa to 10kPa), Young's moduli (15kPa to 35kPa) and storage shear moduli at high frequency (1000-2000Pa) that are consistent with the reported mechanical properties of vocal fold tissue. The RLP hydrogels showed little hysteresis under tensile deformation (resilience >90%, shown in Figure 3),54 and facilitated the survival and proliferation of NIH 3T3 fibroblasts in vitro.51,54
Figure 3.
Schematic and amino acid sequence of highly elastomeric and biologically active resilin-like polypeptide with 12 repeats of the resilin consensus sequence and is hence referred to as RLP12 in the text (left). Oscillatory rheological and uniaxial tensile testing characterizations on hydrated RLP-based hydrogels (right). Reprinted with permissions from Charati, M. B.; Ifkovits, J. L.; Burdick, J.A.; et al, Soft Matter, 2009, 5, 3412. Copyright (2009) Royal Society of Chemistry and Li, L. Q.; Teller, S.; Clifton, R. J.; et al, Biomacromolecules, 2011, 12, 2302. Copyright (2011), American Chemical Society.
The independent tailoring of specific cell-matrix interactions is a critical parameter in regulating cellular responses.18,55 Such needs have motivated the engineering of the RLP12-based sequences to increase their versatility; multiple RLP-based constructs, in which each RLP construct contains the 12 resilin consensus motifs but bears a different biologically active module, were produced recombinantly.56 The resulting polypeptides exhibited essentially identical conformational behavior as the original RLP12 constructs and allowed independent manipulation of the concentrations of cell-binding, MMP-sensitive, and heparin-binding domains in cross-linked materials of targeted mechanical properties (Figure 4).56 The rapid gelation reaction enables 3D encapsulation of hMSCs; the mechanical integrity of the non-degradable hydrogels and viability of encapsulated cells were both maintained over 21 days. This straightforward approach offers substantial opportunities for fabricating scaffolds that can be systematically tailored for either implantable or injectable vocal fold tissue therapies.
Figure 4.
Schematic of various RLP constructs with oscillatory mechanical properties at either 10wt% or 20wt% with different material compositions and biological cell attachment responses at 100% RLP alone or 50% RLP +50% RLP-RGD compositions. Reprinted with permission from Li, L. Q.; Tong, Z. X.; Jia, X. Q.; et al, Soft Matter, 2013, 9, 665. Copyright (2013), Royal Society of Chemistry.
In order to take advantage of the versatility of polypeptide-polymer hybrid materials,57 we have also produced cysteine-containing RLPs that can be cross-linked into hydrogels via Michael-type reaction of the RLP with vinyl sulfone-functionalized four-arm PEG. This approach combines the chemical versatility and chain architecture flexibility of PEG macromers with the specificity and inherent bioactivity of the RLP component. Solid hydrogels with elastic shear moduli ranging from 7 to 12kPa were easily produced. The benign cross-linking reaction and fast hydrogel formation conditions allowed the successful encapsulation of human aortic adventitial fibroblast cells, with the encapsulated cells showing high viability and adopting a spread morphology over 7 days of culture. These encouraging results suggest a promising strategy to the development of resilin-based materials for cardiovascular applications.58
In order to understand the functional and structural properties of native resilin more completely, Kaplan and coworkers have produced recombinant resilin (containing exons 1, 2, and 3) from the D. melanogaster CG15920 gene; the sequence includes the cuticular chitin-binding domain (ChBD) that allows binding between resilin and chitin during resilin deposition and construction of the cuticle composite.59,60 This recombinant resilin was demonstrated to bind strongly to chitin-coated beads, while the resilin lacking the ChBD sequence showed no affinity. A horseradish peroxidase-mediated cross-linking reaction was employed to generate an RLP-ChBD-based hydrogel with comparable structural and mechanical behavior to other resilins; the introduction of the chitin-binding domain provides additional opportunities for in vitro formation and mimicry of resilin-chitin composite structures. Subsequent work compared the mechanical properties of peroxidase-cross-linked and photo-catalyzed Fenton-reaction cross-linked hydrogels produced with exon I- and III-derived RLPs. Under both cross-linking conditions, the exon I-RLP materials exhibited higher resilience (93%) compared to that of exon III-RLP materials (86%), suggesting that the exon I region is critical to the mechanical function of natural resilin.61 Recently, Qin et al explored the self-assembly properties of exon I, exon III and full-length resilins and identified reversible beta-turn transitions in full-length resilins during energy input and release that may be correlated with the rapid deformation of resilin during function in vivo.62 The exon I regions of resilin were indicated to respond immediately to mechanical deformation and transfer energy to the exon III regions for storage, with concomitant formation of beta-turn structures in the exon III regions and reversible recovery after removal of force. The contribution of beta-turn configurations to the elastomeric behavior is consistent with other models such as beta-spiral, sliding beta-turn and PPII that are proposed as mechanisms of elasticity in natural elastomeric structural proteins.32,62
Interest in combining the unique mechanical properties of multiple structural proteins into a single, multifunctional material has motivated the recombinant production of chimeric resilin-elastin-collagen-like polypeptides (REC).63 This 11kDa chimeric polypeptide comprises a resilin-mimetic sequence (SDTYGAPGGGNGGRP)4, an elastin-mimetic section ((LGGVG)3K(LGGVG)4), and a collagen-like domain (PKG(PGG)5K(PAG)5PKG). Atomic force microscopy characterization revealed that the REC forms fibers with Young's moduli ranging from 0.1 to 3MPa, likely due to the differences in the polarities of the compositionally dissimiliar sequences of each block (Figure 5). Further characterization, via AFM-based single-molecule force spectroscopy, demonstrated that a single REC polypeptide exhibited quasi-ideal elastomeric behavior with resilience of nearly 100%. MSCs extracted from healthy donors were able to adhere and proliferate on the surface of glass coverslips coated with RECs, suggesting the potential use of this polypeptide in directing cell growth and guiding differentiation.64 Lv et al. adopted a similar modular approach to design a novel multi-block protein in which folded GB1 domains (from the streptococcal B1 immunoglobulin-binding domain of protein G) were interspersed with random-coil resilin-based sequences. This design mimics the complex molecular spring structure of the muscle protein titin, which comprises folded immunoglobin-like domains and unstructured sequences that act as two distinct entropic springs. This multiblock construct combines the unique mechanical properties of each element and yields a rubber-like biomaterial with a measured Young's modulus (50-70kPa) that is similar to that of myofibrils (60-100kPa), that exhibits high resilience at low strain (<15%), but shows shock absorbing-like properties at high strain (>35%), comparable to the passive elastic characteristics of muscle.65
Figure 5.
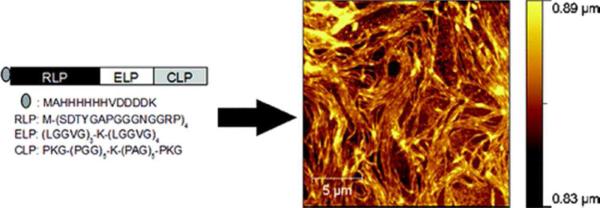
Schematic of the modular structure of an REC polypeptide and the amino acid sequences of each domain (left). Assembly of the REC polypeptide (0.5mg/mL) characterized via AFM after 5 days of incubation in water at 50 °C (right). Reprinted with permission from Bracalello, A.; Santopietro, V.; Vassalli, M.; et al. Biomacromolecules, 2011, 12, 2957. Copyright (2011), American Chemical Society.
This increasing amount of knowledge gained from studies on natural resilin and resilin-like polypeptides continues to inspire new designs and applications of recombinant resilin-based biopolymers in biomedical and biotechnological applications. Further advances in the production of RLPs with various sequences and amino acid substitutions, coupled with the characterization of their mechanical properties, will permit the development of more detailed models of elasticity. Standardization of facile and efficient purification protocols to scale up protein yield from bench-top to production facilities will reduce the cost and significantly increase the scope of materials available, and exploring other expression hosts will provide opportunities for post-translational modification to increase the complexity of RLPs. Investigating the impact of mechano-transduction of these resilin-based biomaterials on cellular behavior and cell phenotype would also be of significant value in the engineering of new mechanically active tissue constructs. Altogether, the prospects are promising for the expansion of RLPs in a variety of biotechnology applications.
ACKNOWLEDGMENT
Related work in the author's laboratories has been supported by grants from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (P20-RR017716 (K.L.K)), and the National Institute for Deafness and Communication Disorders (RO1 DC011377A to K.L.K).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Maskarinec SA, Tirrell DA. Curr. Opin. Biotechnol. 2005;16:422–426. doi: 10.1016/j.copbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Kiick KL. Polym. Rev. 2007;47:1–7. [Google Scholar]
- 3.DiMarco RL, Heilshorn SC. Adv. Mater. 2012;24:3923–3940. doi: 10.1002/adma.201200051. [DOI] [PubMed] [Google Scholar]
- 4.Buck ME, Tirrell DA. In: Polymer Science: A Comprehensive Reference. Krzysztof M, Martin M, editors. Elsevier; Amsterdam: 2012. pp. 117–136. [Google Scholar]
- 5.MacEwan SR, Chilkoti A. Nano Lett. 2012;12:3322–3328. doi: 10.1021/nl301529p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta D, Gilbert PM, Johnson KJ, Blau HM, Heilshorn SC. Adv. Healthcare Mater. 2012;1:785–789. doi: 10.1002/adhm.201200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv S, Bu T, Kayser J, Bausch A, Li H. Acta Biomater. 2013;9:6481–6491. doi: 10.1016/j.actbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Brenckle MA, Tao H, Kim S, Paquette M, Kaplan DL, Omenetto FG. Adv. Mater. 2013;25:2409–2414. doi: 10.1002/adma.201204678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Li HB. Nat. Mater. 2007;6:109–114. doi: 10.1038/nmat1825. [DOI] [PubMed] [Google Scholar]
- 10.Lai YT, Cascio D, Yeates TO. Science. 2012;336:1129–1129. doi: 10.1126/science.1219351. [DOI] [PubMed] [Google Scholar]
- 11.Thomas CS, Glassman MJ, Olsen BD. Acs Nano. 2011;5:5697–5707. doi: 10.1021/nn2013673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong Po Foo CTS, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22067–22072. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partridge SM, Davis HF. Biochem. J. 1955;61:21–30. doi: 10.1042/bj0610021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JC, Heilshorn SC, Tirrell DA. Biomacromolecules. 2004;5:497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 15.Simnick AJ, Lim DW, Chow D, Chilkoti A. Polym Rev. 2007;47:121–154. [Google Scholar]
- 16.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. J. Am. Chem. Soc. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu XY, Sallach RE, Caves JM, Conticello VP, Chaikof EL. Biomacromolecules. 2008;9:1787–1794. doi: 10.1021/bm800012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straley KS, Heilshorn SC. Soft Matter. 2009;5:114–124. [Google Scholar]
- 19.Kyle S, Aggeli A, Ingham E, McPherson MJ. Trends Biotechnol. 2009;27:423–433. doi: 10.1016/j.tibtech.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LQ, Charati MB, Kiick KL. Polym. Chem. 2010;1:1160–1170. doi: 10.1039/b9py00346k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Eldijk MB, McGann CL, Kiick KL, Van Hest JM. Peptide-Based Materials. Vol. 310. Springer; Berlin Heidelberg: 2012. pp. 71–116. [Google Scholar]
- 22.Li L, Kiick KL. In: Polymer Science: A Comprehensive Reference. Krzysztof M, Martin M, editors. Elsevier; Amsterdam: 2012. pp. 105–116. [Google Scholar]
- 23.Weis-Fogh T. J. Exp. Biol. 1960;37:889–907. [Google Scholar]
- 24.Bailey K, Weis-Fogh T. Biochim. Biophys. Acta. 1961;48:452–459. doi: 10.1016/0006-3002(61)90043-9. [DOI] [PubMed] [Google Scholar]
- 25.Bennet-Clark HC. J. Exp. Biol. 1997;200:1681–1694. doi: 10.1242/jeb.200.11.1681. [DOI] [PubMed] [Google Scholar]
- 26.Bennet-Clark H. J. Exp. Biol. 2007;210:3879–3881. doi: 10.1242/jeb.001339. [DOI] [PubMed] [Google Scholar]
- 27.Weis-Fogh T. J. Mol. Biol. 1961;3:648–667. [Google Scholar]
- 28.Jensen M, Weis-Fogh T. Philos. Trans. R. Soc. London Ser B-Biol. Sci. 1962;245:137–169. [Google Scholar]
- 29.Fonseca PJ, Bennet-Clark HC. J. Exp. Biol. 1998;201:717–730. [Google Scholar]
- 30.Elliott GF, Huxley AF, Weis-Fogh T. J. Mol. Biol. 1965;13:791–795. [Google Scholar]
- 31.Weis-Fogh T. J. Mol. Biol. 1961;3:520–531. [Google Scholar]
- 32.Bochicchio B, Pepe A, Tamburro AM. Chirality. 2008;20:985–994. doi: 10.1002/chir.20541. [DOI] [PubMed] [Google Scholar]
- 33.Urry DW, Long MM, Ohnishi T, Jacobs M. Biochem. Biophys. Res. Commun. 1974;61:1427–1433. doi: 10.1016/s0006-291x(74)80442-0. [DOI] [PubMed] [Google Scholar]
- 34.Lyons RE, Nairn KM, Huson MG, Kim M, Dumsday G, Elvin CM. Biomacromolecules. 2009;10:3009–3014. doi: 10.1021/bm900601h. [DOI] [PubMed] [Google Scholar]
- 35.Ardell DH, Andersen SO. Insect Biochem. Mol. Biol. 2001;31:965–970. doi: 10.1016/s0965-1748(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 36.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 37.Kim M, Elvin C, Brownlee A, Lyons R. Protein Express. Purif. 2007;52:230–236. doi: 10.1016/j.pep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Dutta NK, Truong MY, Mayavan S, Choudhury NR, Elvin CM, Kim M, Knott R, Nairn KM, Hill A. J. Angew. Chem. Int. Ed. 2011;50:4428–4431. doi: 10.1002/anie.201007920. [DOI] [PubMed] [Google Scholar]
- 39.Lyons RE, Elvin CM, Taylor K, Lekieffre N, Ramshaw JA. M. Biotechnol. Bioeng. 2012;109:2947–2954. doi: 10.1002/bit.24565. [DOI] [PubMed] [Google Scholar]
- 40.Truong MY, Dutta NK, Choudhury NR, Kim M, Elvin CM, Hill AJ, Thierry B, Vasilev K. Biomaterials. 2010;31:4434–4446. doi: 10.1016/j.biomaterials.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Dutta NK, Choudhury NR, Truong MY, Kim M, Elvin CM, Hill AJ. Biomaterials. 2009;30:4868–4876. doi: 10.1016/j.biomaterials.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Mayavan S, Dutta NK, Choudhury NR, Kim M, Elvin CM, Hill AJ. Biomaterials. 2011;32:2786–2796. doi: 10.1016/j.biomaterials.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 43.Renner JN, Cherry KM, Su RSC, Liu JC. Biomacromolecules. 2012;13:3678–3685. doi: 10.1021/bm301129b. [DOI] [PubMed] [Google Scholar]
- 44.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 45.Nagase H, Fields GB. Biopolymers. 1996;40:399–416. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C399::AID-BIP5%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.Lutolf MR, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Nat. Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 47.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Furst EM, Kiick KL. J. Controlled Release. 2006:130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL. J. Am. Chem. Soc. 2007;129:3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiick KL. Soft Matter. 2008;4:29–37. doi: 10.1039/b711319f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charati MB, Ifkovits JL, Burdick JA, Linhardt JG, Kiick KL. Soft Matter. 2009;5:3412–3416. doi: 10.1039/b910980c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nairn KM, Lyons RE, Mulder RJ, Mudie ST, Cookson DJ, Lesieur E, Kim M, Lau D, Scholes FH, Elvin CM. Biophys. J. 2008;95:3358–3365. doi: 10.1529/biophysj.107.119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamburro AM, Panariello S, Santopietro V, Bracalello A, Bochicchio B, Pepe A. ChemBioChem. 2010;11:83–93. doi: 10.1002/cbic.200900460. [DOI] [PubMed] [Google Scholar]
- 54.Li LQ, Teller S, Clifton RJ, Jia XQ, Kiick KL. Biomacromolecules. 2011;12:2302–2310. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 56.Li LQ, Tong ZX, Jia XQ, Kiick KL. Soft Matter. 2013;9:665–673. doi: 10.1039/C2SM26812D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopeček J, Yang J. Angew. Chem. Int. Ed. 2012;51:7396–7417. doi: 10.1002/anie.201201040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGann CL, Levenson EA, Kiick KL. Macromol. Chem. Phys. 2013;214:203–213. doi: 10.1002/macp.201200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burrows M, Shaw SR, Sutton GP. Bmc Biol. 2008;6:41–57. doi: 10.1186/1741-7007-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin GK, Lapidot S, Numata K, Hu X, Meirovitch S, Dekel M, Podoler I, Shoseyov O, Kaplan DL. Biomacromolecules. 2009;10:3227–3234. doi: 10.1021/bm900735g. [DOI] [PubMed] [Google Scholar]
- 61.Qin GK, Rivkin A, Lapidot S, Hu X, Preis I, Arinus SB, Dgany O, Shoseyov O, Kaplan DL. Biomaterials. 2011;32:9231–9243. doi: 10.1016/j.biomaterials.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin GK, Hu X, Cebe P, Kaplan DL. Nat. Commun. 2012;3 doi: 10.1038/ncomms2004. DOI:10.1038/ncomms2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bracalello A, Santopietro V, Vassalli M, Marletta G, Del Gaudio R, Bochicchio B, Pepe A. Biomacromolecules. 2011;12:2957–2965. doi: 10.1021/bm2005388. [DOI] [PubMed] [Google Scholar]
- 64.Sbrana F, Fotia C, Bracalello A, Baldini N, Marletta G, Ciapetti G, Bochicchio B, Vassalli M. Bioinspir. Biomim. 2012;7 doi: 10.1088/1748-3182/7/4/046007. DOI:10.1088/1748-3182/7/4/046007. [DOI] [PubMed] [Google Scholar]
- 65.Lv S, Dudek DM, Cao Y, Balamurali MM, Gosline J, Li HB. Nature. 2010;465:69–73. doi: 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]






