Abstract
Background: Titanium (Ti) implants are commonly coated with hydroxyapatite (HA). However, HA has some disadvantages such as brittleness, low tensile strength and fracture toughness. It is desirable to combine the excellent mechanical properties of ZrO2 and the chemical inertness of Al2O3 with respect to the purpose of this project which was coating Ti implants with HA-ZrO2-Al2O3 to modify the surface of these implants by adding ZrO2 and Al2O3 to HA. The purpose of this study was to evaluate the efficacy of hydroxyapatite coating nonocomposite.
Methods: From September 2009 to January2011, functionally graded HA-Al2O3-ZrO2 and HA coatings were applied on Ti samples. HA-Al2O3-ZrO2 and HA sols were orderly dip coated on the substrates and calcined. Scanning electron microscopy and EDS were used to estimate the particle size of the surfaces and for morphological analysis. The morphology of non-coated HA-coated HA-Al2O3-ZrO2 (composite-coated) and double-layer composite coated samples were compared with one other. Mechanical test (heat & quench) was also done for comparing single-phase (HA), composite and double-layer composite samples.
Results: The morphology of HA-Al2O3-ZrO2 coating is more homogenous than HA-coated and uncoated samples. Furthermore, single-layer coating is more homogenous than double-layer coating. EDS analysis was done on HA-coated sample and showed that the Ca/P ratio in the film was similar to the theoretical value 1.67 in HA.
Conclusion: Surface modification of Ti implants can be done by coating them with single-layer of HA-Al2O3-ZrO2. Single-layer hydroxyapatite-alumina-zirconia coated sample has the most homogenous morphology on the surface.
Key Words: Surface modification, Ti Implants, Hydroxyapatite, Nanocomposite
Metallic implants used in plastic and reconstructive surgery, orthopedic surgery, craniofacial surgery, and oral implantology can be regarded as scaffolds for load-bearing, bone-replacing/contacting applications such as joint and tooth replacement, fracture healing, and reconstruction of congenital skeletal abnormalities (1). Titanium (Ti) and its alloys are widely used as base materials for orthopedic or dental implants because they are materials with excellent mechanical properties and corrosion resistance. They also show good biocompatibility and the oxide layer exhibits corrosion resistance, but not sufficient for a long term use in a corrosive environment like body fluid.
The release of ions in the blood stream could be detrimental to the patient, inducing inflammatory, allergic or carcinogenic reactions (1, 2). However, by generating a coating onto a titanium surface that mimics the organic and inorganic components of living bone tissue, a physiological transition between the non-physiological titanium surface and surrounding bone tissue can be established. Research efforts have focused on modifying the surface properties of titanium to control the interaction between the implant and its biological surrounding (1, 3).
Ti implants are commonly coated with hydroxyapatite [Ca10 (PO4)6 (OH) 2, HA], a bioceramic which resembles the mineral constituents of human bones and teeth. The HA coating would reduce the release of metallic ions by acting like a barrier, and at the same time enhance the bone bioactivity by virtue of its chemical constituents (3-5). In particular, the use of HA is promising since it has very similar chemical and crystallographic structures to those of the human bone, which effectively eliminates biocompatibility problems. However, HA has some disadvantages, such as brittleness, low tensile strength and fracture toughness. In practical use, If HA is produced in the form of coating; however, its advantages can be properly exploited (6).
To solve these problems, hydroxyapatite composite coatings especially nanocomposite coatings are used. Nanophase materials, by their very nature, possess greater numbers of atoms at the surface, higher surface areas, larger portions of surface defects (such as edge/corner sites), increased electron delocalization, and greater numbers of grain boundaries at the surface have an advantage over conventional larger grain size materials for many biological applications. All of these factors contribute to higher surface reactivity of nanophase compared to conventional materials (7). There is an increasingly interest in hydroxyapatite nanoparticles for its similarity to bioapatite and enhanced biomedical properties (5). It has been reported that nano- sized HA exhibits better bioactivity than coarser crystals (8). This nanotechnology makes the HA particle finer, which means that a remineralization effect of a demineralized tooth surface can be expected (9).
High technology ceramics have different crystal phases as well as superior characteristics such as high temperature resistance and high chemical stability. Alumina is one of the widely used structural ceramics. Additive interaction can modify and achieve tailor made properties of alumina ceramics. Zirconia is one such additive, which can increase the strength and toughness of the alumina matrix either by stress-induced transformation toughening or microcrack toughening (10). Zirconia has been commonly used as reinforcement for many ceramics because of its high strength and fracture toughness. Bioinertness is another merit of the ZrO2. However, extensive reaction between the HA and the ZrO2 to from TCP and fully stabilized ZrO2 is a serious disadvantage of this approach. Alumina, which is also classified as a bioinert material, has been widely investigated as a reinforcing agent for HA. Therefore, it is desirable to combine the advantages of both materials as reinforcements for HA: the excellent mechanical properties of ZrO2 and the chemical inertness of Al2O3 (11). In this study, we tried to find out the efficacy of hydroxyapatite coating nonocomposite.
Methods
Coating Procedure: Four commercially pure grades 2 titanium with 6×2 mm dimension and 0.02% iron content, (Dr Nik Biomedic Engineering Research Center) were used. The cleaning was followed by an ultrasonic rinse in distilled water and ethanol then was washed with acetone. One sample was put away without coating on it. It was the uncoated sample that was compared with coated samples. This study was conducted from September 2009 to January 2011.
Hydroxyapatite sol was prepared by using Ca(NO3)2. 4H2O (Merck No. 2121) and (NH4)2HPO4 (Merck No. 1207) as starting materials and ammonia solution as the agent for pH adjustment. A suspension of Ca (NO3)2.4H2O was vigorously stirred and a solution of (NH4)2HPO4 was slowly added dropwise to the Ca (NO3)2.4H2O solution.
The so-obtained slurry was aged for 20 hours under stirring at 60°C. After ageing, the solution was moved for dip coating of the specimen in 60mm/min. The obtained coating was dried at 40°C and calcined at 675°C to get adherent crystalline coating (In this stage, one sample was coated with hydroxyapatite sol).
In the next step, AlCl3.6H2O (Scharlau) and ZrOCl2.8H2O (Merck No. 8917) were used as the starting materials for preparation of ZrO2-Al2O3 sol. The matrix solution (HA) and the reinforcement solution (ZrO2-Al2O3) were constantly stirred for 24 hours. Then HA solution was finally added to the sol of ZrO2-Al2O3 and stirred for 2 hours.
After ageing (20 hours under stirring at 60°C), the samples (2 samples) were dip coated in 60 mm/min, dried at 40°C and calcined at 675 °C. Double-Layer coated sample was prepared by re-drying the sample at 40°C for 15 minutes and doing calcination on it after that.
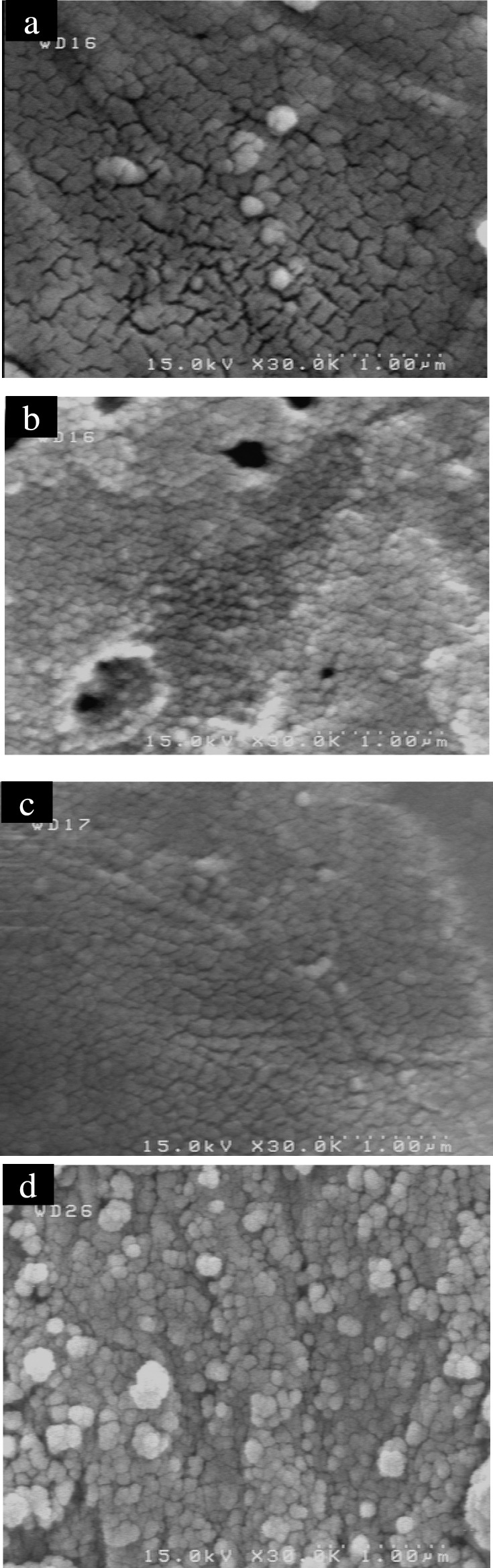
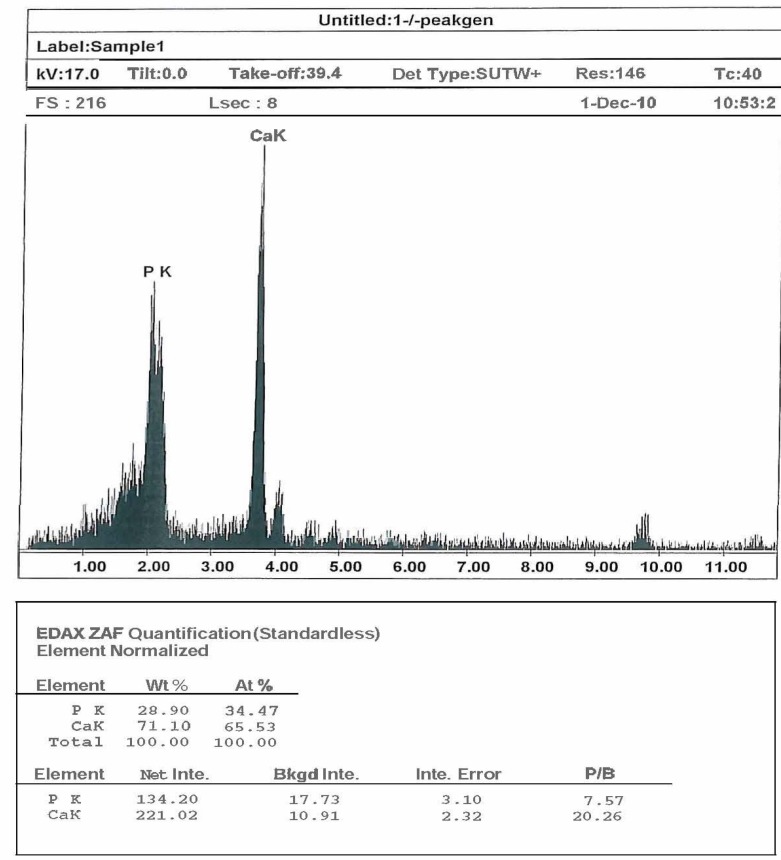
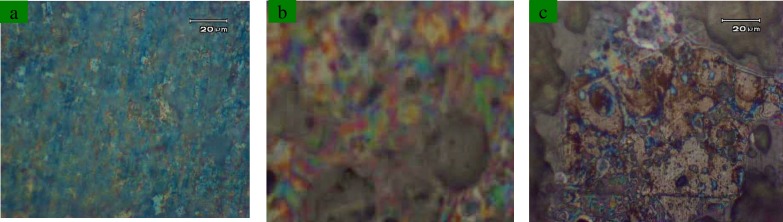
Characterization of coatings: The morphological analysis has been performed by Field-Emission scanning electron microscopy (FE- S4160 SEM). Figure 1 shows SEM image of uncoated sample (1a), hydroxyapatite-coated sample (1b), composite-coated sample (1c) and double-layer composite coated sample (1d). The composition analysis by energy dispersive X-ray spectroscopy (EDS) was done on hydroxyapatite-coated sample to show the molar ratio of Ca and P (figure 2 and table 1). Furthermore, heat -and -quench test (Mechanical test) was done on coated samples for analyzing the morphology of coatings. It was based on BS 4601 standard where the samples were put in the oven at 80°C for 1 hour and quenched in the air. This cycle was repeated for 4 times. Then the surface of the coatings was analyzed by an optical microscope (figure 3).
Figure 1.
SEM Pictures of Non-Coated sample (a), Hydroxyapatite-Coated sample (b), Double-Layer composite coated sample (c) and Single-Layer composite coated sample (d)
Figure 2.
Scanning electron microscopy energy dispersive X-ray analysis of the Hydroxyapatite-Coated sample
Table 1.
Result of Scanning electron microscopy energy dispersive X-ray of the Hydroxyapatite-Coated sample
| Sample | Weight of Ca | Moles of Ca | Weight of p | Moles of P | Molar tatio of Ca/P |
|---|---|---|---|---|---|
| HA | 0.711 | 0.0177 | 0.289 | 0.0093 | 1.9 |
Figure 3.
Optical Microscope Pictures of Double-Layer composite coated sample (a), Composite-Coated sample (b) and Hydroxyapatite-Coated sample (c)
Results
Morphological studies: Figure 1 shows SEM image of uncoated sample (a), hydroxyapatite-coated sample (b), double-layer composite coated sample (c) and single-layer composite coated sample (d).
Figure 1 (b) shows that the film is not smooth and consists of some pores, and the grain boundary is obvious. There is a clear surface texture in the film which will decrease the combination strength of the film. The grain boundary is also obvious in uncoated sample and its surface, is not smooth and is rougher than HA-coated sample and single and double-layer samples (figure 1 (a)). Figure 1 (c, d) shows the surface morphologies of the sol-gel films for single-layer and double-layer coating are clear to see because the surface texture disappears and the dimension of the pores decreases. The coating is homogenous and covers the surface after one step of dipping. For the thicker film (double-layer composite coating) some tiny cracks are sometimes visible on the SEM pictures.
EDX analysis: The EDX scanning analysis in an HA deposited layer on a Ti implant is shown in figure 2. The results in table 1 show that the Ca/P ratio in the HA-coated film is 1.9 which is similar to the theoretical value 1.67 in HA.
Mechanical properties: Figure 3 shows the optical microscope pictures of HA-coated sample, single-layer composite coated sample and double-layer composite coated sample. By paying attention to the scanning electron, the microscope pictures and optical microscope images in this research have shown that the mechanical properties of HA coatings can be improved by the reinforcement of alumina and zirconia.
On the basis of BS 4601 in heat- and quench test, the samples must not have any cracks or bubbles on their surfaces after the test, and the samples in this research did not have any cracks or bubbles on their surface although in the image that corresponded to a single layer sintered at 675 °C for 1 h can be seen a very homogeneous crystalline grain structure of about 80 nm has developed [figure 1(c) and figure 3 (b)].
Discussion
In this work, the application of the sol-gel technique for the realization of ceramic coatings on metals has been studied, with the aim of obtaining composite biomaterials to be used in the coating of dental implants. The study was focused on the deposition of hydroxyapatite-alumina-zirconia films by dip-coating on a titanium substrate. This process was allowed to obtain films that SEM analyses revealed good homogeneity and high surface roughness, and this latter parameter was particularly important, as it guaranteed a wide contact surface between the implant and the surrounding bony tissue.
In this project, the morphology of uncoated titanium, HA-coated titanium, HA-alumina-zirconia coated titanium and double HA-alumina-zirconia coated titanium has been studied. It means that HA and the reinforced coatings are more corrosion resistant than uncoated titanium (12).
Double layer coating makes the film thicker without affecting the morphology of the coating. Based on the structural and morphological results the single-layer HA-alumina-zirconia coated sample has the most homogenous morphology on the surface.
The study showed that the bond strength of HA coating/metal substrate interface has been the point of potential weakness in prosthesis because it is limited by the strength of hydroxyapatite, porosity, and inclusion in the lamellar structure of the coating (13).
The EDX analysis shows the surface chemical composition of HA coating. The analysis reveals that calcium and phosphorus is in the desired ratio and no alteration is noticed in the stoichiometric HA. The spectrum reveals the presence of HA crystal phase and results show that the Ca/P ratio in the HA-coated film is similar to the theoretical value 1.67 in HA.
At the molecular level, the high adhesion strength values are possible with a single layer of HA-ZrO2-Al2O3 sintered at 675 °C for 1 h. According to the optical microscope analysis HA coating shows the lowest strength, and the single layer of HA-ZrO2-Al2O3 coating shows the highest strength. The strength of the coating has been improved by the addition of ZrO2 and Al2O3. In conclusion, surface modification of Ti implants can be done by coating them with singe-layer of HA-Al2O3-ZrO2. Single-layer hydroxyapatite-alumina-zirconia coated sample has the most homogenous morphology on the surface.
Acknowledgment
The authors wish to thank Dr Nik of the Engineering Research Center for supplying the titanium samples.
Finding: This study was self-funded
Conflict of Interest: There was no conflict of interest.
References
- 1.De JongeLT, Leeuwenburgh SC, Wolke JG, Jansen JA. Organic–inorganic surface modifications for titanium implant surfaces. Pharm Res. 2008;25:2357–69. doi: 10.1007/s11095-008-9617-0. [DOI] [PubMed] [Google Scholar]
- 2.Arnould C, Denayer J, Planckaert M, Delhalle J, Mekhalif Z. Bilayers coating on titanium surface: the impact on the hydroxyapatite initiation. J Colloid Interface Sci. 2010;341:75–82. doi: 10.1016/j.jcis.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Chou BY, Chang E. Influence of deposition temperature on mechanical properties of plasma-sprayed hydroxyapatite coating on titanium alloy with ZrO2 intermediate layer. J Therm Spray Technol. 2003;12:199–207. [Google Scholar]
- 4.Kwok CT, Wong PK, Cheng FT, Man HM. Characterization and corrosion behavior of hydroxyapatite coatings on Ti6Al4V fabricated by electrophoretic deposition. Applied Surface Sci. 2009;255:6736–44. [Google Scholar]
- 5.Shi HB, Zhong H, Liu Y, Gu JY, Yang CS. Effect of precipitation method on stoichiometry and morphology of hydroxyapatite nanoparticles. J Key Eng Mater. 2007;19:271–4. [Google Scholar]
- 6.Lim YM, Hwang KS, Park YJ. Sol-gel derived functionally graded TiO2/HAP films on Ti-6Al-4V implants. J Sol-Gel Sci Technol. 2001;21:123–8. [Google Scholar]
- 7.Webster TJ. Nanotechnology: better materials for all implants. J Mater Sci Forum. 2007;539-543:511–16. [Google Scholar]
- 8.Meng XC, Lv KL, Zhang JX, Qu DL. Caries inhibitory activity of the Nano-HA in Vitro. J Key Eng Mater. 2007;330-332:251–4. [Google Scholar]
- 9.Jeong SH, Jang SO, Kim KN, et al. Remineralization potential of new toothpaste containing Nano-hydroxyapatite. J Key Eng Mater. 2006;309-311:537–40. [Google Scholar]
- 10.Ergu OB, Guru M, Cabbar C. Preparation and characterization of alumina zirconia composite material with different acid ratios by the sol-gel method. Cent Eur J Chem. 2008;6:482–7. [Google Scholar]
- 11.Mobasherpour I, Solati HashjinM, Razavi ToosiSS, Darvishi KamachaliR. Effect of the addition ZrO2AI2O3 on nanocrystalline hydroxyapatite bending strength and fracture toughness. J Ceram Int. 2009;35:1569–74. [Google Scholar]
- 12.Balamurugan A, Balossier G, Kannan S, et al. Electrochemical and structural characterisation of zirconia reinforced hydroxyapatite bioceramic sol-gel coatings on surgical grade 316L SS for biomedical applications. Ceram Int. 2007;33:605–14. [Google Scholar]
- 13.Li J, Hermansson L, So¨remark R. High strength biofunctional zirconia: mechanical properties and static fatigue behaviour of zirconia–apatite composites. J Mater Sci Mater Med. 1993;4:50–4. [Google Scholar]