Abstract
Background: Bacillus anthracis may usually cause three forms of anthrax: inhalation, gastrointestinal and cutaneous. The gastrointestinal (GI) anthrax develops after eating contaminated meat. Thus, in this paper were report 5 cases of intestinal anthrax.
Case Presentation: We report a case series of intestinal anthrax, with history of consumption of raw or poorly cooked liver of sheep. One patient was female and 4 were males with the age range between 17 and 26 years. All patients were admitted with abdominal pain, nausea, and vomiting. Examination revealed abdominal distention on the right lower quadrant or diffuse tenderness. Laboratory examination in all patients showed leukocytosis with polymorphonuclear of >80%. Because of the unclear and questionable diagnosis, exploratory laparotomy was performed on several patients, invariably showing an abundant yellowish and thick ascitic fluid, soft hypertrophied mesenteric lymph nodes (3-5 cm) mostly in the ileocecal region, and substantial edema involving one segment of small bowel, cecum or ascending colon. Anthrax was diagnosed on the epidemiologic basis (ingestion history of half cooked liver of sheep) or microbiologic (microscopy with bacterial culture) and pathologic testing (post surgery in four patients or autopsy in one patient). With appropriate treatment, 4 survived and one patient died.
Conclusion: Gastrointestinal anthrax is characterized by rapid onset, fever, ascitis and septicemia. The symptoms can mimic those seen in an acute surgical abdomen. Rapid diagnosis and prompt initiation of antibiotic therapy and then exploratory laparotomy (right hemicolectomy) are keys to survival.
Key Words: Anthrax, Gastrointestinal, Bacillus anthracis
Bacillus anthracis is a gram-positive rod 4 μm by 1 μm that is aerobic or facultatively anaerobic anthrax has been a disease of animals primarily with a long history of animal-associated disease in humans. Infection occurs most frequently by exposure of the spore of B. anthracis, although when the vegetative from of the organism is ingested in the meat of an infected animal the clinical disease can also occur (1). Bacillus anthracis causes three forms of anthrax: inhalation, gastrointestinal and cutaneous (1). Anthrax is characterized by both toxemia which is caused by secretion of immunomodulating toxins (lethal toxin and edema toxin) and septicemia which is associated with bacterial encapsulation (1). The incubation period for the gastrointestinal form is usually 2 to 5 days but may be as short as 15 hours. The gastrointestinal (GI) anthrax develops after eating contaminated meat. When spores germinate in the intestinal tract, they cause ulcerative lesions. These lesions can occur anywhere and may in severe cases, result in hemorrhage, obstruction or perforation (2-3). GI anthrax occurs 2 to 5 days after spores are ingested usually from raw or under cooked meat or liver of infected animals. Because anthrax is more common in the developing world where reporting is not common, the true incidence of GI anthrax is unknown. Likewise, the mortality rate is not precisely known. Although rates have been estimated at 4 to 50% (2).
This study reports 5 cases of intestinal anthrax from 2001 to 2009 in Ghaem Medical Center of Mashhad, where the consumption of raw or poorly cooked liver of sheep is customary.
Case Presentation
Case 1: A 26- year-old woman was admitted to the hospital with abdominal pain, generalized weakness, hematemesis and high-grade fever. She had eaten poorly cooked liver of sheep 5 day ago. Physical examination on his admission was as follows; temperature= 38°C, pulse rate= 120 beats/min, blood pressure= 110/60 mmHg, respiratory rate= 25 breaths / min.
On examination, the abdomen was distended with ascitis and tenderness in the right lower quadrant. The patient underwent laparotomy in other centers for suspected acute appendicitis that showed edema of the cecum and ascending colon, and enlargement of the mesenteric lymph nodes, then with continuous clinical symptoms, the patient referred to our center with abdominal distention and leakage of ascitics of abdominal incision.
Her peripheral leukocyte count was 32000/mm3 with 89% neutrophils. After 6 hours of admission, the clinical condition of the patient deteriorated and because of the unclear and questionable diagnosis, relaparotomy was performed, invariably showing an abundant yellowish and ascitic fluid, soft hypertrophied mesenteric lymph nodes mostly in the ileocecal region and edema involving in cecum and ascending colon. Right hemicolectomy with ileostomy and mucus fistula was performed. Gross pathology evaluation revealed extremely edematous bowel wall, necrotic hemorrhagic mucosa and enlarged mesenteric lymph nodes.
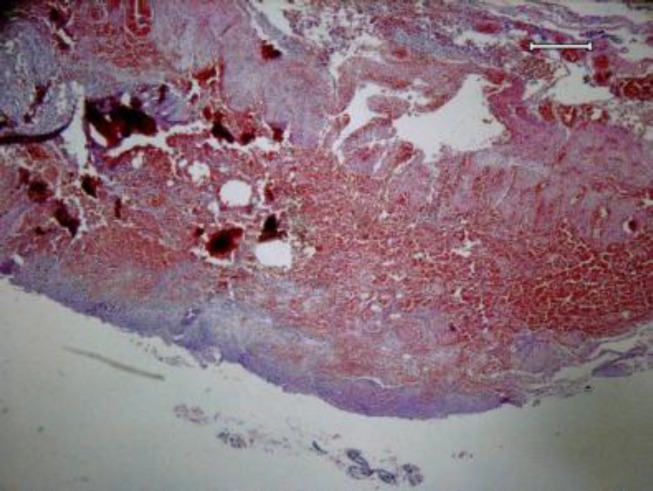
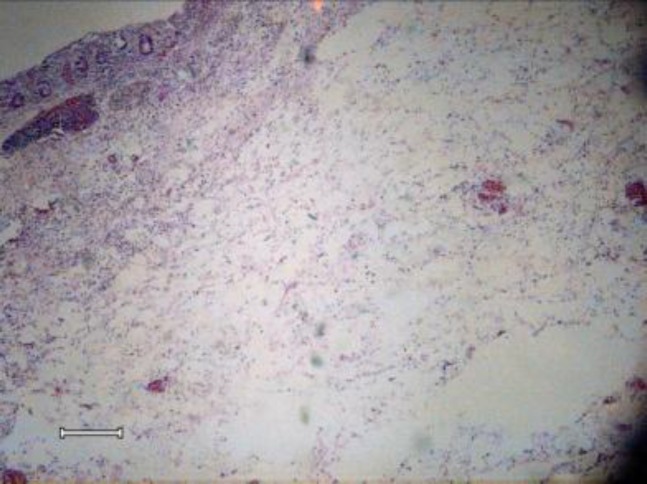
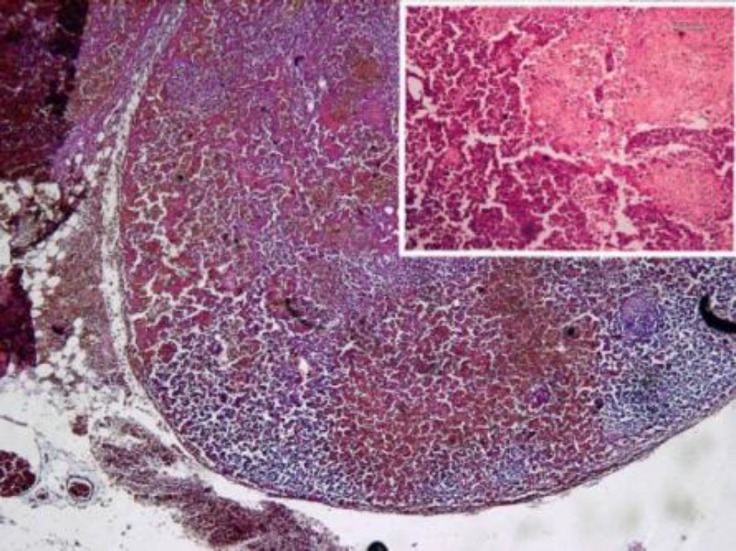
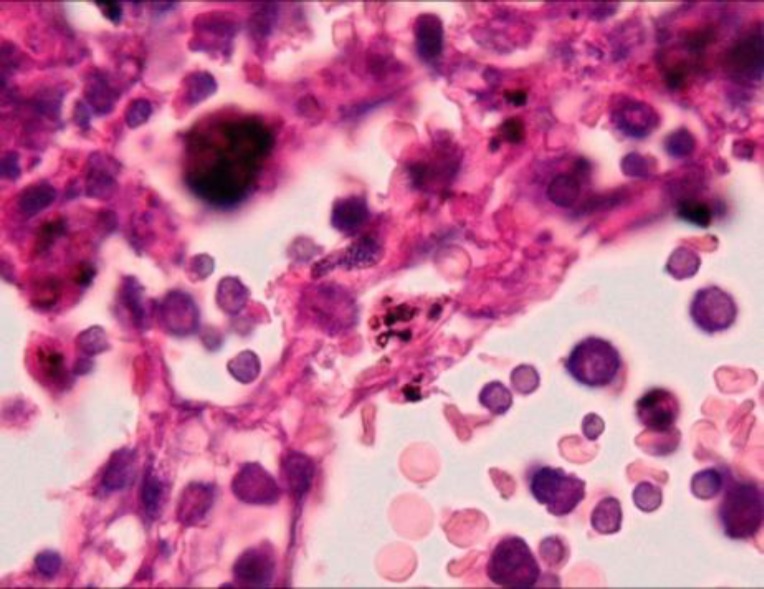
Microscopic pathology report was severe transmural edema, diffuse non-specific inflammation, foci of ulceration, and coagulative and hemorrhagic transmural necrosis as well as hemorrhagic and necrotic lymphadenitis (figures 1, 2, 3). Thick bacilli were shown inside the macrophages in submucosa and also lymph nodes (figure 4).
Figure 1.
Transmural hemorrhagic necrosis in bowel wall -x 40, Hematoxillin & Eosin staining
Figure 2.
Marked transmural edema of bowel wall - x40, Hematoxillin &Eosin staining
Figure 3.
Hemorrhagic necrotic lymphadenitis- x40, Hematoxillin & Eosin staining. [inset: necrotic areas –x100, Hematoxillin & Eosin staining]
Figure 4.
Presence of thick bacterial bacilli inside macrophages cytoplasm of lymph node– x1000, Hematoxillin & Eosin staining
After surgery, the patient revealed rapid clinical improvement, culture from fluid grew B. anthracis, attempts at resuscitation with intravenous fluids and treatment with penicillin. She was discharged 10 days later in good condition.
Case 2: A 25-year-old man was admitted with abdominal pain and distention, dyspnea, bloody diarrhea with 7 days duration. Physical examination on his admission was as follows:
Temperature= 36oC, pulse rate= 110 beats/min and regular blood pressure 120/80 mmHg, respiratory rate= 20 breaths/min. On abdominal examination, he had generalized abdominal tenderness and evidence of ascites. CT scan of abdomen revealed free fluid and mass in ascending colon, in colonoscopic examination, ascending colon showed evidence of inflammation, necrosis and ulcerative lesions. His periphral leukocyte count was 21000/mm3 with 95% neutrophils. He underwent laparotomy which yielded approximately 2 liters of fluid and fibrin. The cecum and ascending colon were edematous with ulcerative and necrotic lesions, pericolic and interloop abscess. Right hemicolectomy with ileostomy was performed, pathologic examination showed a central mucosal necrotic lesion along with hemorrhage and extensive edema.
In this particular case, the clinical and surgical findings suggested intestinal anthrax. Although, no microbiologic evidence was obtained, he improved slowly and was discharged on day 8.
Case 3: A 17-year-old young boy was admitted with history of abdominal pain and distention, developed 4 days after ingestion of half-cooked liver of sheep. The following results were obtained in the physical and laboratory examinations on his admission: temperature= 36.5oC, pulse rate= 70 beats/min heart and lung= normal, and presence of the generalized abdominal tenderness and rigidity. His peripheral leukocyte count was 22000/mm3, with 75% neutrophils and the patient underwent laparotomy in other centers for suspected acute appendicitis that showed yielded approximately 2 liters of fluid and edema of the cecum and ascending colon. Then, with continuous clinical symptoms, the patient referred to our center with abdominal distention and pain. In sonography, ileus in intestinal loop and edema of intestine with presence of free fluid were showed.
Because of the unclear and questionable diagnosis, relaparotomy was performed showing an abundant yellowish and thick ascitis fluid, soft hypertrophied mesenteric lymph nodes mostly in the ileocecal region and substantial edema involving one segment of cecum and ascending colon. The right hemicolectomy with ileostomy and mucus fistula was performed. The examination of the bowel segment disclosed a central necrotic lesion encircled by small soft red nodules surrounded by severe edema of the bowel wall with areas of hemorrhage. He was discharged 5 days later in good condition.
Case 4: A 17-year-old young boy was admitted with generalized abdominal pain and distention; he had eaten poorly cooked liver of sheep. The patient underwent appendectomy in other centers for suspected acute appendicitis and then he was referred to our center with abdominal distention and continuous clinical symptoms. His pulse was 80 beats/min, respiratory rate of 20 breath/min, systolic blood pressure 110 mmHg, and temperature of 37.5oC. On physical examination, he had abdominal tenderness and evidence of ascites. Peripheral leukocyte count was 18000/mm3 with 80% neutrophils. He underwent laparotomy, with approximately 2 liters of hemorrhagic fluid. The cecum and ascending colon were edematous with a hemorrhagic mucosa and patchy necrosis, mesenteric lymphadenopathy was noted. Right hemicolectomy with ileostomy and mucus fistula was performed. After surgery, the patient revealed rapid clinical improvement. His pathologic evaluation showed a central mucosal necrotic lesion along with hemorrhage and extensive edema. Mesenteric hemorrhagic edema was also observed.
Case 5: A 23-year-old man was admitted to our hospital with history of fever, abdominal pain and bloody diarrhea. On examination, the abdomen was distended with ascites He was very weak and with intravascular depletion. Attempts at resuscitation with intravenous fluids and treatment penicillin failed. The patient died 6 hours after admission. In autopsy (liver and lungs), gram positive thick rods were visible.
Discussion
In our study, all patients were admitted with abdominal pain, nausea, and vomiting. The examination usually revealed abdominal distention on the right lower quadrant or diffuse tenderness (table 1).
Table 1.
Demographic characteristics, presentation and outcome
| Age | Sex | Symptom | WBC | Surgery | Survival | |
|---|---|---|---|---|---|---|
| Patient 1 | 26 | F | Abdominal pain | 32000 | Right hemicolectomy | alive |
| Patient 2 | 25 | M | Abdominal pain | 21000 | Right hemicolectomy | alive |
| Patient 3 | 17 | M | Abdominal pain | 22000 | Right hemicolectomy | alive |
| Patient 4 | 17 | M | Abdominal pain | 18000 | Right hemicolectomy | alive |
| Patient 5 | 23 | M | Abdominal pain | ----- | ----- | expired |
Clinical presentation has three stages for abdominal anthrax; phase I, including low grade fever, syncope and malaise, then about 24 hours later, phase II is characterized by mild to severe abdominal pain, nausea, and vomiting (4). Examination usually reveals abdominal distention and an ill-defined mass in the right lower quadrant or periumbilical area. Unlike early appendicitis, the patients with intestinal anthrax have evidences of ascites, intravascular depletion and severe weakness following an initial phase of syncope and malaise. Phase III was characterized by paroxysmal abdominal pain, rapidly increasing abdominal girth, ascites, flushed face, red conjunctiva and often shock (4-5). Children with anthrax present with a wide range of clinical signs and symptoms, somewhat differ from the presenting features of adults with anthrax. Like adults, the children with gastrointestinal anthrax have two distinct presentations, upper tract disease characterized by dysphagia and oropharyngeal findings and lower tract disease characterized by fever, abdominal pain, nausea and vomiting (6-7).
Because of ulceration of the gastrointestinal mucosa, patients often vomit material that is blood-tinged or has a coffee-ground appearance. Ascites develops with concomitant reduction in abdomen two to four days after the onset of symptoms (7(. The appearance of ascites fluid ranges from hemorrhagic to purulent and it often yields colonies of bacillus anthracis when cultured (8-9).
In a study in Kazakhistan, confirmed 29 (55%) of patients had measurable fever with a median maximum temperature of 39 oC, headache (25%) and myalgias (43%) were also frequently reported (10). The diagnosis of GI anthrax requires a high index of suspicion may be diagnosed on the basis of epidemiology or microbiologic, pathologic or serologic testing. Laboratory diagnosis may be obtained from stool, blood or peritoneal cultures, tissue samples or serologic evidence of infection (11).
Accurate and timely diagnosis of anthrax is essential and the range of differential diagnosis is extensive specimen from those patients suspected to have anthrax, they should be stained by both gram stain and polychrome methylene blue. A direct gram-staining of any tissue or fluid will reveal large number of the characteristic bacilli anthracis seen as large non spore-forming rods (12).
Serologic diagnosis is available only in a few research laboratories. Pathologic evaluation requires surgery or necroscopy in an appropriate hospital, microbiologic testing requires at least microscopy and preferably bacterial culture capacity and epidemiology requires a level of suspicion and an ability to properly perform out break investigations (13).
Pathologic examination of anthrax lesions with entry via the GI tract shows that the mucosa is always involved as regional lymph nodes, which are enlarged and hemorrhagic. (13). A study in Shiraz University of Medical Sciences has records of 7130 autopsies performed in the past 40 years, 33 of which were anthrax cases. They reviewed all the pathology slides of these cases and classified the organs involved in a search for unrecognized microscopic findings (14). The GI anthrax may be diagnosed on the epidemiologic basis (ingestion history of half cooked liver of sheep) or microbiologic (microscopy and bacterial culture) and pathologic testing (post-surgery in four patients or autopsy in one patient). In our study, the laboratory examination in all patients showed leukocytosis with WBC>10000/mm3 and with PMN>80%. Anthrax can be successfully treated if the disease is promptly recognized and appropriate therapy is initiated early (surgery and antibiotic therapy).
Penicillin G (24.000.000 IU/day, IV) is the best choice for therapy. Gastrointestinal anthrax are characterized by rapid onset, fever septicemia and a high fatality rate without early antibiotic treatment if not, most patients will require some degree of critical care in the form of ventilator of hemodynamic support (15, 16). All patients with acute abdominal pain, rapid onset and history of consumption of raw or poorly cooked sheep meat must be suspicious to GI Anthrax and then treatment and evaluation should be initiated.
In summary, gastrointestinal anthrax is characterized by rapid onset, fever, ascites, and septicemia. The symptoms can mimic those seen in an acute surgical abdomen. Rapid diagnosis and prompt initiation of antibiotic therapy and then exploratory laparotomy (right hemicolectomy) are keys to survival.
Acknowledgments
The authors thank Ms. M. Hassanpour for editing the manuscript.
Conflict of interest: There was no conflict of interest.
References
- 1.Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. Primary involvement of pharynx and peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 2007;3:e76. doi: 10.1371/journal.ppat.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of physicians. Anthrax. 2007. [Dec 26, 2011]. Availible at: URL: http://pier.acponline.org/physicians.
- 3.Schmid G, Kaufmann A. Anthrax in Europe: its epidemiology, clinical characteristics, and role in bioterrorism. Clin Microbiol Infect. 2002;8:479–88. doi: 10.1046/j.1469-0691.2002.00500.x. [DOI] [PubMed] [Google Scholar]
- 4.Lucey D. Bacillus anthracis (Anthrax) In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6th ed. Pennsylvania: Churchill Livingstone; 2005. pp. 2485–91. [Google Scholar]
- 5.Bravata DM, Wang E, Holty JE, et al. Pediatric anthrax: implications for bioterrorism preparedness. Evid Rep Technol Assess. 2006;141:1–48. [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty ME, Ashford DA, Griffin PM, Tauxe RV, Sobel J. Gastrointestinal anthrax: review of the literature. Arch Intern Med. 2003;163:2527–31. doi: 10.1001/archinte.163.20.2527. [DOI] [PubMed] [Google Scholar]
- 7.Spencer RC. Bacillus anthracis. J Clin Pathol. 2003;56:182–7. doi: 10.1136/jcp.56.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med. 1999;341:815–26. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 9.Anthrax. 2004. [Dec 26, 2011]. Availible at: URL: http://emergency.cdc.gov/agent/anthrax/index.asp.
- 10.Woods CW, Ospanov K, Myrzabekov A, et al. Risk factors for human anthrax among contacts of anthrax-infected livestock in Kazakhstan. Am J Trop Med Hyg. 2004;71:48–52. [PubMed] [Google Scholar]
- 11.Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;2:247–56. doi: 10.1016/j.det.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Oncü S, Oncü S, Sakarya S. Anthrax--an overview. Med Sci Monit. 2003;9:276–83. [PubMed] [Google Scholar]
- 13.Sirisanthana T, Brown AE. Anthrax of the gastrointestinal tract. Emerg Infect Dis. 2002;8:649–51. doi: 10.3201/eid0807.020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabei SZ, Amin A, Mowla A, Nabavizadeh SA, Razmkon A. Anthrax: pathological aspects in autopsy cases in Shiraz, Islamic Republic of Iran, 1960-2001. East Mediterr Health J. 2004;10:27–36. [PubMed] [Google Scholar]
- 15.Ozkaya E, Kirimi E, Berktas M, Odabas D. Bacillus anthracis sepsis in a newborn. Pediatr Infect Dis J. 2000;19:487–8. doi: 10.1097/00006454-200005000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Kalamas AG. Anthrax. Anesthesiol Clin North America. 2004;22:533–40. doi: 10.1016/j.atc.2004.05.009. [DOI] [PubMed] [Google Scholar]