Abstract
A number of anti-cancer drugs have their targets localized to particular intracellular compartments. These drugs reach the targets mainly through diffusion, dependent on biophysical and biochemical forces that allow cell penetration. This means that both cancer cells and normal cells will be subjected to such diffusion; hence many of these drugs, like chemotherapeutics, are potentially toxic and the concentration achieved at the site of their action is often suboptimal. The same relates to radiation that indiscriminately affects normal and diseased cells. However, nature-designed systems enable compounds present in the extracellular environment to end up inside the cell and even travel to more specific intracellular compartments. For example, viruses and bacterial toxins can more or less specifically recognize eukaryotic cells, enter these cells, and direct some protein portions to designated intracellular areas. These phenomena have led to creative thinking, such as employing viruses or bacterial toxins for cargo delivery to cells and, more specifically, to cancer cells. Proteins can be genetically engineered in order to not only mimic what viruses and bacterial toxins can do, but also to add new functions, extending or changing the intracellular routes. It is possible to make conjugates or, more preferably, single-chain proteins that recognize cancer cells and deliver cargo inside the cells, even to the desired subcellular compartment. These findings offer new opportunities to deliver drugs/labels only to cancer cells and only to their site of action within the cells. The development of such dual-specificity vectors for targeting cancer cells is an attractive and potentially safer and more efficacious way of delivering drugs. We provide examples of this approach for delivering brain cancer therapeutics, using a specific biomarker on glioblastoma tumor cells.
1. Introduction
Traditional cancer treatment can be classified into two main approaches. One approach is a specific recognition of cancer cells by means of plasma-membrane receptor-binding drugs, such as monoclonal antibodies or naturally occurring ligands against tumor-associated or tumor-specific receptors, and/or oncogenic receptors.[1] The other approach of either indiscriminate or more targeted chemotherapy involves cellular delivery of the drugs through diffusion. The specificity of such an approach can be assigned when a target is a unique factor or a mutated oncogenic protein, reachable by a drug that binds exclusively to this form of oncogene. In most cases, the plasma membrane must be permeable to these drugs and, in the case of brain tumors, they need to cross the blood-brain barrier.[2] Most anti-cancer therapeutics have defined targets such as oncogenes, enzymes, or DNA, all of which are localized to distinct intracellular compartments like cytosol, mitochondria or nuclei. Hence, vectors providing a direct delivery of therapeutics/labels to these subcellular compartments may lead to increased specificity and efficacy, with less toxicity.
We have developed ‘double specificity’ vectors to transport therapeutics not only to a subset of cells, but also into their specific intracellular compartments. These ‘double specificity’ vectors are designed to achieve specificity by recognition of the tumor-associated antigen IL-13Rα2, which is specifically overexpressed on cells of glioblastoma (GBM) tumors. After being internalized into the tumor cell through this receptor, intracellular organelle localization signals in these vectors will exert the next level of specificity by transporting therapeutics, including radioisotopes, into that particular intracellular compartment. Organelle-specific delivery of some of the therapeutics, such as in the nucleus, is expected to lead to an increased efficiency of glioma cell killing, as most drugs and radioisotopes have a small range of effectiveness or influence inside the cell. Specifically transporting such therapeutics into the nucleus, i.e. to the core of DNA synthesis machinery inside the cell, should lead to rapid and enhanced killing with decreased drug dosage and minimal harm to the normal cells. This strategy has potential to improve therapy of GBM and other cancers.
2. Treatment Options for Glioblastoma (GBM)
GBM is a high-grade astrocytoma representing the most common, and most treatment resistant, form of primary brain tumor. Primary brain tumors account for 2.4% of all yearly cancer-related deaths and are one of the top ten causes of death in the US. The treatment of patients with GBM is still a major challenge; the median survival rate for patients with GBM is usually in the range of 12–14 months and has improved only marginally over the past 30 years.[3] Currently, GBMs are treated primarily by surgical resection, followed by radiotherapy and chemotherapy.[4–6] It is apparent from the survival statistics that the current multimodality treatment approach is inadequate.
GBM tumors are composed of a heterogenous mixture of poorly differentiated neoplastic astrocytes that are located preferentially in the cerebral hemispheres. Surgical debulking is the mainstay treatment for such tumors, though tumor recurrence remains the leading cause of death in GBM patients. In more than 90% of cases, after surgical resection and adjuvant treatment, the residual glioma cells from the periphery of the tumor resection cavity give rise to recurrent tumor immediately adjacent to it or within 2 cm of the cavity.[7] Current therapies for GBM such as chemotherapy, radiation therapy, and monoclonal antibody therapeutics do not effectively eliminate residual tumor cells after surgical resection.[5,8] This is because the majority of these therapeutics are non-specific in nature, and they face delivery restrictions into the tumor because of their large sizes and complex chemical constitutions. In fact, most of these agents do not cross the compromised blood-brain barrier, leading to inefficient delivery of the drug to the tumor cells.[9] In addition, the non-targeted and non-specific nature of chemotherapeutics and radiation therapy leads to killing of normal brain cells, resulting in cognitive impairment in GBM patients.
To increase survival of GBM patients, there is an urgent need to develop alternative and effective therapies to eliminate GBM tumor cells. To effectively manage GBM, a combination therapy will have to be used, i.e. after surgical resection and irradiation, multiple targeted therapies will be able to kill even the residual GBM tumor cells and prevent the recurrence of the disease. An efficient therapeutic against GBM tumor would be one with enhanced specificity to GBM tumor cells, which is also efficiently delivered to the tumor cells and one with maximal drug efficacy. An effective approach to make such therapeutics is to take advantage of tumor-associated phenotypic changes, such as elevated cell surface receptors or antigens. The relative selectivity of cell surface markers on cancer cells makes them fascinating molecules for local delivery of some of the conventional cancer therapeutics. A more favorable therapeutic approach for GBM tumors would involve conjugating cancer chemotherapeutics, biological toxins, or radioactive isotopes to ligands for over-expressed brain tumor cell surface antigens (such as receptor ligands, monoclonal antibodies, peptides or growth factors) in hope of promoting specific recognition and localization in tumor cells.
One of our goals is to specifically recognize target biomarkers and effectively penetrate the GBM tumor cells. These intracellular compartment-specific targeted molecules may be valuable in the construction of various diagnostic/prognostic and therapeutic tools for GBM. These are modern, new age, targeted diagnostics and treatment modalities which will lead to successful killing of GBM tumor cells, protection of normal brain cells and tissues and hence efficient treatment and prognosis for brain tumor patients.
3. Intracellular Delivery Strategy
3.1 Employing Bacterial Toxins for Targeting
In the field of non-viral vectors based on recombinant proteins, we have pioneered the use of proteinaceous compounds for the targeted intracellular transport of proteins and nonproteinaceous agents.[10] The system exploits nature-designed bacterial toxins, such as Pseudomonas aeruginosa exotoxin A (PE) or diphtheria toxin (DT). These type A-B bacterial toxins first bind to a plasma membrane receptor, which in turn induces internalization of the ligand-receptor complex. Once in the endocytic compartment, proteolytic cleavage releases a portion of the toxin, which contains a signal for the exit from the endocytic compartment into the cytosol. This portion is a catalytic unit that shuts down new protein synthesis. This ‘get cleaved and exit the endocytic compartment’ property is a result of the presence of a specialized domain of PE, domain II.[11,12] Initially, we exploited the ability of PE to translocate other, non-PE, or multiplied PE peptide sequences into the cell cytosol.[10] We demonstrated for the first time that PE can serve as a vector for intra-cytosolic delivery of various proteins/peptides. This approach has served as a basis for further developments of intracellular vaccines[13] and prompted the use of DT protein, having very similar properties to PE toxin, for the same purpose of intra-cytosolic delivery.[14]
PE and DT have similar complex multi-domain structures that reflect the multiple-step killing pathways of eukaryotic cells.[15,16] Both possess receptor binding domains. In PE, the receptor binding domain is the N-terminal domain la (amino acids 1–252)[15] and in DT it is the C-terminal domain.[17] PE- or DT-receptor complexes are internalized and are first sequestered to the endocytic vesicles. Domain II of PE (amino acids 253–364) is a site of proteolytic cleavage by furin, which is necessary for the toxin’s intra-endosomal activation,[11] and the sequences in this domain are important for the toxin’s translocation into the cytosol.[12,18–22] Domain Ib (amino acids 365–404) has an unidentified function and is dispensable.[10] The last domain of PE (domain III; amino acids 405–613) is an enzyme that catalyzes ADP-ribosylation of elongation factor-2 (EF-2), followed by arrest of protein synthesis, and subsequent cell death.[15,23] Prior to translocation, PE must be cleaved by furin at its disulfide bond-formed loop, next to Arg279, in order to produce a 37-kDa C-terminal fragment comprising all of domain lb and III, and a major portion of domain II that reaches the cytosol.[19] The C-terminal end of PE, REDLK (amino acids 609–613), is absolutely necessary for the toxin to be active. This sequence resembles the endoplasmic reticulum (ER) retention signal, KDEL.[24] A recombinant form of PE, PE40, lacks the receptor binding domain Ia, but retains all the functions of domains II and III,[15] although it does not possess specific cytotoxic activity on its own. PE38 is a PE40 molecule with additional deletion of the portion of dispensable domain Ib.[25] These variants of PE can only be toxic to eukaryotic cells when another targeted ligand is built in.[26]
We have utilized the unique features of the PE toxin to target specific sub-cellular organelles of GBM cancer cells through the GBM-specific plasma membrane biomarker, interleukin-13 receptor alpha 2 (IL-13Rα2).
3.2 Targeting Interleukin-13 Receptor-α2 on GBM Cells
It has been demonstrated that IL-13Rα2, a tumor-associated plasma membrane receptor, is highly overexpressed in GBM.[27,31] This is one of the receptors for IL-13, an immune regulatory cytokine, mainly produced by T-helper type 2 (Th2) cells.[32] The other receptor for the IL-13 ligand is a heterodimeric receptor complex consisting of IL-13Rα1 and IL-4Rα components. IL-13 ligand binds with a low affinity to the IL-13Rαl receptor, but, in the presence of IL-4Rα protein, it forms a high affinity receptor complex for the IL-13 ligand and is thought to signal through the signal transduction and activator of transcription (STAT)-6 signaling pathway.[32] It is thought that the IL-13Rα2 receptor acts as a decoy receptor for the IL-13 ligand, and therefore as negative regulator of IL-13, as it does not mediate any downstream signal transduction.[33]
Our laboratory has designed various IL-13 ligand mutants which specifically bind to IL-13Rα2, the cancer-associated receptor, and which do not bind to the other physiological receptor for IL-13 that is also shared with homologous cytokine, IL-4[34–36] IL-13.E13K is one such IL-13 mutant that has an amino acid glutamate residue at position 13 substituted for lysine.[34] IL-13 binds to the IL-13Rα2 receptor followed by internalization through receptor-mediated endocytosis.[37] Hence, drugs attached to the IL-13 ligand or its mutant forms can be internalized and delivered specifically inside GBM cells. One early example of such a construct is the first generation of IL-13-based cytoxin, IL-13-PE38QQR, which was also evaluated clinically.[37]
3.3 Intracytosolic Bio-Engineered Delivery Vectors
An interesting question is whether something else can replace native PE sequences and be trafficked inside the cell. It was envisioned that PE fragments could be used to target intracellular compartments of GBM cells using IL-13Rα2 as an entry site and deliver various polypeptides into the cytosol. A proof-of-principle study documented the possibility of replacing the dispensable portion of PE40, domain Ib, with hormonal polypeptides in a chimeric cytotoxin composed of transforming growth factor α (TGFα) and PE40, which uses the epidermal growth factor receptor (EGFR) as a site of entry into malignant cells.[10] The N-terminal portion of domain Ib has been successfully replaced with two somatostatin peptides and a very hydrophobic methionine-rich peptide. Replacement of domain Ib with such peptides did not hamper activity of the chimeric cytotoxin.[10] Also, PE40 has been significantly enlarged by another domain III engineered into domain Ib region, downstream of the processing site at Arg279. This maneuver made the toxin cleavable at one site as in the wild type of PE, however, the cleaved fragment was expected to be of ~62 kDa compared with 37 kDa normally produced from PE.[10,19] Interestingly, the activity of the chimeric toxins with two domain IIIs in tandem was similar to that of the original chimera. These studies inferred an efficient transport of a considerably larger proteinaceous entity or even non-PE polypeptides through intracellular membrane(s).
3.4 Fusion Proteins Designed with Intracellular Localization Signals
In order to accomplish a higher multi-specificity order of targeting, one can envision the development of fusion proteins consisting of targeting ligands like IL-13 mutants, together with proteins/peptides that possess the desired intracellular localization propensities:
cytosolic localization proteins (to deliver, or self-deliver, bacterial toxins – already advanced in the development; oncogene inhibitors; pro-apoptotic proteins; and antisense nucleotides),
lysosomal localization signal peptide (to deliver labels; chemotherapeutics), or
nuclear localization signal peptide (to deliver auger-electron emitting isotopes, α-emitting labels, photosensitizers).
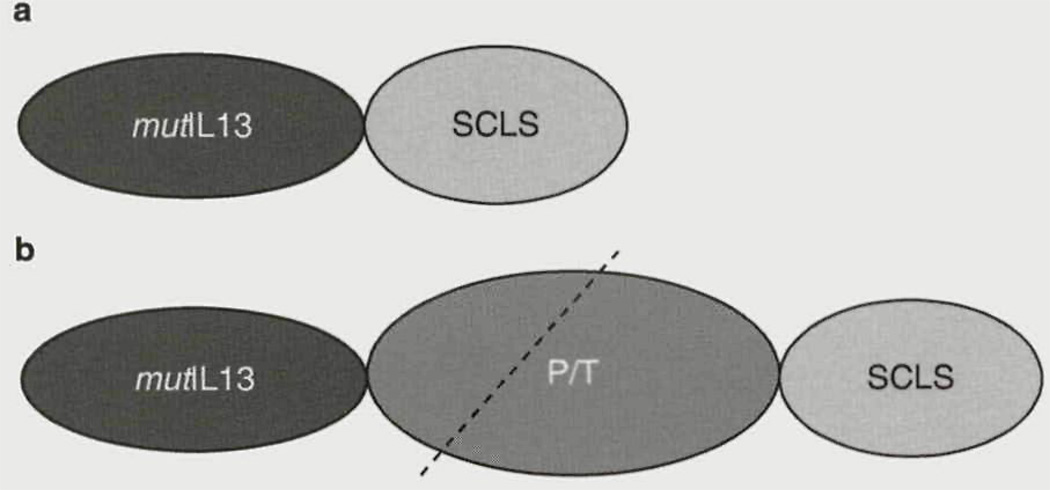
The subcellular compartment (nuclei, endosomes, lysosomes, mitochondria, etc.) recognition polypeptides are relatively short-length peptides and they can potentially all be incorporated into the sequences of the doubly targeted recombinants [figure 1].
Fig. 1.
Cancer-associated receptor-targeted fusion proteins for controlled intracellular routing in malignant cells, (a) An interluekin-13 ligand mutant is fused to a sub-cellular compartment localization signal polypeptide. For this construct to work, it needs to be released from the endocytic compartment after receptor-mediated endocytosis, which is unlikely to take place, (b) Same as (a), but the processing/translocation domain of Pseudomonas aeruginosa exotoxin A is incorporated between the ligand and SCLS. This construct will be cleaved within the PAT domain (interrupted line) and the C-terminal portion of the cleaved protein will enter the cytosol. Once in the cytosol, the SCLS will redistribute the protein into the targeted compartment of the cell, mutIL13 = interleukin-13 ligand mutant; P/T=processing/translocation; SCLS = sub-cellular compartment localization signal polypeptide.
Intracellular localization signals help proteins traffic to various specific organelles/subcellular compartments. The nuclear localization signals (NLS) are employed by a large number of proteins that travel to and from the nucleus, and are important for cell function. NLS derived from the simian virus 40 (SV40) large T antigen is a well characterized sequence enabling nuclear transport.[38–42] The SV40 NLS is a classical monopartite NLS and comprises a characteristic stretch of basic, positively charged amino acids like arginines and lysines. The NLS binds to importins/karyopherins that transport NLS-containing proteins through the nuclear pore machinery.[40] Multiple NLS have been identified. NLS have already been used to distribute an agent from the extracellular space into the cell nucleus.[43] The optimized SV40 T antigen NLS is as follows: SSDDEATADAQHAAPPKKKRKVEDP. This sequence is recognized by a high affinity importin α/β hetero-dimer that catalyzes import into the nucleus. Many other NLS are known and available to choose from to exploit in the designer constructs.[44] There are also many identified types of lysosomal localization sequences (LLS). These include the lysosome-associated membrane protein 1 (LAMP-1) tail sequence RKRSHAGYQTI, the lysosomal acid phosphatase (LAP) sequence RLKRMQAQPPGYRHVADGEDHAV, and the lysosomal integral membrane protein 2 (LIMP-2) sequence RGQGSTDEGTADERAPLIRT. LAP has a propensity to accelerate the internalization of the molecule that contains the sequence.
These prototype fusion proteins are expected to closely follow the intracellular trafficking pathways of PE. The detached, proteolytic cleaved C-terminal end of PE, containing a specific endosomal exit sequence, would enable the fusion protein to travel to a chosen site in the cell.[26] In a simplified form, there is a general order in these designer proteins, which is dependent on the type of bacterial toxin used. For example, counting from N terminus to C terminus, they will be represented by the order as follows: A-B-C-D-E, A-B-C-E-D, A-B-E-D-C, E-D-C-B-A, A-B-C-D, and D-C-B-A, where A is a specific cancer cell-binding ligand; B* is a cytosol localization element; C is a subcellular compartment localization signal element; D is an effector molecule; and E is a second effector molecule (may be present or absent). B* must always be between A and C in either direction.
Alternative approaches are available for the construction of intracellularly deliverable proteins. The DT molecule contains the processing site and a defined sequence, which is responsible for the translocation of an active DT fragment into the cytosol. Thus, by using engineered fragments of DT, one can direct growth factor fusion protein into the cytosol.[14,45,46] Furthermore, some of the viral proteins serve as controlled distributors within the eukaryotic cells. Their structural features have been used to modulate the passage through some intracellular membranes.[47]
3.5 Targeting GBM Cell Nuclei
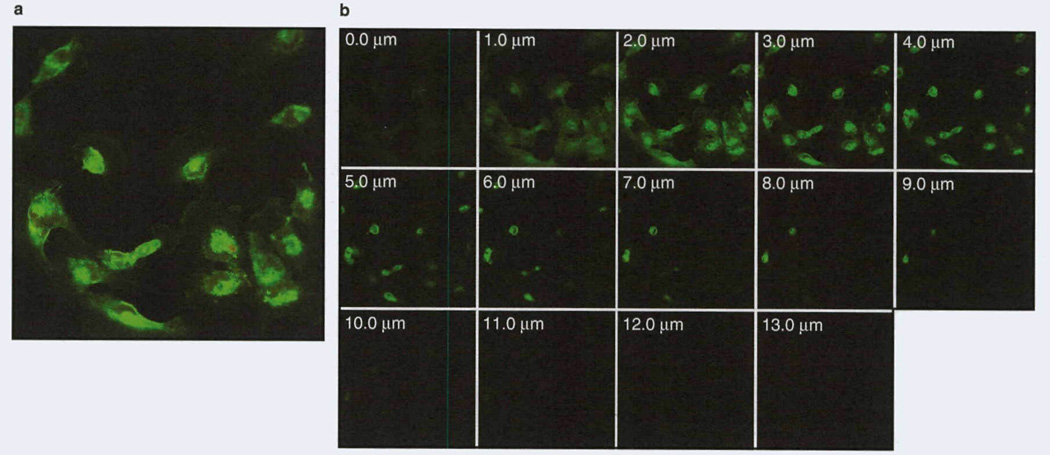
For targeted delivery to specific intracellular compartments, we designed a single-chain protein containing sequences for (i) receptor recognition, (ii) endocytic vesicles/departing, and (iii) nuclear transport ligands/signals. The single-chain protein, a vector for double-level targeting of cancer cells, consists of IL-13.E13K (for specific recognition of the IL-13Rα2) followed by domain II of PE (for endosomal cleavage and then exit), and NLS from the SV40 T antigen to guide the portion of PE remaining after furin cleavage (for nuclear transport). We have provided direct evidence that this molecularly targeted designer protein recognizes GBM cancer cells specifically, and travels to and accumulates in these cells’ nuclei[48] (figure 2). This was the first direct demonstration of delivery to a specific cell and a cell-specific cellular compartment, such as the nucleus. Thus, we have designed a universal module that binds cancer cells specifically and also travels specifically to the cells’ nuclei. We have shown directly that the universal module, a vector for intracellular delivery, binds to plasma membranes of GBM cells, enters the endocytic compartment, concentrates in the perinuclear region and then enters nuclei in a time-dependent manner’481 (figure 2). The vector accumulates in the nuclei for prolonged periods of time. The journey of a designer protein-based vector takes place from the cell surface to the nucleus of GBM cells as intended.[48]
Fig. 2.
Biotin-labeled IL-13.E13K-D2-NLS retains nuclear localization ability in glioblastoma multiforme cells, (a) Biotin-labeled IL-13.E13K-D2-NLS was added to U-251 MG (human neuronal glioblastoma) cells for 8 hours. The biotin signal was amplified by tyramide-alexa fluor 488 labeling, (b) The Z-stack confocal analysis of IL-13.E13K-D2-NLS nuclear accumulation in cells shown in (a).
The components of our fusion single-chain ‘double-specificity’ protein construct can be switched with other protein/peptide domains exhibiting similar functions as the original construct. We can shorten and use just the basic SV40 T antigen nuclear localization signal (i.e. PPKKKRKVEDP) or use other NLS sequences such as that of human nucleoplasmin protein.[42] These NLS sequences have been shown to efficiently transport proteins into the nucleus.[43] NLS sequence vectors have been used in transporting photosensitizers such as chlorin e6,[49] and radioisotopes such as the auger electron emitter indium-111 (111In) in acute myeloid leukemia (AML) cells[50] and techne-tium-99 (99mTc) in B16F1 mouse melanoma cells.[51]
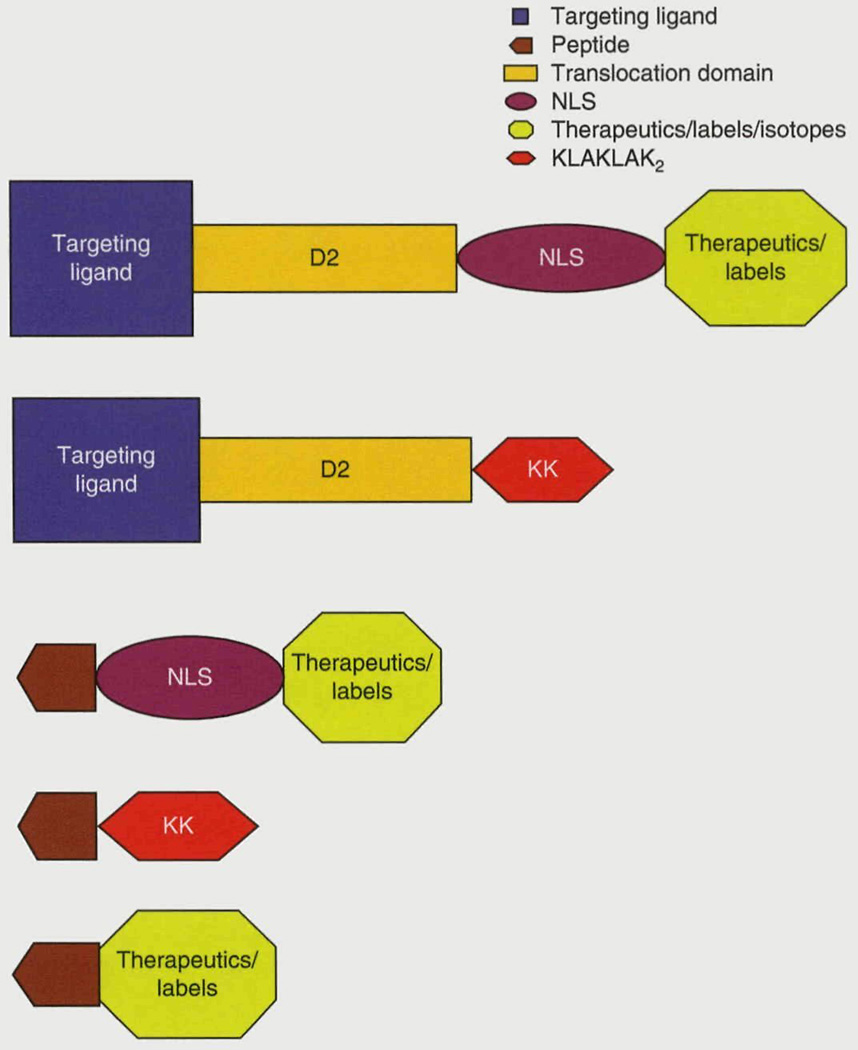
Our design strategy for the intracellular delivery vector is very versatile (figure 3). The IL-13 targeting ligand can be exchanged for any targeting ligand (including monoclonal antibodies, peptides, and natural receptor ligands) against tumor-associated cell surface receptors. In addition, the PE translocating domain can be exchanged for other endosomal translocating structures, such as a 30-amino-acid synthetic peptide with a glutamic acid-alanine-leucine-alanine repeat, termed GALA, and electroneutral lipids like l,2-dioleolyl-sn-glycero-3-phos-phoethanolamine (DOPE), as well as the translocation protein domain from DT. Moreover, localization signal sequences for many intracellular organelles such as lysosomes,[52] peroxisomes,[53] or mitochondria,[54] as well as other nuclear localization signals,[44] can replace the SV40 T-NLS for targeted drug delivery to one or more of the intracellular compartments of the cell.
Fig. 3.
Diagrammatic representation of some potential future therapeutics. Targeting ligands/peptides against brain tumor-associated receptors will be fused to a nuclear localization signal, pro-apoptotic (KLAKLAK)2 peptides, or various radioisotopes and labels. D2=translocation/processing domain of Pseudomonas aeruginosa exotoxin A; NLS=nuclear localization signal.
4. Therapeutic Potential of Recombinant Protein Therapeutics for Targeted Intracellular Compartment Delivery in GBM Cells
Some isotopes and therapeutics such as photosensitizers have a small radius of action (e.g. photosensitizers, radius of action ~0.01 µm to 0.02 µm due to the short half-life of the activated drug of ~0.4 µs compared with tumor cell size of −10 µm). One to two molecules of radioisotopes such as α-emitters are enough to kill a GBM cell, if they are targeted and left in the nucleus. One can envision developing a delivery vehicle with an intracellular organelle localization signal fused to the IL-13 ligand or peptides binding to the IL-13Rα2 receptor that allows passage of attached therapeutics such as α-emitters/ auger electrons into the nucleus, or delivery of a pro-apoptotic peptide, such as (KLAKLAK)2 [figure 3]. Prototype vehicles that specifically deliver therapeutics to GBM nuclei using the IL-13 ligand will be discussed in more detail in this section.
4.1 Development of Intracellularly Targeted α-Emitters in GBM Tumors
Conventionally, β-particle-emitting radioisotopes (e.g. iodine-131, yttrium-90 or rhenium-186) are been used in cancer radiotherapy. β-emitters have also been predominantly used for the purposes of radioimmunotherapy (RIT) trials, wherein these radionuclides are conjugated to monoclonal antibodies and peptides against various tumor-associated antigens.[55] Low linear energy transfer (LET) β-emitters have been efficacious for treatment of small solid tumors and hematologic malignancies with considerable success. However, β-emitters with mean ranges of 50–200 cell diameters in tissues would be favorable for treatment of large tumors, whereas it is believed that α-emitting radionuclides, with their inherent high LET and short path lengths (5–10 cell diameters), could be advantageously used against micrometastatic diseases such as lymphomas and leukemias, small tumor clusters, and resected tumor cell boundaries.[56] α-emitters have a high LET of around lOOKeV/µm and a short path length of 50–80 µm compared with β-particle emitters with a far lower LET of 0.2 KeV/µm and path length of 4–11 mm in tissues.[57] For an equal amount of radiotherapeutic fixed on cancer cells, the cytotoxicity of α -particles is 5–100 times that of β-particles. This high energy and small range can potentially kill all the tumor cells while sparing most of the normal cells, whereas β-particles cause damage to normal bystander cells because of their long path lengths. Several processes have been implicated as causes of radiation-mediated cell death, including double-stranded DNA breaks, apoptosis, and over expression of tumor protein p53 (TP53), leading to delays in the Gl phase of the cell cycle.[58,59] It has been shown that death due to α-particle emission occurs only when the α-particle traverses the cell nucleus, irrespective of high concentrations of α-particles directed at the cytoplasm.[60] Radiation generated by α-particles has maximal efficacy, as high LET of these particles causes maximum double-stranded breaks. Also, high LET results in more severe chromosomal damage and more complex chromosomal rearrangements than low LET radiations.[61] Directing these particles to the nucleus will not only result in immediate cell death but will also decrease the amount of isotope required to mediate this cell killing, as only 1–2 α-particles in the nucleus will be enough to cause cell death. This translates to a lower required dosage and decreased radiation-induced brain damage.
Such α-particle therapeutics can be successfully and specifically used to kill residual tumor cells left behind after surgical GBM tumor resection. This should lead to increased survival for GBM patients, as tumor recurrence is the main cause of the low survival rates prevalent in GBM. α-particles like astatine-211 (211At),[62] bismuth-213 (213Bi), and actinium-225 (225Ac),[63,64] are currently being developed for use in α-radionuclide-mediated RIT. α-Particles have been conjugated to monoclonal antibodies to make therapeutics like 211At-labeled MX35 F(ab′)2 for studies of ovarian cancer in nude mice,[65] 213Bi-labeled CO 17-1A Fab′ fragment in a colon cancer model,[66] 213Bi-labeled HuM195 (anti-CD33) for studies in patients with leukemia,[67] and 225Ac conjugates of Mab CC49 and ΔCH2CC49 in studies of human carcinomas.[68] 213Bi α-emitters have also been conjugated peptides like the somatostatin analog peptide, octreotide, for studies in a pancreatic tumor mouse model.[69] ‘Double-specificity’ α-particle-based therapeutics will not only recognize the IL-13Rα2 on GBM tumors, but will also get transported into the nucleus, and will act as ‘bombs’ which will specifically and efficiently kill the targeted tumor cells with decreased dosage, while causing minimal harm to normal bystander cells.
4.2 Delivery of Auger Electrons to the Nuclei of a Cell
Auger electron-emitting isotopes like Indium-1ll (111In) have a very short path length ranging from nanometers to micrometers (mostly <1 µm) in tissues. They also have high LET values ranging from 4 to26keV/µM.[70] For 111In, the dose of radiation absorbed to the nucleus is 2- to 35-fold higher when it decays in the nucleus than when 111In decays on the cell surface or in the cytoplasm,[71,72] indicating that 111In is very cytotoxic when delivered directly to the DNA of the cell. Moreover, when targeted to a particular tumor-associated antigen, it can be highly specific in destruction of cancer cells while sparing the surrounding normal cells. These short-range emitters can be advantageous when targeting individual cells, small clusters of tumor cells, or micrometastases. Efforts have been carried out to target HER2-positive human breast cancer with 111In-NLS-trastuzumab.[73,74] The indium-conjugated trastuzumab significantly slowed the growth of HER2-positive breast cancer xenografts in mice compared with the unconjugated trastuzumab.[75] In these studies, the NLS peptides were chemically conjugated to the trastuzumab antibody. Other studies, including one in which the anti-CD33 antibody HuM195 was used to target 111In to the nucleus of human leukemia cells, have utilized NLS peptides that were conjugated to the antibody through a linker; however, no direct evidence of nuclear localization was provided.[76]
4.3 Pro-Apoptotic Peptides – (KLAKLAK)2
Cationic amphipathic peptides can cause lipid matrix deformation in negatively charged plasma membranes of prokar-yotes.[77] (KLAKLAK)2 is a synthetic, cationic, amphipathic L-configuration peptide designed by Javadpour et al.[78] that was found to have increased cytotoxic activity on bacteria. These peptides do not act on eukaryotic cells because of phospholipids in the eukaryotic cell membrane structure. Because of similarities between the bacterial cell membranes and the mitochondrial membranes, the (KLAKLAK)2 peptide has been found to mediate cell killing in eukaryotic cells by disrupting the mitochondrial membrane and causing the release of cytochrome c into the cytosol.[79] The released cytochrome c then leads to formation of the ‘apoptosome’, causing activation of procaspase 9, which in turn leads to activation of effector caspases 3 and 7, finally leading to induction of apoptosis in eukaryotic cells. The peptide (KLAKLAK)2, if targeted specifically to a cancer cell, can induce the cell to undergo apoptosis, leading to the tumor cell destruction. This is yet another example of a potentially effective, molecularly targeted therapy. The (KLAKLAK)2 peptide has been fused to various peptidomimetics like DPI in MCA205 murine sarcomas,[80] RGD-4C peptide against the integrin receptors in human breast carcinoma cells,[79] HN-1 peptide in human head and neck squamous cell cancer,[81] and SMSIARL peptide in prostate cancer,[82] as well as to monoclonal antibodies like anti-CD33 and anti-CD 19[83] in hematologic malignancies.
One can think of a potent pro-apoptotic anti-cancer therapeutic agent which is specific for GBM tumor cells. The majority of tumor cells are apoptosis-resistant due to mutations in various signaling molecules that act to induce apoptosis in the cells. Since the pro-apoptotic peptide (KLAKLAK)2 acts through the mitochondrial membrane, we rationalize that these therapeutics will be able to induce tumor cell death even in the majority of apoptotic-resistant tumor cells. We can use alternative translocation domains, such as the GALA peptide, and the translocation domain of DT if this design of the therapeutic is unable to mediate apoptotic cell death. If the (KLAKLAK)2 peptide is ineffective in inducing apoptosis in GBM cells, then other pro-apoptotic peptides targeting the mitochondria, and inhibitor of apoptosis proteins (IAPs) such as the BH3 peptide of Bid (EDIIRNIARHLAQVGDSMDR) can be used.[84]
As mentioned earlier, there is a strong requirement in the field of brain tumor therapy for specifically targeted small molecular weight therapeutics that have the advantages of small size and better pharmacokinetic properties. The next step in the direction of development of clinically applicable agents would be to fuse various drugs such as chemotherapeutics, pro-apoptotic peptides, fluorescent labels, radioisotopes and pro-drugs such as photosensitizers to these targeted delivery vehicles. One is to fuse pro-apoptotic peptides such as (KLA-KLAK)2 and radioisotopes such as α-particle emitters to these delivery vectors. One can fuse this peptide to the double-targeted vector, which binds to the IL-13Rα2 plasma membrane receptor. This will enable delivery of (KLAKLAK)2 peptides inside the GBM tumor cell, wherein the (KLAKLAK)2 peptide can mediate its effect. In a similar vein, we plan to develop IL-13Rα2-targeted radiotherapeutics by fusing our ‘double specificity’ delivery vehicles, such as the IL13.E13K-D2-NLS and the pep-IL-13Rα2-NLS vectors to an α-particle emitter like 225Ac (half-life = 10 days) so they can be specifically targeted to the nucleus of the glioma tumor cells, i.e. to the site of their action. 225Ac decays through a process of four a emissions and two β emissions to a stable isotope of 209Bi releasing approximately 28 MeV of energy to the surroundings. If used for radiotherapy of tumor tissues, emission of such high energies would be expected to lead to greater toxicity to the cells. Delivery of such high energy isotopes to the nucleus in the glioma cells should lead to efficient action of these therapeutics since they have a small radius of action.
5. Summary
Proof-of-principle studies have documented the feasibility of using proteinaceous compounds for targeting to intracellular compartments of cancer cells, and more recently, to specific organelles of specific cells. Nuclear delivery vectors are now available and they can be conjugated (single conjugation) to various radioisotopes, chemotherapeutics, and other therapeutics, thus delivering these cytotoxic agents specifically to their sites of action. These delivery vectors can also be used to transport DNA into the nuclei and hence be used as effective gene therapy delivery agents. Furthermore, they can be applied for genetically mediated therapy like siRNA or anti-sense gene therapy. The IL-13Rα2-specific nuclear delivery vectors described in this review can also be utilized for delivery of photosensitizers as their optimal site of action resides in the nucleus of GBM cells. Our IL-13-based cytotoxin, IL-13-PE38QQR, has shown anti-GBM tumor activity under both in vitro and in vivo conditions.[37] This cytotoxin entered phase III clinical trials worldwide and showed an increased overall survival in GBM patients treated by physicians who were experienced with the drug-delivery technology [85,86] In addition to nuclear targeting, mitochondria are potential pharmaceutically targeted organelles.
Several of these biotherapeutics are under pre-clinical and clinical development. They offer high specificity and are potentially safer than existing therapeutics and thus may exhibit larger therapeutic windows. At this time, these constructs need to be delivered loco-regionally to brain tumors using convection-enhanced delivery (CED).[87,88] This method of delivery has already produced significant clinical responses[86,89] and is undergoing continuous improvement.[90]
Acknowledgments
This study was supported by the NCI grant R01 CA74145.
Wake Forest Office of Technology Asset Management has filed a patent application for the ‘IL-13Rα2-targeted intracellular delivery proteins’ (assignees: Waldemar Debinski, Hetal Pandya and Denise Gibo).
References
- 1.Wick W, Weller M, Weiler M, et al. Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro Oncol. 2011 Jun;13(6):566–579. doi: 10.1093/neuonc/nor039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009 Mar;373(9668):1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Dietrich PY, Ostermann KS, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002 Mar;20(5):1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 4.Kristiansen K, Hagen S, Kollevold T, et al. Combined modality therapy of operated astrocytomas grade III and IV: confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival times - a prospective multicenter trial of the Scandinavian Glioblastoma study Group. Cancer. 1981 Feb;47(4):649–652. doi: 10.1002/1097-0142(19810215)47:4<649::aid-cncr2820470405>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Stern JI, Raizer JJ. Chemotherapy in the treatment of malignant gliomas. Expert Rev Anticancer Ther. 2006 May;6(5):755–767. doi: 10.1586/14737140.6.5.755. [DOI] [PubMed] [Google Scholar]
- 6.Reardon DA, Rich JN, Friedman HS, et al. Recent advances in the treatment of malignant astrocytoma. J Clin One. 2006 Mar;24(8):1253–1265. doi: 10.1200/JCO.2005.04.5302. [DOI] [PubMed] [Google Scholar]
- 7.Lefranc F, Brotchi J, Kiss R. Present and future issues in the treatment of malignant gliomas, with a special emphasis on cell migration and the resistance of migrating glioma cells to apoptosis. J Clin Oncol. 2005 Apr;23(10):2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith SJ. Receptor imaging: competitive or complementary to antibody imaging? Semin Nucl Med. 1997 Apr;27(2):85–93. doi: 10.1016/s0001-2998(97)80041-4. [DOI] [PubMed] [Google Scholar]
- 9.Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004 Jan;54(1):131–140. doi: 10.1227/01.neu.0000097715.11966.8e. [DOI] [PubMed] [Google Scholar]
- 10.Debinski W, Siegall CB, FitzGerald D, et al. Substitution of foreign protein sequences into a chimeric toxin composed of transforming growth factor alpha and Pseudomonas exotoxin. Mol Cell Biol. 1991 Mar;11(3):1751–1753. doi: 10.1128/mcb.11.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogata M, Chaudhary VK, Pastan I, et al. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990 Nov;265(33):20678–20685. [PubMed] [Google Scholar]
- 12.Jinno Y, Ogata M, Chaudhary VK, et al. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989 Sep;264(27):15953–15959. [PubMed] [Google Scholar]
- 13.London SD, Schmaljohn AL, Dalrymple JM, et al. Infectious enveloped RNA virus antigenic chimeras. Proc Natl Acad Sci U S A. 1992 Jan;89(1):207–211. doi: 10.1073/pnas.89.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenmark H, Moskaug JO, Madshus IH, et al. Peptides fused to the aminoterminal end of diphtheria toxin are translocated to the cytosol. J Cell Biol. 1991 Jun;113(5):1025–1032. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang J, Fitzgerald DJ, Adhya S, et al. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E coli. Cell. 1997 Jan;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- 16.Allured VS, Collier RJ, Carroll SF, et al. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Hall WA, Jin N, et al. Targeting glioblastoma multiforme with an IL-13/diphtheria toxin fusion protein in vitro and in vivo in nude mice. Protein Eng. 2002 May;15(5):419–427. doi: 10.1093/protein/15.5.419. [DOI] [PubMed] [Google Scholar]
- 18.Chiron MF, Fryling CM, FitzGerald D. Furin-mediated cleavage of Pseudomonas exotoxin-derived chimeric toxins. J Biol Chem. 1997 Dec;272(50):31707–31711. doi: 10.1074/jbc.272.50.31707. [DOI] [PubMed] [Google Scholar]
- 19.Inocencio NM, Moehring JM, Moehring TJ. Furin activates Pseudomonas exotoxin A by specific cleavage in vivo and in vitro. J Biol Chem. 1994 Dec;269(50):31831–31835. [PubMed] [Google Scholar]
- 20.Moehring JM, Inocencio NM, Robertson BJ, et al. Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J Biol Chem. 1993 Feb;268(4):2590–2594. [PubMed] [Google Scholar]
- 21.Siegall CB, Ogata M, Pastan I, et al. Analysis of sequences in domain II of Pseudomonas exotoxin A which mediate translocation. Biochemistry. 1991 Jul;30(29):7154–7159. doi: 10.1021/bi00243a016. [DOI] [PubMed] [Google Scholar]
- 22.Theuer CP, Buchner J, FitzGerald D, et al. The N-terminal region of the 37-kDa translocated fragment of Pseudomonas exotoxin A aborts translocation by promoting its own export after microsomal membrane insertion. Proc Natl Acad Sci U S A. 1993 Aug;90(16):7774–7778. doi: 10.1073/pnas.90.16.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iglewski BH, Rabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhary VK, Jinno Y, FitzGerald DJ, et al. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegall CB, Chaudhary VK, FitzGerald DJ, et al. Functional analysis of domains II, Ib, and III of Pseudomonas exotoxin. J Biol Chem. 1989 Aug;264(24):14256–14261. [PubMed] [Google Scholar]
- 26.Pastan I, Chaudhary VK, Fitzgerald D. Recombinant toxins as novel therapeutic agents. Ann Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- 27.Debinski W, Gibo DM, Hulet SW, et al. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999 May;5(5):985–990. [PubMed] [Google Scholar]
- 28.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000 May;6(5):440–449. [PMC free article] [PubMed] [Google Scholar]
- 29.Debinski W, Obiri NI, Pastan I, et al. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995 Jul;270(28):16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 30.Mintz A, Gibo DM, Slagle-Webb B, et al. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002 Sep;4(5):388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debinski W, Slagle B, Gibo DM, et al. Expression of a restrictive receptor for Interleukin 13 is associated with glial transformation. J Neurooncol. 2000 Jun;48(2):103–111. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 32.Keegan AD, Johnston JA, Tortolani PJ, et al. Similarities and differences in signal transduction by interleukin 4 and interleukin 13: analysis of Janus Kinase activation. Proc Natl Acad Sci U S A. 1995 Aug;92(17):7681–7685. doi: 10.1073/pnas.92.17.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernard J, Treton D, Vermot-Desroches C, et al. Expression of Interleukin 13 receptor in glioma and renal cell carcinoma: IL13Ralpha2 as a decoy receptor for IL13. Lab Invest. 2001 Sep;81(9):1223–1231. doi: 10.1038/labinvest.3780336. [DOI] [PubMed] [Google Scholar]
- 34.Debinski W, Gibo DM, Obiri NI, et al. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998 May;16(5):449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JP, Debinski W. Mutants of interleukin 13 with altered reactivity toward interleukin 13 receptors. J Biol Chem. 1999 Oct 15;274(42):29944–29950. doi: 10.1074/jbc.274.42.29944. [DOI] [PubMed] [Google Scholar]
- 36.Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004 Jan;6(1):15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Debinski W, Obiri NI, Powers SK, et al. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995 Nov;1(11):1253–1258. [PubMed] [Google Scholar]
- 38.Kalderon D, Roberts BL, Richardson WD, et al. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 39.Hubner S, Xiao CY, Jans DA. The protein kinase CK2 site (Serlll/112) enhances recognition of the simian virus 40 large T-antigen nuclear localization sequence by importin. J Biol Chem. 1997 Jul;272(27):17191–17195. doi: 10.1074/jbc.272.27.17191. [DOI] [PubMed] [Google Scholar]
- 40.Rihs HP, Peters R. Nuclear transport kinetics depend on phosphorylation-site-containing sequences flanking the karyophilic signal of the Simian virus 40 T-antigen. EMBO J. 1989 May;8(5):1479–1484. doi: 10.1002/j.1460-2075.1989.tb03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rihs HP, Jans DA, Fan H, et al. The rate of nuclear cytoplasmic protein transport is determined by the casein kinase II site flanking the nuclear localization sequence of the SV40 T-antigen. EMBO J. 1991 Mar;10(3):633–639. doi: 10.1002/j.1460-2075.1991.tb07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao CY, Hubner S, Jans DA. SV40 large tumor antigen nuclear import is regulated by the double-stranded DNA-dependent protein kinase site (serine 120) flanking the nuclear localization sequence. J Biol Chem. 1997 Aug 29;272(35):22191–22198. doi: 10.1074/jbc.272.35.22191. [DOI] [PubMed] [Google Scholar]
- 43.Rosenkrnaz AA, Lunin VG, Gulak PV, et al. Recombinant modular transporters for cell-specific nuclear delivery of locally acting drugs enhance photosensitizer activity. FASEB J. 2003 Jun;17(9):1121–1123. doi: 10.1096/fj.02-0888fje. [DOI] [PubMed] [Google Scholar]
- 44.Jans DA, Xiao CY, Lam MHC. Nuclear targeting signal recognition: a key point in nuclear transport. Bio Essays. 2000 Jun;22(6):532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Beaumelle B, Bensammar L, Bienvenue A. Selective translocation of the A chain of diphtheria toxin across the membrane of purified endosomes. J Biol Chem. 1992 Jun;267(16):11525–11531. [PubMed] [Google Scholar]
- 46.Madshus IH, Olsnes S, Stenmark H. Membrane translocation of diphtheria toxin carrying passenger protein domain. Infect Immun. 1992 Aug;60(8):3296–3302. doi: 10.1128/iai.60.8.3296-3302.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chignola R, Anselmi C, Serra M, et al. Self-potentiation of ligand-toxin conjugates containing Ricin A chain fused with viral structures. J Biol Chem. 1995 Oct;270(40):23345–23351. doi: 10.1074/jbc.270.40.23345. [DOI] [PubMed] [Google Scholar]
- 48.Pandya H, Gibo DM, Debinski W. Molecular targeting of intracellular compartments specifically in cancer cells. Genes Cancer. 2010;1(5):421–433. doi: 10.1177/1947601910375274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akhlynina TV, Jans DA, Rosenkranz AA, et al. Nuclear targeting of chlorine e6 enhances its photosensitizing activity. J Biol Chem. 1997 Aug;272(33):20328–20331. doi: 10.1074/jbc.272.33.20328. [DOI] [PubMed] [Google Scholar]
- 50.Chen P, Wang J, Hope K, et al. Nuclear localizing sequences promote nuclear translocation and enhance the radiotoxicity of the anti-CD33 monoclonal antibody HuM 195 labelled with 111In in human myeloid leukemia cells. J Nucl Med. 2006 May;47(5):827–836. [PubMed] [Google Scholar]
- 51.Haefliger P, Agorastos N, Renard A, et al. Cell uptake and radiotoxicity studies of an nuclear localization signal peptide-intercalator conjugate labeled with [99mTc(CO)3]+ . Bioconjug Chem. 2005 May-Jun;16(3):582–587. doi: 10.1021/bc0500084. [DOI] [PubMed] [Google Scholar]
- 52.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007 Aug;59(8):748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terlecky SR, Koepke JI. Drug delivery to peroxisomes: employing unique trafficking mechanisms to target protein therapeutics. Adv Drug Deliv Rev. 2007 Aug;59(8):739–747. doi: 10.1016/j.addr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Mukhopadhyay A, Weiner H. Delivery of drugs and macromolecules to mitochondria. Adv Drug Deliv Rev. 2007 Aug;59(8):729–738. doi: 10.1016/j.addr.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song H, Du Y, Sgouros G, et al. Therapeutic potential of 90Y-and 131I-labeled anti-CD20 monoclonal antibody in treating non-Hodgkin’s lymphoma with pulmonary involvement: a Monte Carlo-based dosimetric analysis. J Nucl Med. 2007 Jan;48(1):150–157. [PMC free article] [PubMed] [Google Scholar]
- 56.Zalutsky MR. Targeted α-particle therapy of microscopic disease: providing a further rationale for clinical investigation. J Nucl Med. 2006 Aug;47(8):1238–1240. [PubMed] [Google Scholar]
- 57.Couturier O, Supiot S, Degraef-Mougin M, et al. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med Mol Imaging. 2005 May;32(5):601–604. doi: 10.1007/s00259-005-1803-2. [DOI] [PubMed] [Google Scholar]
- 58.Nunez MI, Villalobos M, Olea N, et al. Radiation-induced DNA doublestranded break rejoining in human tumor cells. Br J Cancer. 1995 Feb;71(2):311–316. doi: 10.1038/bjc.1995.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pandita TK, Lieberman HB, Lim DS, et al. Ionizing radiation activates ATM kinase throughout the cell cycle. Oncogene. 2000 Mar;19(11):1386–1391. doi: 10.1038/sj.onc.1203444. [DOI] [PubMed] [Google Scholar]
- 60.Munro TR. The relative radiosensitivity of the nucleus and cytoplasm of Chinese hamster fibroblasts. Radiat Res. 1970 Jun;42(3):451–570. [PubMed] [Google Scholar]
- 61.Kampf G. Induction of DNA double-strand breaks by ionizing radiation of different quality and their relevance for cell inactivation. Radiobiol Radiother. 1988;29(6):631–658. [PubMed] [Google Scholar]
- 62.Rosenkranz AA, Vaidyanathan G, Pozzi OR, et al. Engineered modular recombinant transporters: application of new platform for targeted radio-therapeutic agents to alpha-particle emitting 211 At. Int J Radiat Oncol Biol Phys. 2008 Sep;72(1):193–200. doi: 10.1016/j.ijrobp.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chappell LL, Deal KA, Dadachova E, et al. Synthesis, conjugation, and radiolabeling of a novel bifunctional chelating agent for 225-Ac radio-immunotherapy applications. Bioconjugate Chem. 2000 Jul-Aug;11(4):510–519. doi: 10.1021/bc990153f. [DOI] [PubMed] [Google Scholar]
- 64.Deal KA, Davis IA, Mirzadeh S, et al. Improved in vivo stability of Actinium225 macrocyclic complexes. J Med Chem. 1999 Jul;42(15):2988–2992. doi: 10.1021/jm990141f. [DOI] [PubMed] [Google Scholar]
- 65.Back T, Andersson H, Divgi CR, et al. 211At radioimmunotherapy of subcutaneous human ovarian cancer xenografts: evaluation of relative biologic effectiveness of an α-emitter in vivo. J Nucl Med. 2005 Dec;46(12):2061–2067. [PubMed] [Google Scholar]
- 66.Behr TM, Behe M, Stabin MG, et al. High-linear energy transfer (LET) α versus low-LET β emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled C017-1A Fab’ fragments in a human colonic cancer model. Cancer Res. 1999 Jun;59(11):2635–2643. [PubMed] [Google Scholar]
- 67.Sgouros G, Ballangrud AM, Jurcic JG, et al. Pharmacokinetics and dosimetry of an α-particle emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J Nucl Med. 1999 Nov;40(11):1935–1946. [PubMed] [Google Scholar]
- 68.Kennel SJ, Brechbiel MW, Milenic DE, et al. Actinium-225 conjugates of Mab CC49 and humanized ΔCH2CC49. Cancer Biother Radiopharm. 2002 Apr;17(2):219–231. doi: 10.1089/108497802753773847. [DOI] [PubMed] [Google Scholar]
- 69.Norenberg JP, Krenning BJ, Konings IRHM, et al. 213Bi-[DOTA0, Tyr3] octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res. 2006 Feb;12(3):897–903. doi: 10.1158/1078-0432.CCR-05-1264. [DOI] [PubMed] [Google Scholar]
- 70.Buchegger F, Perillo-Adamer F, Dupertuis YM, et al. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging. 2006 Nov;33(11):1352–1363. doi: 10.1007/s00259-006-0187-2. [DOI] [PubMed] [Google Scholar]
- 71.Faraggi M, Gardin I, de Labriolle-Vaylet C, et al. The influence of tracer localization on the electron dose rate delivered to the cell nucleus. J Nucl Med. 1994 Jan;35(1):113–119. [PubMed] [Google Scholar]
- 72.Goddu SM, Howell RW, Rao DV. Cellular dosimetry: absorbed fractions for monoenergetic electron and alpha particle sources and S-values for radionuclides uniformly distributed in different cell compartments. J Nucl Med. 1994 Feb;35(2):303–316. [PubMed] [Google Scholar]
- 73.Costantini DL, Chan C, Cai Z, et al. (11l)In-labeled trastuzumab (herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007 Aug;48(8):1357–1368. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 74.Costantini DL, Villani DF, Vallis KA, et al. Methotrexate, paclitaxel, and doxorubicin radiosensitize HER2-amplified human breast cancer cells to the Auger electron-emitting radiotherapeutic agent (11l)In-NLS-trastuzumab. J Nucl Med. 2010 Mar;51(3):477–483. doi: 10.2967/jnumed.109.069716. [DOI] [PubMed] [Google Scholar]
- 75.Costantini DL, McLarty K, Lee H, et al. Antitumor effects and normal-tissue toxicity of 111 In-nuclear localization sequencetrastuzumab in athymic mice bearing HER-positive human breast cancer xenografts. J Nucl Med. 2010 Jul;51(7):1084–1091. doi: 10.2967/jnumed.109.072389. [DOI] [PubMed] [Google Scholar]
- 76.Kersemans V, Cornelissen B, Minden MD, et al. Drug resistant AML cells and primary AML specimens are killed by lllIn-anti-CD33 monoclonal antibodies modified with nuclear localizing peptide sequences. J Nucl Med. 2008 Sep;49(9):1546–1554. doi: 10.2967/jnumed.107.047399. [DOI] [PubMed] [Google Scholar]
- 77.Leuschner C, Hansel W. Membrane disrupting lytic peptides for cancer treatments. Curr Pharm Des. 2004;10(19):2299–2310. doi: 10.2174/1381612043383971. [DOI] [PubMed] [Google Scholar]
- 78.Javadhpour MM, Juban MM, Lo WC, et al. De novo antimicrobial peptides with low mammalian cell toxicity. J Med Chem. 1996 Aug;39(16):3107–3113. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- 79.Ellerby HM, Arap W, Ellerby LM, et al. Anti cancer activity of targeted proapoptotic petides. Nature Med. 1999;5(9):1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 80.Mai JC, Mi Z, Kim SH, et al. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001 Nov;61(21):7709–7712. [PubMed] [Google Scholar]
- 81.Hong FD, Clayman GL. Isolation of a peptide for targeted drug delivery into the human head and neck solid tumors. Cancer Res. 2000 Dec;60(23):6551–6556. [PubMed] [Google Scholar]
- 82.Arap W, Haedicke W, Bernasconi M, et al. Targeting the prostrate for destruction through a vascular address. Proc Nat Acad Sci U S A. 2002 Feb;99(3):1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marks AJ, Cooper MS, Anderson RJ, et al. Selective apoptotic killing of malignant hemopoietic cells by antibody-targeted delivery of amphipathic peptide. Cancer Res. 2005 Mar;65(6):2373–2377. doi: 10.1158/0008-5472.CAN-04-2594. [DOI] [PubMed] [Google Scholar]
- 84.Chipuk JE, Fisher JC, Dillon CP, et al. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci U S A. 2008 Dec;105(51):20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sampson JH, Archer G, Pedain C, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2010;113(2):301–309. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 86.Kunwar S, Prados M, Chang SM, et al. Direct intracerebral delivery of cintredekin besodotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Bsedotox Intraparenchymal Study Group. J Clin Oncol. 2007 Mar;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 87.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009 Oct;9(10):1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Debinski W, Tatter SB. Convection-enhanced delivery to achieve widespread distribution of viral vectors: predicting clinical implementation. Curr Opin Mol Ther. 2010 Dec;12(6):647–653. [PubMed] [Google Scholar]
- 89.LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011 Apr;10(4):309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 90.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neurooncol. 2010 Sep;12(9):928–940. doi: 10.1093/neuonc/noq046. [DOI] [PMC free article] [PubMed] [Google Scholar]