Abstract
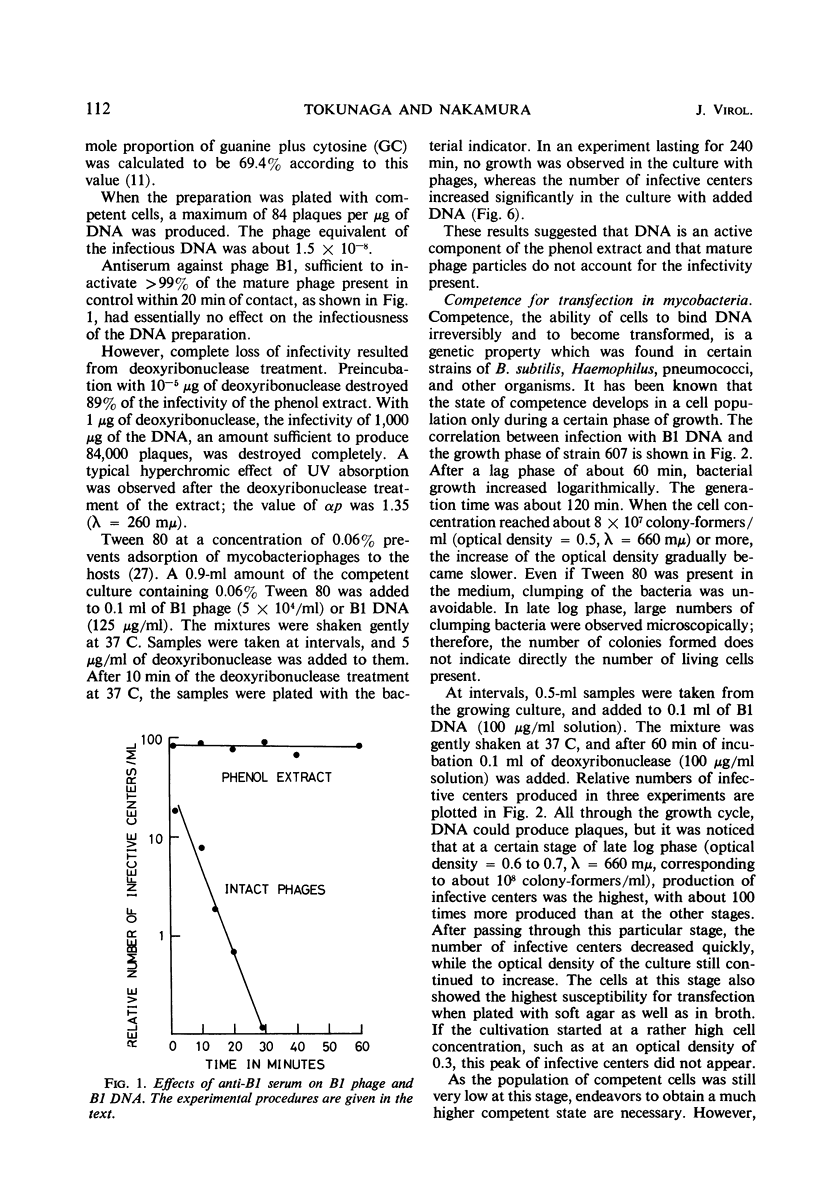
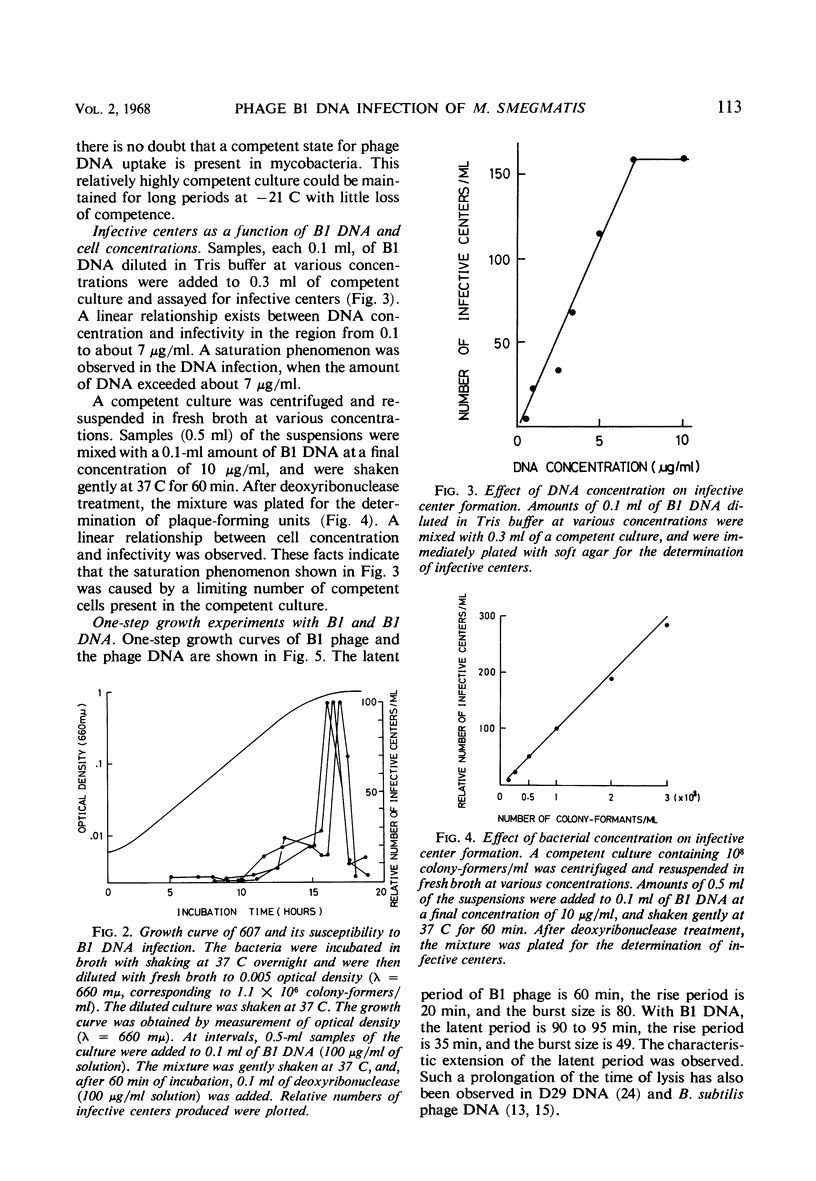
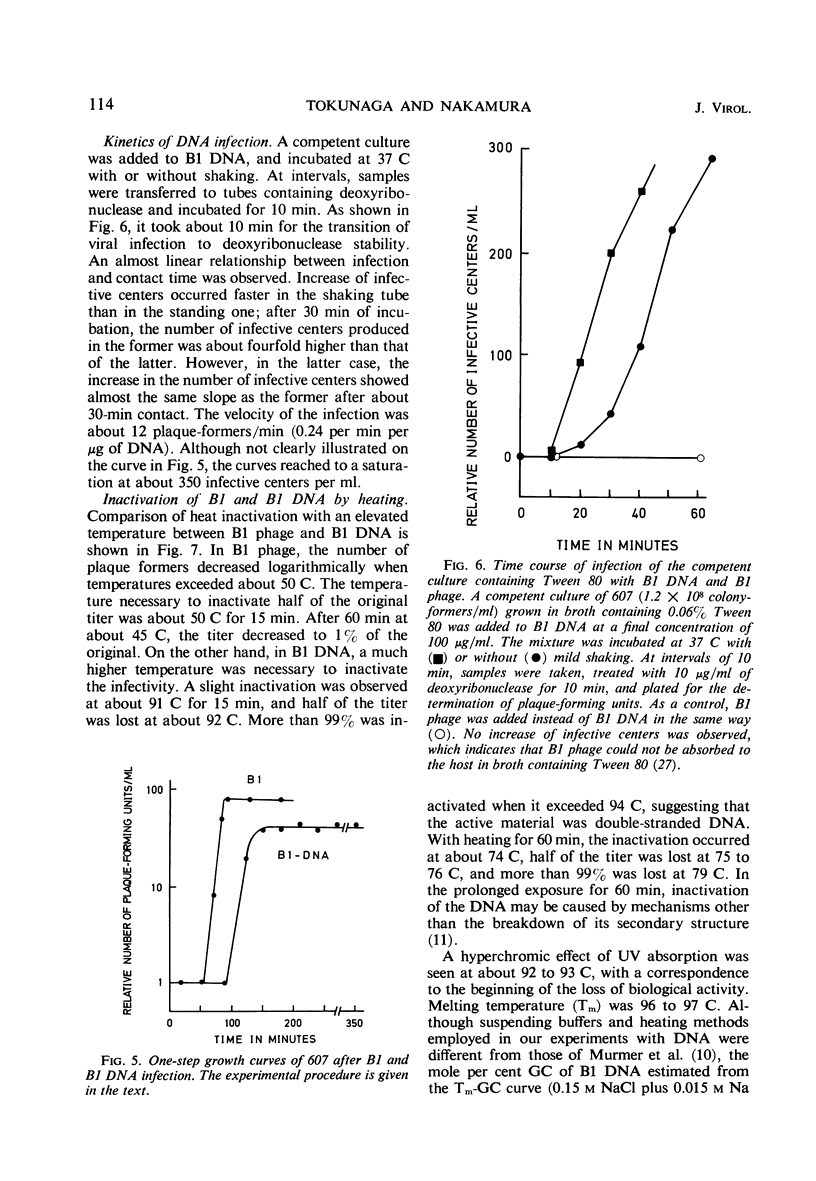
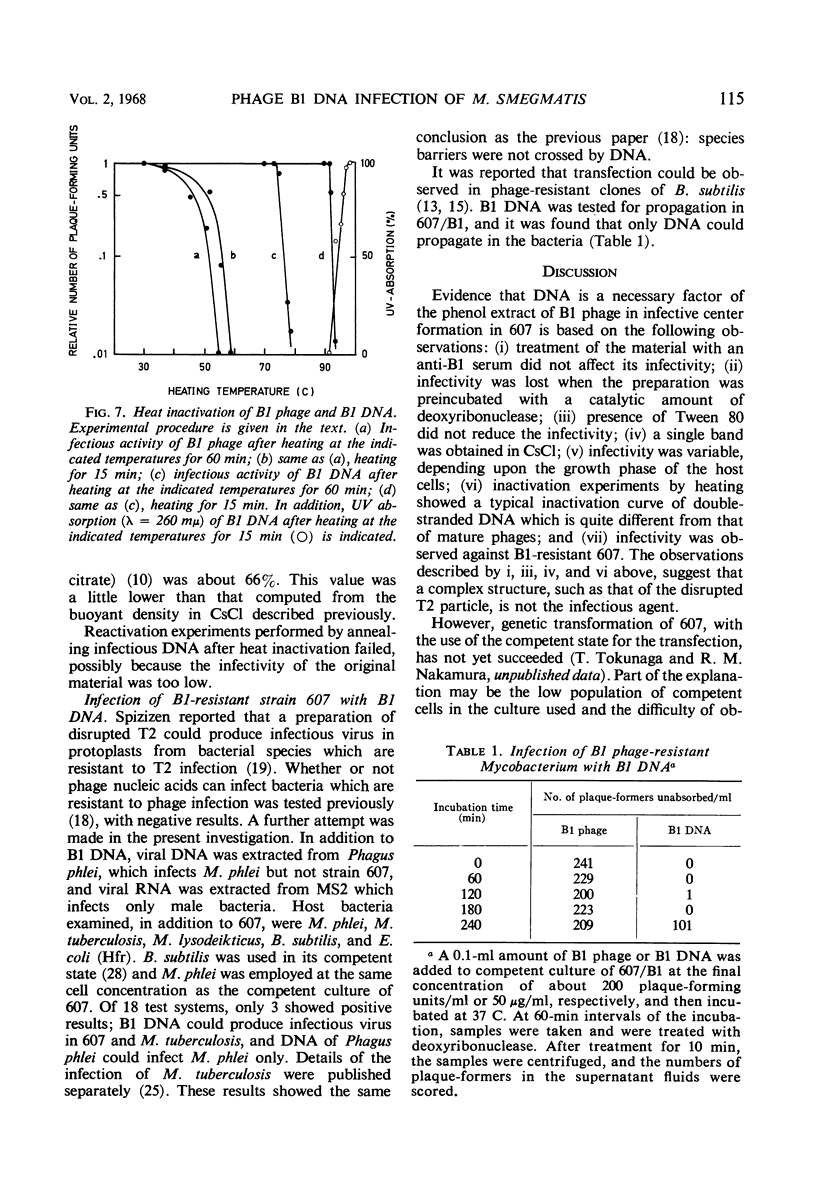
A relatively competent state of Mycobacterium smegmatis for infection with deoxyribonucleic acid (DNA) extracted from phage B1 was found in the late log phase of bacterial growth. This state of the culture was used in quantitative studies on the infectivity of the DNA. The buoyant density of B1 DNA was 1.728 g/cc in CsCl, and 1 μg of the DNA produced 84 infective centers, the phage equivalent of which was 1.5 × 10−8. The infectivity was destroyed by catalytic amounts of deoxyribonuclease but not by specific B1 antiserum. Tween 80, which prevents phage adsorption, did not prevent DNA infection. The response of plaque-forming ability to DNA concentration suggested that two or more molecules are required to initiate an infective center. The low efficiency of DNA infection in mycobacteria was considered to be caused by a limiting population of competent cells in the culture employed; in this experiment less than 10−5 of the cells were infected with DNA. A typical cycle of infection was observed, although the latent period was prolonged and the burst size reduced after DNA infection. The transition of B1 DNA infection to deoxyribonuclease insensitivity had a lag period of about 10 min, and increased linearly with a velocity of about 0.24 infective centers per min per μg of DNA. Half of the infective titer was inactivated by heating at 92 C for 15 min. The melting temperature was about 96 C. Species barriers were not crossed by B1 DNA; however, the DNA was infectious for a B1-resistant mutant of the host.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL P., TRAUTNER T. A. FORMATION OF AN ANIMAL VIRUS WITHIN A BACTERIUM. Z Vererbungsl. 1964 Apr 10;95:66–72. doi: 10.1007/BF00898185. [DOI] [PubMed] [Google Scholar]
- ALEXANDER H. E., LEIDY G., HAHN E. Studies on the nature of hemophilus influenzae cells susceptible to heritable changes by desoxyribonucleic acids. J Exp Med. 1954 Jun 1;99(6):505–533. doi: 10.1084/jem.99.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYREUTHER K. E., ROMIG W. R. POLYOMA VIRUS: PRODUCTION IN BACILLUS SUBTILIS. Science. 1964 Nov 6;146(3645):778–779. doi: 10.1126/science.146.3645.778. [DOI] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. The production of phage chromosome fragments and their capacity for genetic transfer. J Mol Biol. 1962 Apr;4:275–287. doi: 10.1016/s0022-2836(62)80005-9. [DOI] [PubMed] [Google Scholar]
- LIE S. STUDIES ON THE PHENOTYPIC EXPRESSION OF COMPETENCE IN NEISSERIA MENINGITIDIS. Acta Pathol Microbiol Scand. 1965;64:119–129. doi: 10.1111/apm.1965.64.1.119. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Perry D., Slade H. D. Effect of filtrates from transformable and nontransformable streptococci on the transformation of streptococci. J Bacteriol. 1966 Jun;91(6):2216–2222. doi: 10.1128/jb.91.6.2216-2222.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMIG W. R. Infection of Bacillus subtilis with phenol-extracted bacteriophages. Virology. 1962 Apr;16:452–459. doi: 10.1016/0042-6822(62)90226-x. [DOI] [PubMed] [Google Scholar]
- SELLERS M. I., BAXTER W. L., RUNNALS H. R. Growth characteristics of mycobacteriophages D28 and D29. Can J Microbiol. 1962 Jun;8:389–399. doi: 10.1139/m62-051. [DOI] [PubMed] [Google Scholar]
- SPIZIZEN J. Genetic activity of deoxyribonucleic acid in the reconstitution of biosynthetic pathways. Fed Proc. 1959 Dec;18:957–965. [PubMed] [Google Scholar]
- Sellers M. I., Tokunaga T. Further studies of infectious DNA extracted from mycobacteriophages. J Exp Med. 1966 Feb 1;123(2):327–340. doi: 10.1084/jem.123.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. INFECTION OF PROTOPLASTS BY DISRUPTED T2 VIRUS. Proc Natl Acad Sci U S A. 1957 Aug 15;43(8):694–701. doi: 10.1073/pnas.43.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., YOSHIMURA T., YAMAURA K., TODA T. Studies on the biologic properties of mycobacteriophage. Am Rev Respir Dis. 1959 Oct;80:543–553. doi: 10.1164/arrd.1959.80.4P1.543. [DOI] [PubMed] [Google Scholar]
- TOKUNAGA T., SELLERS M. INFECTION OF MYCOBACTERIUM SMEGMATIS WITH D29 PHAGE DNA. J Exp Med. 1964 Jan 1;119:139–149. doi: 10.1084/jem.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Thorne C. B. Concurrent changes in transducing efficiency and content of transforming deoxyribonucleic acid in Bacillus subtilis bacteriophage SP-10. J Bacteriol. 1966 Jan;91(1):81–88. doi: 10.1128/jb.91.1.81-88.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga T., Nakamura R. M. Infection of Mycobacterium tuberculosis with deoxyribonucleic acid extracted from mycobacteriophage B1. J Virol. 1967 Apr;1(2):448–449. doi: 10.1128/jvi.1.2.448-449.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga T., Nakamura R. [Infectiosity of Mycobacteriophage DNA. 2. Competence of Mycobacteria]. Igaku To Seibutsugaku. 1966 Jan 1;72(1):51–55. [PubMed] [Google Scholar]
- Tomasz A. Model for the mechanism controlling the expression of competent state in Pneumococcus cultures. J Bacteriol. 1966 Mar;91(3):1050–1061. doi: 10.1128/jb.91.3.1050-1061.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE A., KNIGHT V. Effect of tween 80 and serum on the interaction of mycobacteriophage D-29 with certain mycobacterial species. Am Rev Tuberc. 1958 Jan;77(1):134–145. doi: 10.1164/artpd.1958.77.1.134. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J Bacteriol. 1961 May;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]



