Abstract
Background: Tramadol is a synthetic analgesic. Seizures have been reported in patients receiving this drug. In this study we evaluated the correlation between tramadol consumption and seizure occurrence.
Methods: Twenty-eight subjects with a history of tramadol consumption and seizure were studied. Electroencephalograms (EEG) were performed in the first 24 hours and again one week later. Subjects were followed up for a mean of 18 months after the initial attack.
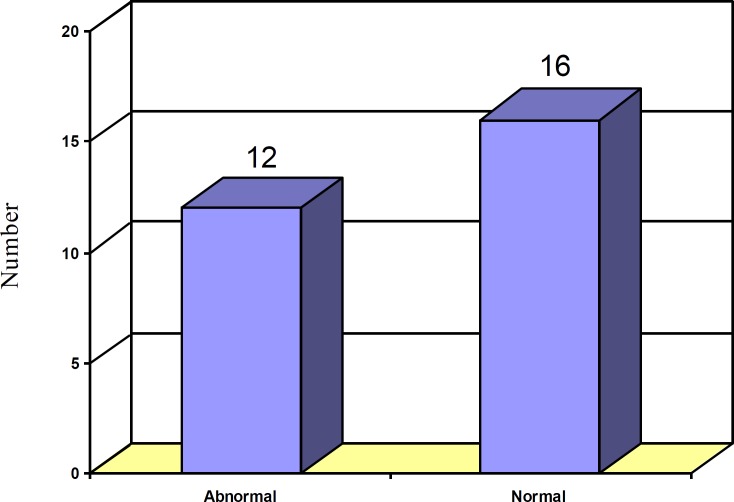
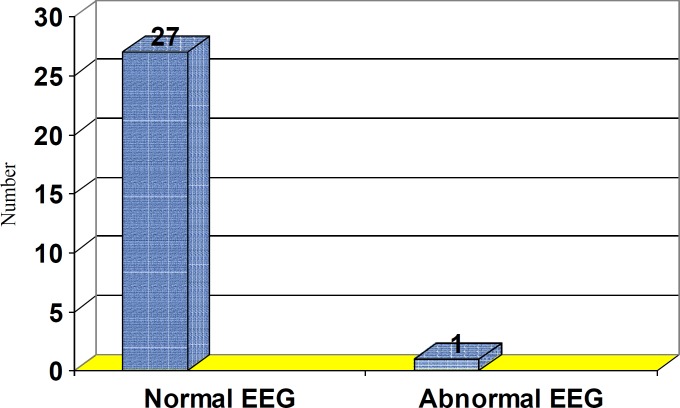
Results: In the 28 subjects, 26 (92.8%) were males and 2 (7.2%) were females. The mean age of the subjects was 28.4 years. Thirteen patients had abused more than 400 mg/day of tramadol. Sixteen subjects concomitantly used other drugs. The seizures occurred within the first 24 hours of tramadol intake in 25 of the subjects. The first EEG was abnormal in 12 cases, but the second EEG was abnormal in only one case. Neuroimaging of only one subject displayed patchy white matter lesions.
Conclusion: In conclusion, the neurotoxicity of tramadol commonly manifests as generalized tonic clonic seizures most frequently within 24 hours after tramadol intake and was more common in subjects concomitantly consuming alcohol, illicit drugs, anti-psychotics, or anti-depressants.
Key Words: Tramadol, Seizure, Electroencephalograms
Tramadol is a synthetic analogue of codeine and acts as a pure opioid agonist. Analgesia results also by inhibition of reuptake of nor-epinephrine and serotonin, endogenous neurotransmitters that modulate pain (1). Tramadol completely penetrates the blood-brain barrier. The peak plasma levels occur at about 1.5 hours after intake and plasma elimination half-life is 5-6 hours. Most excretion takes place through the kidneys. Therapeutic blood levels in adults are about 100-300 ng/ml (0.1- 0.2 μg/ml) (2). The maximum recommended dose is 400 mg/day (3). Acute overdose may induce miosis, respiratory depression, seizures, hypotonicity, and acidosis. Chronic side effects include fatigue, dizziness, vertigo, headache, visual disorders, nausea, vomiting, sweating, dry mouth, constipation, premature heartbeats, euphoria, dysphoria, and hallucinations (2). Seizures have been reported even with the recommended dosages (4). Some studies demonstrate that seizures may also occur in therapeutic ranges –especially in association with consumption of other drugs such as alcohol, selective serotonin reuptake inhibitors, tricyclic antidepressants, and antipsychotics (1, 2).
Chronic side effects include fatigue, dizziness, vertigo, headache, visual disturbance, nausea, vomiting, dry mouth, constipation, and hallucinations (5). There are a variety of tramadol preparations manufactured by different drug companies in Iran. In response to the increasing number of tramadol abusers in recent years, we decided to study tramadol induced seizures among the Iranian population and compare the results with other reports globally.
Methods
One hundred individuals with a history of tramadol induced seizures recorded between September 2004 and September 2007 were evaluated in Imam Khomeini and Shahid Motahari Hospitals at Urmia University of Medical Sciences. Among these subjects, 28 agreed to return for a follow up evaluation. All subjects involved in this study presented with a history of seizures after tramadol consumption and have no past medical history of epilepsy, structural brain lesion, or head trauma. Metabolic etiologies of seizure such as hypocalcemia and hypoglycemia were ruled out in these patients by measuring the serum level of sugar and calcium after admission for seizure attack in the hospital.
Data collection sheets were designed to survey the subjects’ sex, age, dose and duration of tramadol consumption, type of seizures experienced, number of attacks, concurrent medications during attacks (selective serotonin reuptake inhibitors, illicit drugs, tricyclic antidepressants, anti- psychotics and alcohol), time lag between tramadol intake and the onset of the attack, and the frequency of seizures. Comprehensive neurological examinations were carried out on each subject. Additionally, brain CT scan and/or MRI were obtained.
Electroencephalograms (EEG) were obtained both during the first 24-hours after admission and one week later. The following are the characteristics of electroencephalography machine and recording protocols: SAN.EI, model 92-A; High frequency filter = 70 Hz; Low frequency filter =1Hz; paper speed=30 mm/second; Sensitivity: 50 microvolt = 7mm. Both monopolar and bipolar montages were obtained. Normal EEG characterization in 20-60 years old awake adult person with closed eyes was accepted as displaying a posterior dominant alpha rhythm of 8-13 Hz. with an amplitude of 20-100 µV. In the frontocentral regions of adult subjects, normal EEG recordings were characterized by β rhythms (rhythms more than 13 Hz frequency and 5-20 µV amplitude).
Abnormal EEG patterns were characterized by any of the following EEG features: (1) spike discharges; (2) sharp wave discharges; (3) polyspike waves; (4) spike and wave or sharp and wave complexes; (5) abnormalities of the background rhythms; (6) abnormal periodic patterns; (7) focal or generalized abnormal slow activity. All the patients were followed up at any time between 9-31 months (Mean: 18 months) following initial seizure episode while consuming tramadol. This study was initially approved by the regional Ethics Committee. The data were collected and analyzed.
Results
Twenty-eight subjects with a history of tramadol induced seizures were evaluated within 24 hours of seizure attack and again, 9-31 months (Mean: 18 months) after the attack. From the 28 subjects who agreed to participate in a follow up evaluation, 26 were males (92.8%) and 2 were females (7.2%). The average age of subjects was 28.4 years (17-65 years). According to the patients’ history, four subjects consumed more than 1 g/day of tramadol, nine 400 mg/day and 1g/day, and fifteen in the therapeutic range of 50 mg/day to 400 mg/day. We did not have any laboratory equipment to measure the serum tramadol level. Sixteen patients concurrently used other medications. Two subjects consumed selective serotonin reuptake inhibitors, one, tricyclic antidepressants and antipsychotics, seven illicit drugs, and six patients alcohol abusers.
None of these patients had any previous history of seizures. In the follow up evaluation, only one subject was noted to have experienced another seizure episode. However, this subject continued to abuse tramadol. The seizures were entirely generalized tonic - clonic without aura or focal neurological deficit. Twenty seven subjects experienced only one attack of generalized tonic - clonic seizure. One patient experienced three attacks of tonic – clonic seizures without regaining consciousness (status tonic – clonic seizure). The tonic - clonic phase of seizures lasts less than 5 minutes in all 28 subjects. The seizure attacks occurred within the first 24-hours of tramadol consumption in 25 subjects and after 24-hours post-consumption in 3 subjects.
Initial EEG recordings (during the first 24-hours after admission) depicted generalized slowing in 12 subjects which may be seen between the attacks of idiopathic generalized tonic – clonic epilepsy (figure 1). Delayed EEG (one week after admission), however, showed scattered sharp waves in only one subject (figure 2). This pattern can also be seen between the attacks of idiopathic generalized tonic – clonic epilepsy.
Figure 1.
First day EEG
Figure 2.
Second week EEG
Brain MRI showed signal abnormality in subcortical white matter in one subject who had abused more than 1g/day tramadol over the course of one month. This subject experienced three attacks of generalized tonic clonic seizures within 24 hours from the time of admission.
Discussion
Tramadol is a synthetic 4-phenyl piperidine analogue of codeine whose selectivity for μ-receptors has recently been demonstrated. It is a central analgesic that has a low affinity for opioid receptors (1). Conversely, the major (M1) metabolite of tramadol produced by liver via o-demethylation shows a higher affinity for opioid receptors than the parent drug. The rate of production of this M1 derivative (o-demethylated tramadol) is determined by a polymorphic isoenzyme of debrisoquine type, cytochorome P450 2 D6 (CYP2D6) (6). Nevertheless, the affinity of the M1 derivative for μ-receptors of the CNS remains 6000 times lower than that of morphine (5, 6).
Interestingly, and in contrast to other opioids, the analgesic action of tramadol is only partially inhibited by the opioid antagonist naloxone. This suggests the existence of another mechanism of action by which tramadol exerts its analgesic effects. Support for this hypothesis was made through the characterization of tramadol’s monoaminergic activity. Specifically, it was found that tramadol inhibits nor-adrenaline (nor-epinephrine) and serotonin reuptake, making a significant contribution to tramadol’s analgesic action by blocking nociceptive impulses at the spinal level (2, 7).
Cytochrome p-450 plays an important role in the metabolism of tramadol. Genotyping has enabled us to classify subjects into phenotypic groups based on the rate of tramadol metabolism. The subjects were characterized as poor, intermediate, extensive, or ultra-rapid metabolizers – the more rapid the metabolism, the higher the M1 metabolite concentration. This metabolite has serotonergic activity and can induce a serotonin syndrome. A feature of this syndrome is the onset of seizures. Furthermore, the concomitant consumption of selective serotonin reuptake inhibitors and tricyclic antidepressants may increase the probabilitly of seizure occurrence (7). The different patterns of metabolism can explain why seizures may occur in subjects consuming tramadol within the therapeutic range (6).
Several studies had been conducted around the world and also attempted to evaluate tramadol induced seizures. In one study conducted between 2003 and 2004 in Australia, 97 patients with established tramadol induced seizures were assessed. Seizures occurred in the first 24-48 hours in subjects taking 500-750 mg/day of tramadol and between 2-365 days after tramadol intake in subjects taking tramadol within the therapeutic range (50-300mg/day) (8). However, in another study conducted in Serbia and Montenegro, tramadol induced seizures occurred in 84% of patients during the first 24 hours (9). In our study, 89% of subjects experienced a seizure attack within the first 24 hours after taking tramadol. This difference may be attributed to differences in tramadol metabolism among the Iranian population.
In one study, it was observed that all tramadol induced tonic-clonic seizures occurred within 12 hours after tramadol intake and 4.7% of subjects had history of febrile seizures in childhood (10). In our study, no subject had past medical history of seizures.
In some studies, it was shown that the risk of tramadol induced seizure was low unless it was taken by the patients with history of epilepsy (4, 11). In our study, it was seen that seizures occurred both at therapeutic and supratherapeutic ranges without history of epilepsy. Male predominance was seen in our study and in some other studies (9-11). It might be due to more consumption of tramadol in male subjects. There were several limitations in our study. First, despite the 100 subjects who initially presented with tramadol induced seizures, only 28 subjects agreed to participate in our study and to be followed up after their hospitalization. With a larger subject pool, we will be able to evaluate more accurately tramadol induced seizures among the population of Iran. Second, tramadol is manufactured by many different companies and most subjects could not recall the manufacturer names of their specific tramadol medication. This information would allow us to assess any potential correlation between tramadol induced seizures and specific brands of the medication.
In conclusion, the neurotoxicity of tramadol commonly manifests as generalized tonic clonic seizures most frequently within 24 hours after tramadol intake and was more common in subjects concomitantly consuming alcohol, illicit drugs, anti-psychotics, or antidepressants. Tramadol should not be administered to patients receiving SSRIs, TCAs and antipsychotics. In all patients with a first seizure, especially in young adults, the history of tramadol abuse should be taken upon. It is advisable that tramadol should not be used in epileptic patients.
Acknowledgments
We thank Dr. Sorena Nazarbaghi and Dr. Manouchehr Motamedian for their cooperation in following up the patients.
Funding: Self funded
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Raffa RB, Friderichs E, Reimann W, et al. Opioid and non-opioid components independently contribute to the mechanism of action of tramadol, an “atypical” opioid analgesic. J pharmacol Exp Ther. 1992;260:275–5. [PubMed] [Google Scholar]
- 2.Dayer P, Collart L, Desmeules J. The pharmacology of tramadol. Drugs. 1994;47:3–7. doi: 10.2165/00003495-199400471-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bamigbade T, Langford R. The clinical use of tramadol hydrochloride. Pain Rev. 1998;5:155–2. [PubMed] [Google Scholar]
- 4.Jick H, Derby LE, Vasilakis C, Fife D. The risk of seizure associated with tramadol. Pharmacotherapy. 1998;18:607–11. [PubMed] [Google Scholar]
- 5.Grond S, Sablotzki A. Clinical Pharmacology of Tramadol. Clin Pharmacokinet. 2004;43:879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Quetglas E, Azana JR, Sadaba B, et al. Pharmakokinetics of tramadol enantiomers and the respective phase 1 metabolites in relation CYP2D6 phenotype. Pharmacol Res. 2007;55:122–13. doi: 10.1016/j.phrs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Mason BJ, Blackburn KH. Possible serotonin syndrome associated with tramadol and sertraline co-administration. Ann Pharmacother. 1997;31:175–7. doi: 10.1177/106002809703100208. [DOI] [PubMed] [Google Scholar]
- 8.Boyd IW. Tramadol and seizures. Med J Aust. 2005;182:595–6. doi: 10.5694/j.1326-5377.2005.tb06825.x. [DOI] [PubMed] [Google Scholar]
- 9.Javanovic-cupic V, Martinovic Z, Nesic N. Seizures associated with intoxication and abuse of tramadol. J Clin Toxicol ( phila) 2006;44:143–6. doi: 10.1080/1556365050014418. [DOI] [PubMed] [Google Scholar]
- 10.Petramfar P, Borhani H. Tramadol induced seizure: Report of 106 patients. Iran Red Crescent Med J. 2010;12:49–51. [Google Scholar]
- 11.Gasse C, Derby L, Vasilakis –scaramozzaC, Jick H. Incidence of first time idiopathic seizures in users of tramadol. Pharmacotherapy. 2000;20:629–34. doi: 10.1592/phco.20.7.629.35174. [DOI] [PubMed] [Google Scholar]