Abstract
PURPOSE
Delirium affects 50–80% of patients in intensive care units and is associated with long-term cognitive impairment and increased risk of mortality. There are a paucity of data reporting the neuropathologic findings in ICU patients experiencing delirium. The purpose of this pilot, hypothesis-generating study was to evaluate brain autopsies in ICU patients who suffered from delirium in order to explore possible neuroanatomic correlates.
METHODS
Utilizing study databases at Vanderbilt University we retrospectively identified patients who suffered from delirium in the ICU and subsequently died and received a brain autopsy during the same hospitalization. Information was also gathered regarding exposure to sedation and analgesia during ICU stay, number of delirium days, medical conditions surrounding death, and time with hypoxia and hypotension.
RESULTS
Patients’ mean age was 55 (SD±8.4), median number of days spent with delirium was 7 (±5 IQR). In 6 of 7 (86%) patients, pathologic lesions normally attributed to hypoxia or ischemia were noted in the hippocampus, pons, and striatum. Hippocampal lesions represented the most common neuropathological site of injury, present in 5 of 7 (71%) patients.
CONCLUSIONS
Hypoxic ischemic injury in multiple locations of the brain was a common finding in this pilot autopsy study of patients who had demonstrated delirium in the ICU. In particular, study of the hippocampus seems especially warranted in patients experiencing ICU delirium. Further correlation between clinical diagnoses, risk factors for neurological injury, neuroimaging, and neuropathology will help elucidate the mechanisms underlying acute brain dysfunction in our patients and long-term cognitive implications upon survival.
Keywords: Delirium, Brain Autopsy, Neuropathology, Intensive Care, Hippocampus
Introduction
Delirium is defined as an acute change or fluctuation in mental status combined with inattention and either an altered level of consciousness or disorganized thinking1. This is a very prevalent condition with 50–80% of patients in medical, surgical or trauma intensive care units affected1–3. Delirium in ICU patients is associated with longer hospital stays3, new cognitive impairment at discharge4, and an increased risk of mortality5. Given our current knowledge and the emerging importance of this problem, work has begun to address possible interventions to prevent delirium in ICU patients6–8. Despite well-documented accounts of delirium as a problem for our ICU patients and possible strategies to prevent acute brain dysfunction, the neuropathological findings of ICU patients who suffered from delirium have only recently begun receiving attention and very little is currently known.
The purpose of the present exploratory pilot study was to retrospectively examine the brain autopsies of deceased ICU patients in whom we had prospectively measured daily delirium and drug exposure data in order to determine if any common neuropathological lesions were noted in these deceased critically ill patients.
Methods
From 2002 through 2008, a total of 379 patients admitted to a Vanderbilt University Medical Center medical, surgical or trauma ICU suffering from delirium have been enrolled in the MENDS trial5, MIND study and ongoing BRAIN-ICU study. Data were gathered prospectively on these patients in regard to duration of delirium, sedative and analgesic exposure, and clinical outcomes. The diagnosis of delirium was made in these patients using the Confusion Assessment Method for the ICU (CAM-ICU)1,9 and serial assessments were made to determine the duration of delirium. We examined these databases, managed by the ICU Delirium and Cognitive Impairment Study Group (www.ICUdelirium.org), to determine which of these patients previously suffering from delirium had died, the duration of their delirium, and analgesic or sedative medications administered. Of these deceased patients, medical records were obtained from Vanderbilt University Medical Center to determine which of these patients had undergone a routine autopsy of their brain by a Vanderbilt neuropathologist. Records were also examined to determine cause of death, number of days the patient experienced hypotension, the presence of severe sepsis, and hypoxemia. Abnormal gross and histologic autopsy findings were extracted from the medical records and are included in this report. A Vanderbilt neuropathologist(TWA) also retrospectively re-examined H&E-stained slides of these brains to confirm and add to the findings in the initial autopsy reports.
Results
Of the 379 patients available from within the databases, 34% have since died and 7 (2%) had an autopsy of their brain by a Vanderbilt University neuropathologist (Table 1). These 7 patients were identified in the study databases and information regarding the number of days each suffered with delirium, sedatives and/or analgesics received, cause of death, and number of days with hypotension and/or hypoxia are presented in Table 2. All patients included in this study were delirious at the time of death and underwent an autopsy within 24 hours of their delirium episode and death.
Table 1.
Available Pool of Patients from the MENDS, MIND, and BRAIN-ICU studies
| Total # of patients | Alive patients | Deceased patients | Deceased with autopsy | Deceased with autopsy of brain | |
|---|---|---|---|---|---|
| MENDS | 90 | 46(51%) | 44(49%) | 6(6.6%) | 2(2%) |
| BRAIN-ICU | 234 | 161(69%) | 73(31%) | 9(4%) | 4(2%) |
| MIND | 55 | 45(82%) | 10(18%) | 2(4%) | 1(2%) |
|
| |||||
| TOTAL | 379 | 252(66%) | 127(34%) | 17(5%) | 7(2%) |
Table 2.
| # of days with delirium | Sedatives and/or analgesics received | Concurrent conditions associated with episode of delirium* | # of days with hypotension/hypoxia† | Abnormal brain autopsy findings by routine autopsy and retrospective review by neuropathologist | |
|---|---|---|---|---|---|
| 55y/o M | 5 | Fentanyl |
|
0/1 |
Gross Examination: The brain weighs 1460 grams. There is a 0.3 cm
lacuna
in the left striatum. Histologic Examination: Scattered corpora amylacea are present, consistent with age-related changes. |
| 44y/o M | 8 | Fentanyl |
|
14/1 |
Gross Examination: Brain weight 1230grams. Moderate, non-occlusive
atherosclerosis
of the circle of Willis was seen grossly. Histologic Examination: Multifocal parenchymal blood vessel mineralization (primarily putamen) and parenchymal deposits of uncertain clinical significance was appreciated microscopically. Also mild perivascular hemosiderin deposition. Probable acute hypoxic ischemic injury to CA1 of the hippocampus, but not classic “dead and red.” Vague hypercellularity and single microglial nodule, medulla. Congested blood vessels and mild, focal perivascular extravasation of RBCs. |
| 52 y/o F | 4 | Fentanyl |
|
7/1 | Gross Examination: Brain weight 1210 grams. Mild (focal), acute hypoxic ischemic injury to the hippocampus. Mild arteriolosclerosis throughout. |
| 63 y/o F | 17 | Fentanyl Lorazepam Haloperidol |
|
0/2 |
Gross Examination: Brain weight 1090 grams. The cortical surface showed a well-circumscribed area of dull red discoloration involving the left frontal lobe. Serial sectioning of the brain revealed multiple small foci of red discoloration at the gray-white junction involving the right frontal, bilateral occipital, and cerebellar regions. There was also a small focus of discoloration at the level of the thalamus on the left side. Histologic Examination: The left frontal lobe section that corresponded to surface discoloration showed an area of hemorrhage beneath the pia mater with thrombi, infarct and aspergillus organisms. The areas of discoloration at the right frontal cortex and cerebellum showed Hartmannella amoeba organisms by PAS stain and necrosis with fungal elements. The lesion in the left occipital region, left thalamus, bilateral frontal lobe lesions, and cerebellar lesions also showed fungal elements. Near the necrotic fungal lesions were many superficial cortical blood vessels surrounded by prominent perivascular lymphocytic cuffing, including some cells with enlarged, atypical nuclei. CD-3 and CD-20 stains showed a mixed population of B and T cells, suggesting that this exuberant inflammation is likely reactive, but a B-cell neoplasm in the brain could not be fully excluded or ruled out based on immunohistochemical stains. Fairly extensive hemosiderin deposition around blood vessels in the striatum. |
| 69 y/o M | 7 | Fentanyl Haloperidol Lorazepam Midazolam |
|
3/2 |
Gross Examination: Brain weight 1270 grams, no abnormalities Histologic Examination: Sections of the hippocampus and neocortex demonstrate focal spongiosis and eosinophilic degeneration of neurons. Cerebellum with scattered perivascular lymphs and hemosiderin-laden macrophages. Medulla with scattered, vague, microglial nodules. Possible acute hypoxic ischemic neuronal injury, hippocampus. |
| 60 y/o F | 9 | Fentanyl Lorazepam Haloperidol |
|
5/2 |
Gross Examination: A Subdural hematoma is present. There is dural involvement by Chronic Lymphocytic Leukemia. Histologic Examination: Axonal swelling is observed in the pons and midbrain. The pons also contains intraparenchymal hemorrhages with macrophage infiltration (microinfarcts). The hippocampus and middle frontal gyrus, specifically CA1 and the neocortex, reveal eosinophilic degeneration of neurons and reactive astrocytosis. Petechial perivascular hemorrhages. Rare granulovacuolar degeneration, neurofibrillary tangle, hippocampus. Prominent perivascular spaces (edema). Bona fide acute hypoxic ischemic injury, hippocampus and cortex. This is mostly a global hypoxic ischemic injury picture vs more focal hypoxic ischemic injury secondary to watershed infarcts. |
| 46 y/o F | 1 | Haloperidol Lorazepam |
|
2/2 |
Gross Examination: Brain weight 1480 grams. Right occipital/parietal acute watershed infarct, consistent with prolonged hypotension/hypoxia.
Hippocampus
with focal acute terminal ischemic change. Histologic Examination: Probable acute, hypoxic ischemic injury (?anterior horn, CA1, ?striatum). Petechial hemorrhage, thalamus and striatum. Prominent perivascular spaces in cortex (edema). Focal (small) SAH. Mild edema and pallor of white matter. Focal perivascular extravasation of RBCs, medulla. |
As listed on death certificate
Hypotension defined as mean arterial blood pressure average less than 65mmHg in a day or the requirement of vasoactive medications for blood pressure support Hypoxia defined as defined by an arterial oxygen saturation below 90% on a given day
The patients’ mean age was 55 (SD± 8.4), 4 were women, and the conditions associated with their episode of delirium cover a wide spectrum of illnesses, including severe sepsis as the primary cause of death in 6 of the 7 patients(Table 2). These patients spent a median of 7 (± 5 IQR) days with delirium and all received some type of sedative and/or analgesic while in the ICU. The average number of different sedatives and/or analgesics administered to each patient was 2.1. The most common medication used was fentanyl (86%) followed by lorazepam (57%), haloperidol (57%), and midazolam (14%). Of the patients, 57% had received more than one of these medications during their ICU stay. Cumulative dose information was not available for all patients (Table 2).
The most common abnormality found on routine autopsy of the brain from these patients who previously suffered with delirium is evidence of hypoxic ischemic injury in 6 of the 7 patients (86%) in various locations, including the hippocampus, pons, and striatum. Hippocampal lesions were found in 5 of 7 (71%) patients (Figure 1), representing the single most common neuropathological finding in these ICU patients who had suffered from delirium. These patients with hippocampal lesions spent a mean of 5.8 (SD ± 3.27) days with delirium, received 2.2 different sedatives or analgesics, and had varying degrees of hypoxic ischemic injury to the hippocampus. All 5 patients with hippocampal lesions also suffered from ARDS and septic shock with an average of 6.2 (SD ± 4.76) days with hypotension and 1.6 (SD ± 0.54) with hypoxia. Patients without hippocampal lesions spent a mean of 11 (SD ± 8.4) days with delirium, 0 days with hypotension, 1.5 (SD ± 0.7) days with hypoxia, and received 2 different sedatives.
Figure 1.
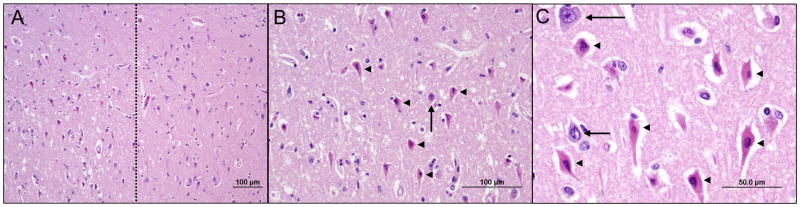
Various degrees of acute hippocampal hypoxic ischemic injury were seen at autopsy in the majority of patients with delirium. In severe cases, irreversibly injured pyramidal neurons (left side of dotted line in panel A) were often sharply demarcated from relatively healthy neurons (right side of dotted line in panel A) at the CA2-CA1 transition. At higher magnification, the injured neurons are characterized by condensed nuclei and brightly eosinophilic cytoplasm (arrowheads in B and C). Arrows indicate morphologically normal, viable neurons.
Discussion
The results of this pilot study, along with past research10–13, support the hypothesis that impaired cerebral blood flow, cerebral hypoxia, and cerebrovascular disease may be involved in the development of delirium in ICU patients. Of the brain autopsies we analyzed, 86% showed evidence of hypoperfusion and vascular changes consistent with cerebrovascular disease. Given the results of past functional studies, these findings were expected and may represent a contributing factor to the development of delirium. SPECT studies suggest that selective hypoperfusion of certain neuroanatomic regions may be a more specific explanation for the development of delirium in these patients and our findings offer support to this theory11–13.
What has not been reported in past imaging and functional studies with similar patients that we have shown here is the possible relationship between acute hypoxic ischemic injury to the hippocampus and delirium in ICU patients. Hippocampal lesions were the only specific neuroanatomic structures that were damaged in the majority of the autopsied brains. Given no evidence was seen on autopsy, the mean age of our patients was 55, and the studies we drew our population from excluded patients with advanced dementia, we can with confidence eliminate advanced Alzheimer’s disease as a confounding factor in the relationship between hippocampal lesions and delirium. As the primary function of the hippocampus relates to forming long-term memories, abnormalities here may provide a neuroanatomic explanation for the long-term cognitive impairment that is twice as common in this population compared to those without delirium5.
These patients had a number of reasons, including ARDS in all 7 patients and septic shock in 6 of the 7, to have suffered hypoxic injury to the hippocampus and other areas of the brain. Given our small sample size it is impossible to determine from our analysis the significance, if any, of these hippocampal lesions as they are known to occur even in the absence of delirium. Sharshar, Annane, and others have described with brain autopsies and imaging studies the neuropathology found in patients suffering from sepsis and septic shock. Although their focus was on patients with sepsis, their findings were generally similar to ours14–16. Although septic shock, ARDS, and ICU delirium are independent markers for severity of illness, it is plausible that ARDS and septic shock induce brain pathology, specifically hippocampal lesions, that leaves the patient with long-term cognitive impairment if they survive.
This method of examining brain autopsies is accompanied by a number of potential problems in determining the risks of developing delirium and possible neuroanatomic consequences of delirium. First, autopsies used in a future study would not always be performed immediately or even soon after a patient suffered from delirium as not all patients would die in the ICU as our patients have. A significant number of patients would survive many years after their episode, making analysis of their brain autopsies fraught with confounding factors. Also, in the effort to elucidate neuroanatomic factors that predispose patients to delirium, imaging and functional studies are the only methods of analysis available to us before the patient actually experiences delirium. Consequently, analysis of brain autopsies retrospectively or prospectively will not be able to differentiate between lesions present prior to delirium acting as a predisposition, or those as a result of the concomitant conditions accompanying delirium. This being said, the post hoc examination of brains from patients who have experienced delirium in an ICU has the potential to expand the understanding the pathophysiology of this problem and will enable us to direct pharmacologic therapy to act on specific neural pathways.
The analysis of routine brain autopsies performed by a Vanderbilt neuropathologist has reinforced the results of past studies examining the neuroanatomic correlates of delirium and has also revealed a possible basis of long-term cognitive impairment in these patients who have developed hippocampal lesions. Future studies should be performed prospectively with larger sample sizes and include a protocolized method to examine specific areas of interest, including the hippocampus and medial temporal lobe. Other areas of interest would include the posterior parietal cortex, prefrontal cortex, brainstem, and their interconnections. The connections and excitatory signals transmitted from the ascending reticular activating system in the brainstem and these other areas mediate arousal and abnormalities found on histologic examination of these areas may shed light on the pathophysiology of delirium in ICU patients17. Special stains and neuronal density in these areas will be of specific interest.
Acknowledgments
No financial funds were needed in preparing this manuscript.
References
- 1.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 2.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. The Journal of Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Critical Care. 2005;9:R375–381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychology Review. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;29:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 6.Pandharipande P, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 7.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomized controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 8.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Naughton BJ, Moran M, Ghaly Y, et al. Computed tomography scanning and delirium in elder patients. Academic Emergency Medicine. 1997;4:1107–1110. doi: 10.1111/j.1553-2712.1997.tb03690.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitabayashi Y, Narumoto J, Shibata K, et al. Neuropsychiatric background of alcohol hallucinosis: a SPECT study. Journal of Neuropsychiatry and Clinical Neuroscience. 2007;19:85. doi: 10.1176/jnp.2007.19.1.85. [DOI] [PubMed] [Google Scholar]
- 12.Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry and Clinical Neurosciences. 2003;57:337–339. doi: 10.1046/j.1440-1819.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 13.Fong TG, Bogardus ST, Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. Journal of Gerontology. 2006;61:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 14.Sharshar T, Annane D, de la Grandmaison GL, et al. The neuropathology of septic shock. Brain Pathology. 2004;14:21–33. doi: 10.1111/j.1750-3639.2004.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharshar T, Hopkinson NS, Orlikowski D, Annane D. Science review: the brain in sepsis – culprit and victim. Critical Care. 2005;9:37–44. doi: 10.1186/cc2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharshar T, Carlier R, Bernard F, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Medicine. 2007;33:798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- 17.Gunther ML, Jackson JC, Ely EW. Loss of IQ in the ICU: brain injury without the insult. Medical Hypotheses. 2007;69:1179–1182. doi: 10.1016/j.mehy.2007.03.039. [DOI] [PubMed] [Google Scholar]


