Abstract
Background
Although the association between exposure to particulate matter (PM) mass and mortality is well established, there remains uncertainty about which chemical components of PM are most harmful to human health.
Methods
A hierarchical approach was used to determine how the association between daily PM2.5 mass and mortality was modified by PM2.5 composition in 25 US communities. First, the association between daily PM2.5 and mortality was determined for each community and season using Poisson regression. Second, we used meta-regression to examine how the pooled association was modified by community and season-specific particle composition.
Results
There was a 0.74% (95% confidence interval = 0.41%–1.07%) increase in nonaccidental deaths associated with a 10 μg/m3 increase in 2-day averaged PM2.5 mass concentration. This association was smaller in the west (0.51% [0.10%– 0.92%]) than in the east (0.92% [0.23%–1.36%]), and was highest in spring (1.88% [0.23%–1.36%]). It was increased when PM2.5 mass contained a higher proportion of aluminum (interquartile range = 0.58%), arsenic (0.55%), sulfate (0.51%), silicon (0.41%), and nickel (0.37%). The combination of aluminum, sulfate, and nickel also modified the effect. These species proportions explained residual variability between the community-specific PM2.5 mass effect estimates.
Conclusions
This study shows that certain chemical species modify the association between PM2.5 and mortality and illustrates that mass alone is not a sufficient metric when evaluating health effects of PM exposure.
Most population-based epidemiologic studies examining the acute health effects associated with exposure to particulate matter (PM) air pollution have used total particle mass as the primary exposure metric.1,2 PM2.5 (particles having aerodynamic diameter ≤2.5 μm) is associated with greater increases in daily mortality than larger particles, and are of greater public health concern.3,4 The magnitude of this association varies with geographic location, suggesting that size is not the only indicator of PM-related health effects.2 Although these studies have identified serious health risks, there is still uncertainty as to which particle components are most harmful.5
The scope of previous studies examining health effects of PM2.5 components6 – 8 has been limited with respect to the number of communities examined and the time series of the speciation data available for analysis. More detailed study into the health effects of specific PM2.5 species has been restricted by temporal sparseness of the PM2.5 speciation data available from the Environmental Protection Agency (EPA) Speciation Trends Network (STN) monitoring sites.
In this multicommunity study, we used STN data to examine whether the chemical composition of fine particle mass plays a role in its toxicity. We have taken a hierarchical approach to overcome the limited exposure data. In the first stage, the seasonal association between PM2.5 mass and mortality was determined for 25 US communities using established time-series methodology. In the second stage, meta-regression was used to determine the extent to which the seasonal proportion of specific chemical components of PM2.5 mass modified the pooled association over all communities. This approach also allowed us to characterize the variability between communities resulting from differences in the chemical composition of particles.
METHODS
Selection of the 25 study communities was based on availability of PM2.5 mass concentrations and daily mortality records for at least 4 years and of PM2.5 speciation data for at least 2 years between 2000 and 2005. The PM2.5 mass and species concentration data were obtained online from the EPA Technology Transfer Network Air Quality System,9 whereas daily mortality records were obtained from the National Center for Health Statistics and various state departments of health. Meteorologic data, including temperature and dew point temperature (necessary to control for confounding in the PM2.5 mass-mortality relationship) were acquired from the National Climatic Data Center.10
Air Pollution and Meteorological Data
In 2000, the EPA established the STN to provide data on the composition of PM2.5 for the nation’s air quality program. This network consists of 54 tightly controlled nationally operated sampling sites operating on a 1-in-3 day schedule and about 175 state and locally run sites operating on a 1-in-3 or 1-in-6 day schedule. Particles are collected on Teflon (DuPont, Wilmington, DE), nylon, and quartz filters that are then analyzed for trace elements using x-ray fluorescence, for ions (nitrate, sulfate, ammonium, sodium, and potassium) using ion chromatography, and for organic carbon and elemental carbon using thermal-optical analysis.
The EPA typically maintains multiple PM2.5 mass sites but only one PM2.5 speciation site within a county. To make use of all information available from monitoring sites within each of the 25 communities, 24-hour integrated mass concentrations were averaged over the county using a method adapted from Schwartz.11 This method accounts for the fact that monitors do not report all concentrations on the same days, resulting in an imbalance in the daily data used to take the county-wide average. Before averaging, any monitor not well correlated with the others (r < 0.8 for 2 or more monitor pairs within a community) was excluded, as it likely measured a local pollution source and would not represent the general population exposure over the entire community.
Because there was only one PM2.5 speciation monitor per community, across-community averaging was not used to characterize the composition of PM2.5 mass. The following data integrity issues were addressed.
Quality flags: All observations with an EPA data quality flag were removed from the analysis.
Organic carbon blank correction: Organic carbon data provided by the EPA are not blank-corrected and thus have a positive bias due to sampling artifacts. In 2003, the EPA made the blank concentrations available for the STN sites. To overcome the many missing values, a monthly correction value was determined by averaging the available blanks (2003–2005) by site and month. Organic carbon concentrations were corrected by subtracting the estimated blank corrections from the reported value.
Data below detection limit: Starting in July 2003, the EPA included a method detection limit (MDL) with each species concentration reported. This information was used to determine which species were frequently undetectable. Due to the paucity of MDL information, it was used cautiously; species with at least 25% of the reported concentrations above the MDL were included in the analysis. No correction was made to reported values below the MDL.
Daily records of temperature and dew point temperature were retrieved from the predominant weather station in each of the 25 communities.
Mortality Data
After 2000 the National Center for Health Statistics stopped providing the date of death, making it necessary to acquire data from state public health departments (CA, MA, MI, MN, MO, OH, PA, TX, WA). Our analysis was conducted at the county level as this was the smallest resolution available for all mortality data; the name of the major city within each of the 25 counties was used as an identifier. The mortality data provided nonconfidential information on decedents including state of death, county of death, age, sex, date of death, and primary cause of death. Only those individuals dying of nonaccidental causes were examined (ie, 10th revision ICD codes V01 through Y98 were excluded). Specific causes were derived from the ICD code for the underlying cause of death: respiratory disease (ICD codes J00 through J99), cardiovascular disease (ICD codes I01 through I52), and stroke (ICD codes I60 through J69). This work was done under an exemption from the Harvard School of Public Health’s Human Subjects Committee.
Statistical Methods
Due in part to different source contributions at different times of the year, a seasonal analysis was conducted. In the first stage, season-specific associations between daily PM2.5 mass concentration and mortality were determined for each community using Poisson regression. The association was examined for several causes of death, and single- and multiple-day exposure lags (eg, PM2.5 averaged over the day before and the day of death). Effects of PM are weaker with concurrent rather than lagged exposures, and the associations at different lags differ by cause of death.2,12,13 Confounding effects of temperature and dew point temperature were controlled for by taking their 3-day running mean and including them in the Poisson model as linear terms; day of the week was controlled for with indicator variables; and time was controlled for with a cubic regression spline with 1.5 degrees of freedom for each season and year. Resultant effect estimates were expressed as a percent increase in mortality with a 10-μg/m3 increase in PM2.5 mass concentration; 95% confidence intervals (CIs) were also computed.
In the second stage, Poisson regression effect estimates and standard errors were combined using random effects meta-analysis to obtain an overall effect across the study domain. Mean seasonal concentration ratios of species to the total PM2.5 mass were determined for each community. These proportions, which reflect particle composition and thus the relative contribution of different sources to the PM2.5 mass, were then used in a meta-regression to quantify to what extent the association between PM2.5 mass and mortality was modified by particle composition.
where βis represents the effect estimates from the first stage for i = 1, …, 25 communities, and s = 1, …, 4 seasons, Xi1s, …, Xips represent the j = 1, …, p seasonal modifier variables. εis is the estimated variance of the estimate βis, and τ2 is the across-community heterogeneity variance over and above what can be explained by the modifier variables. Equation (1) was solved using an extension of the method of DerSimonian and Laird.14,15
People in the United States spend approximately 80% of their time indoors.16 Thus particle penetration, which depends on a building’s ventilation and varies by season and community, may explain some of the differences in seasonal PM2.5-mortality effect estimates.17 This was taken into account by including seasonally averaged temperature in the meta-regression (information on community and season-specific ventilation rates was not available). According to Koutrakis et al,16 at both high and low temperatures ventilation rate and indoor/outdoor ratio of PM will be low, potentially reducing the PM2.5-mortality effect estimate. At more moderate temperatures, ventilation rate and ratio of PM will be high, potentially increasing the PM2.5-mortality effect estimates. To capture this inverted U-shape phenomenon, a quadratic function of temperature was used in the meta-regression and the mean part of Equation (1) was more specifically defined by:
where γ3 represents the change in the estimated association between PM2.5 and mortality as the proportion of sulfate increases. The community-specific prevalence of central air-conditioning was included in the meta-regression to address the potential for any residual heterogeneity associated with ventilation/particle penetration not sufficiently accounted for with the quadratic term for seasonally averaged temperature. These data were obtained from the American Housing Survey18 and were available for all communities except for Harrisburg, PA.
Furthermore, because evidence exists that socioeconomic parameters play a role in the effect of particles,19,20 we addressed the possibility that the effect of the species proportions could be confounded. We examined community-specific parameters (median household income, percent of population below poverty line, percent of adult population having graduated high school, and percent of population older than 65 years of age) obtained from the US Census Bureau21 in the meta-regression along with temperature and each species proportion (separately). Although socioeconomic parameters and central AC prevalence did not vary seasonally, both were included in the seasonal meta-regression.
The gamma coefficients of the species proportions were expressed as an interquartile range (IQR) change, ie, the change in the PM2.5-mortality effect estimate from the 25th to the 75th percentile of the species proportion. In the initial analysis, we examined one species at a time; this was followed by combining all marginally significant species in a multivariate analysis and using backward elimination to determine which combination of species was most strongly associated with mortality. Finally, to test for heterogeneity in the effect estimates, a Q-statistic was computed and compared with a χ2 distribution; the estimated τ2 gave an indication of how much between-community heterogeneity in PM2.5 mass-mortality effects was explained by PM2.5 composition.
RESULTS
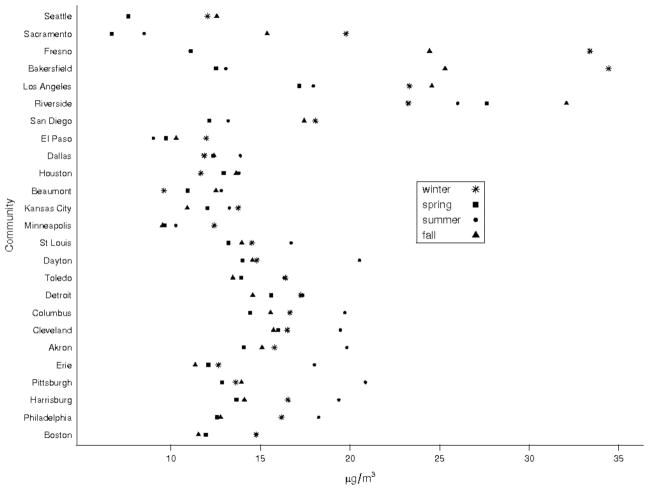
Summary data by community are presented in Table 1. We examined 1,313,983 nonaccidental deaths between 2000 and 2005. Thirty-one percent of deaths were due to cardiovascular, 10% were due to respiratory disease, and 7% were due to stroke. The average number of PM2.5 days examined per community was 1451, whereas the average number of speciation days was 321. Seasonally averaged PM2.5 concentrations ranged from well below the National Ambient Air Quality Standard of 15 μg/m3 in Sacramento, CA in spring (6.7 μg/m3), to over twice the standard, in Bakersfield and Fresno, CA in winter (34.4 and 33.4 μg/m3, respectively) (Table 2). The geographic distribution of the seasonal species proportions is shown in Figure 1. The highest PM2.5 concentrations were observed in California in winter and fall, whereas the peak in eastern communities was in summer. Seasonal variation was largest in California communities and narrowest in the central part of the country communities in (TX, MO, and MN).
TABLE 1.
Summary Statistics by Community
| Communitya | Years of Available Data | Days of PM2.5 Mass No. | Days of PM2.5 Speciationb No. | Nonaccidental Deaths No. | Deaths
|
Daily Temperature (°C) Mean (SD) | ||
|---|---|---|---|---|---|---|---|---|
| Respiratory Diseasec % | Heart Diseasec % | Strokec % | ||||||
| Seattle, WA | 2000–2004 | 1816 | 654 | 59384 | 10.3 | 25.6 | 9.1 | 11.4 (5.5) |
| Sacramento, CA | 2000–2003 | 1341 | 352 | 37377 | 11.7 | 30.8 | 9.1 | 16.5 (6.4) |
| Fresno, CA | 2000–2003 | 1231 | 307 | 21966 | 11.2 | 32.2 | 9.0 | 18.4 (7.7) |
| Bakersfield, CA | 2000–2003 | 1349 | 230 | 17471 | 12.5 | 36.0 | 7.0 | 18.8 (7.8) |
| Los Angeles, CA | 2000–2003 | 1442 | 72 | 227956 | 10.2 | 35.5 | 7.5 | 17.1 (3.3) |
| Riverside, CA | 2000–2003 | 1330 | 264 | 45753 | 11.6 | 35.9 | 7.9 | 17.5 (5.5) |
| San Diego, CA | 2000–2003 | 1424 | 192 | 76082 | 11.0 | 30.6 | 8.4 | 17.4 (3.2) |
| El Paso, TX | 2000–2005 | 1391 | 524 | 24857 | 8.7 | 28.3 | 6.2 | 18.7 (8.6) |
| Dallas, TX | 2000–2005 | 2188 | 559 | 83327 | 8.9 | 30.0 | 7.1 | 19.3 (8.8) |
| Houston, TX | 2000–2005 | 2044 | 635 | 131486 | 7.9 | 28.9 | 7.5 | 21.1 (7.4) |
| Beaumont, TX | 2000–2005 | 1841 | 669 | 17594 | 10.5 | 34.9 | 7.2 | 20.9 (7.2) |
| Kansas City, MO | 2000–2004 | 1306 | 323 | 33044 | 11.1 | 29.5 | 7.3 | 13.0 (10.8) |
| Minneapolis, MN | 2000–2005 | 1262 | 465 | 48248 | 9.4 | 21.0 | 7.3 | 8.4 (12.3) |
| St. Louis, MO | 2000–2004 | 1823 | 567 | 75625 | 9.7 | 32.9 | 7.1 | 14.1 (10.4) |
| Dayton, OH | 2000–2003 | 785 | 131 | 21549 | 9.8 | 29.7 | 6.9 | 11.4 (10.3) |
| Toledo, OH | 2000–2003 | 1362 | 107 | 17596 | 9.9 | 35.3 | 6.7 | 10.6 (10.4) |
| Detroit, MI | 2000–2003 | 1430 | 308 | 70761 | 8.0 | 37.2 | 5.8 | 10.4 (9.5) |
| Columbus, OH | 2000–2003 | 1444 | 108 | 32807 | 9.9 | 28.1 | 7.1 | 11.9 (10.1) |
| Cleveland, OH | 2000–2003 | 1442 | 291 | 59177 | 7.7 | 36.6 | 6.0 | 10.7 (10.0) |
| Akron, OH | 2000–2003 | 1430 | 111 | 20816 | 12.0 | 29.2 | 7.3 | 10.1 (10.1) |
| Erie, PA | 2000–2003 | 910 | 100 | 11054 | 8.9 | 32.9 | 7.4 | 10.1 (9.8) |
| Pittsburgh, PA | 2000–2003 | 1356 | 315 | 67755 | 9.4 | 32.4 | 7.3 | 11.0 (9.8) |
| Harrisburg, PA | 2000–2003 | 1329 | 102 | 11326 | 9.2 | 32.9 | 6.2 | 11.9 (9.7) |
| Philadelphia, PA | 2000–2003 | 1412 | 313 | 64819 | 8.5 | 30.0 | 6.8 | 13.5 (9.6) |
| Boston, MA | 2000–2004 | 1583 | 338 | 36153 | 10.6 | 24.9 | 7.0 | 11.0 (9.5) |
Communities sorted from west to east.
No. days varies by species; determined as no. days when at least one species was measured.
Percent of nonaccidental deaths.
TABLE 2.
Seasonal PM2.5 Concentrations by Community
| Community | Seasonal PM2.5, (μ g/m3) Mean (SD)
|
|||
|---|---|---|---|---|
| Winter | Spring | Summer | Fall | |
| Seattle, WA | 12.0 (6.9) | 7.6 (3.6) | 7.6 (3.3) | 12.6 (7.7) |
| Sacramento, CA | 19.8 (14.6) | 6.7 (2.9) | 8.5 (4.1) | 15.4 (12.1) |
| Fresno, CA | 33.4 (20.5) | 11.1 (6.4) | 11.1 (5.5) | 24.4 (17.8) |
| Bakersfield, CA | 34.4 (24.2) | 12.5 (6.3) | 13.1 (5.2) | 25.3 (18.8) |
| Los Angeles, CA | 23.3 (13.5) | 17.2 (10.1) | 18.0 (6.5) | 24.6 (14.8) |
| Riverside, CA | 23.3 (15.3) | 27.6 (17.9) | 26.0 (10.6) | 32.1 (20.2) |
| San Diego, CA | 18.1 (10.1) | 12.1 (5.8) | 13.2 (4.1) | 17.4 (13.3) |
| El Paso, TX | 12.0 (7.8) | 9.7 (5.2) | 9.0 (3.9) | 10.3 (5.3) |
| Dallas, TX | 11.9 (5.7) | 12.4 (5.4) | 13.9 (6.1) | 12.4 (6.6) |
| Houston, TX | 11.7 (4.8) | 13.0 (4.3) | 13.8 (5.7) | 13.6 (7.5) |
| Beaumont, TX | 9.6 (4.5) | 10.9 (3.8) | 12.8 (6.1) | 12.5 (9.3) |
| Kansas City, MO | 13.8 (6.7) | 12.0 (6.4) | 13.3 (6.2) | 10.9 (5.5) |
| Minneapolis, MN | 12.4 (7.9) | 9.7 (6.3) | 10.3 (6.0) | 9.5 (6.0) |
| St. Louis, MO | 14.5 (6.7) | 13.2 (6.2) | 16.7 (7.8) | 14.0 (7.9) |
| Dayton, OH | 14.8 (7.0) | 14.0 (6.1) | 20.5 (10.1) | 14.6 (7.6) |
| Toledo, OH | 16.4 (8.1) | 13.9 (7.0) | 16.3 (9.4) | 13.5 (8.1) |
| Detroit, MI | 17.3 (9.0) | 15.6 (8.8) | 17.4 (10.0) | 14.6 (8.7) |
| Columbus, OH | 16.6 (7.7) | 14.4 (6.1) | 19.7 (10.2) | 15.6 (8.4) |
| Cleveland, OH | 16.5 (8.5) | 16.0 (8.1) | 19.5 (10.2) | 15.7 (9.3) |
| Akron, OH | 15.8 (7.4) | 14.1 (6.3) | 19.8 (10.1) | 15.1 (8.4) |
| Erie, PA | 12.7 (6.7) | 12.1 (6.2) | 18.0 (10.7) | 11.4 (7.4) |
| Pittsburgh, PA | 13.6 (6.5) | 12.9 (6.0) | 20.9 (10.8) | 13.9 (7.4) |
| Harrisburg, PA | 16.5 (10.8) | 13.7 (8.0) | 19.4 (10.7) | 14.1 (8.6) |
| Philadelphia, PA | 16.2 (8.5) | 12.6 (7.0) | 18.3 (11.5) | 12.8 (7.4) |
| Boston, MA | 14.8 (6.5) | 12.0 (5.8) | 14.8 (8.2) | 11.5 (6.1) |
FIGURE 1.
Season-specific PM2.5 concentrations by community.
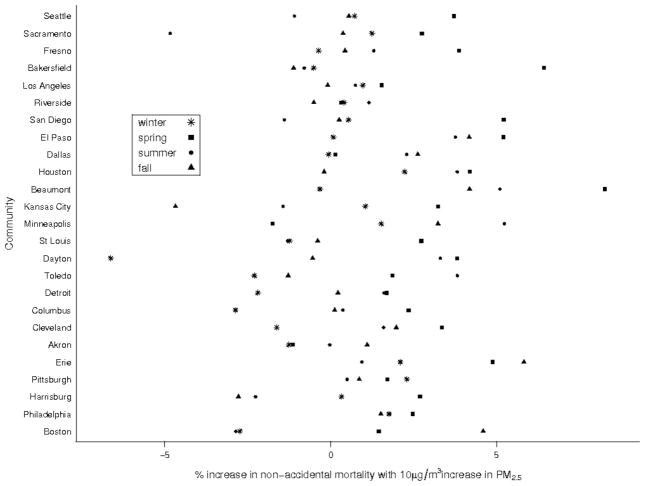
Season-specific Poisson regression estimates for non-accidental mortality by community are shown in Figure 2. Larger effects were seen in spring in western communities and in spring and summer in central communities; in the east there was no distinct pattern. The larger effects in spring in California did not coincide with the highest PM2.5 concentrations, which typically occurred in winter. Similarly, the largest effects in the east were not observed in summer when PM2.5 concentrations peaked.
FIGURE 2.
Season-specific Poisson regression effect estimates by community.
The results of the meta-analysis of these estimates are shown in Tables 3 and 4. Associations between PM2.5 concentrations and nonaccidental mortality (0.74% [95% CI =0.41%–1.07%]), cardiovascular mortality (0.47% [0.02%–0.92%]), and stroke related mortality (0.68% [−0.21% to 1.57%]) were observed with 2-day averaged PM2.5 concentrations (over the day of and the day before death). Respiratory deaths were associated with 2-day averaged PM2.5 concentrations for the 2 days before death (1.01% [−0.03% to 2.05%]). Significant heterogeneity among the community-specific effect estimates was found (P ≤ 0.05) for nonaccidental and respiratory mortality; the estimates for cardiovascular and stroke mortality did not exhibit significant heterogeneity (P = 0.38 and P = 0.91, respectively). A distinct difference in the pooled effect estimates was seen by season and geographic region. The association between nonaccidental mortality and 2-day averaged PM2.5 concentration was higher in spring (1.88% [1.29%–2.48%]) and summer (0.99% [0.31%–1.68%]) than winter or fall. It was also lower in the western region (0.51% [0.10%– 0.92%]) than the rest of the country (0.92% [0.44%–1.39%]).
TABLE 3.
Estimated Percent Increase in Daily Mortality With a 10-μg/m3 Increase in PM2.5
| Cause of Death | Exposure Lag | Effect (95% CI) | Heterogeneity Q (P) |
|---|---|---|---|
| Nonaccidental | 2 d (0,1) | 0.74 (0.41–1.07) | 130.22 (0.02) |
| Cardiovascular | 2 d (0,1) | 0.47 (0.02–0.92) | 102.63 (0.38) |
| Respiratory | 2 d (1,2) | 1.01 (−0.03–2.05) | 123.99 (0.05) |
| Stroke | 2 d (0,1) | 0.68 (−0.21–1.57) | 80.98 (0.91) |
TABLE 4.
Seasonal and Regional Estimates of the Percent Increase in Nonaccidental Daily Mortality With a 10-μg/m3 Increase in PM2.5
| Stratum | Effect (95% CI) |
|---|---|
| Season | |
| Winter | 0.15 (−0.42 to 0.72) |
| Spring | 1.88 (1.29 to 2.48) |
| Summer | 0.99 (0.31 to 1.68) |
| Fall | 0.19 (−0.25 to 0.64) |
| Region | |
| West | 0.51 (0.10 to 0.92) |
| East and central | 0.92 (0.44 to 1.39) |
The smaller number of deaths attributed to specific causes reduced the statistical power to detect an association of PM2.5 with respiratory and stroke-related mortality. Although an association was found between PM2.5 and cardiovascular mortality, there was insufficient heterogeneity in the effect estimates among communities to examine effect modification in the second stage. Thus, only the estimates of the association between nonaccidental mortality and PM2.5 were examined for effect modification by particle composition in the second stage.
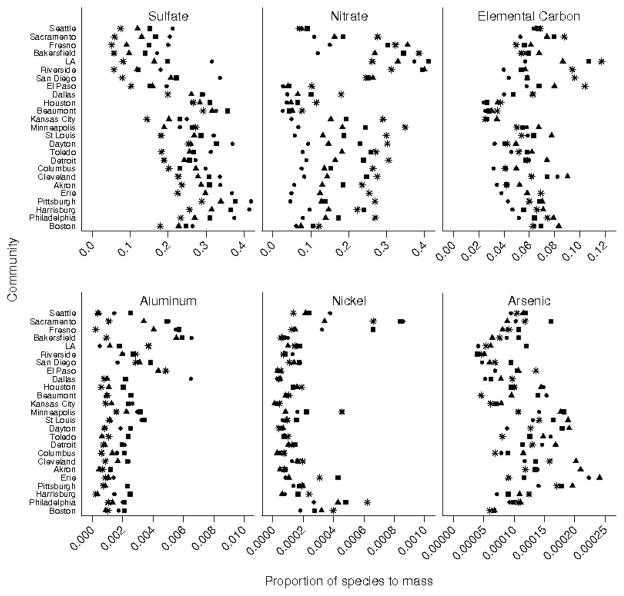
The species examined include elements, ions, elemental carbon, and organic carbon (Table 5). Average species proportions were calculated by community and season, and selected proportions are illustrated in Figure 3; all seasonal proportions are in eTable 1. Correlations between seasonally averaged species proportions (eTable 2) showed strong similarities to the correlations between unaveraged species proportions (eTable 3). Communities with similar seasonal patterns (eg, Dayton and Columbus, OH) had similar subseasonal temporal patterns (day of the week and month) in the species proportions (data not shown).
TABLE 5.
Effect Modification of Composition on the Estimated Percent Increase in Nonaccidental Mortality With a 10-μg/m3 Increase in PM2.5
| Model | Species | P for Effect Modification by Species to PM2.5 Mass Proportion | % Increase in Nonaccidental Mortality Per 10-μg/m3 Increase in PM2.5 for an Interquartile Increase in Species to PM2.5 Mass Proportiona | Heterogeneity Explained (%)b |
|---|---|---|---|---|
| Univariate | Aluminum | <0.001 | 0.58 | 45 |
| Arsenic | 0.02 | 0.55 | 35 | |
| Bromine | 0.11 | 0.38 | 5 | |
| Chromium | 0.12 | 0.33 | 16 | |
| Elemental carbon | 0.79 | 0.06 | 0 | |
| Iron | 0.43 | 0.12 | 3 | |
| Potassium | 0.10 | 0.41 | 28 | |
| Manganese | 0.42 | 0.14 | 10 | |
| Sodium ion | 0.22 | 0.20 | 14 | |
| Nickel | 0.01 | 0.37 | 41 | |
| Nitrate | 0.07 | −0.49 | 28 | |
| Ammonium ion | 0.84 | 0.04 | 3 | |
| Organic carbon | 0.59 | −0.02 | 4 | |
| Lead | 0.31 | 0.17 | 11 | |
| Silicon | 0.03 | 0.41 | 25 | |
| Sulfate | 0.01 | 0.51 | 33 | |
| Vanadium | 0.28 | 0.30 | 3 | |
| Zinc | 0.19 | 0.23 | 15 | |
| Multivariate model 1c | Aluminum | <0.001 | 0.79 | |
| Nickel | 0.01 | 0.34 | 100 | |
| Sulfate | <0.001 | 0.75 | ||
| Multivariate model 2c | Aluminum | <0.001 | 0.61 | |
| Nickel | 0.01 | 0.35 | 100 | |
| Arsenic | <0.001 | 0.58 |
Adjusted for temperature.
Includes heterogeneity explained by temperature.
These models include the 3 species listed.
FIGURE 3.
Season-specific proportions of species to PM2.5 mass for 6 selected species by community. Key for season as in Figure 2.
Sulfate proportions were consistently highest in summer in the eastern communities of Pennsylvania (Pittsburgh, Philadelphia, Harrisburg) and Ohio (Akron, Cleveland, and Dayton), but were lowest in California in winter. The highest proportions of nitrate occurred in California with no distinct seasonal pattern, although in eastern communities it peaked in winter. Elemental carbon was also highest in California, particularly Los Angeles in winter. Aluminum proportions were highest in arid communities of Houston, Dallas, Bakersfield, and Fresno, peaking in spring and summer. Nickel proportions were highest in Sacramento and Fresno in spring and summer, but in the east, were highest in Boston and Philadelphia in winter. Across all communities, there was a greater amount of variation in the seasonal proportions of elements than in carbonaceous species. The averaged coefficients of variation over all seasons and communities for aluminum, nickel, silicon, nitrate, and sulfate were 1.8, 1.4, 0.9, 0.6, and 0.4, respectively, whereas it was 0.5 for both elemental carbon and organic carbon proportions (eTables 5 and 6).
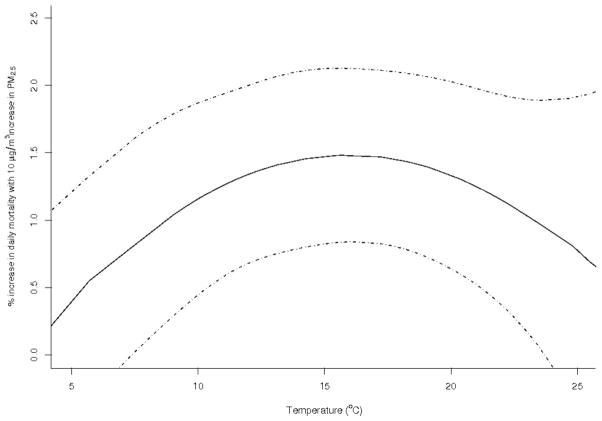
Figure 4 illustrates the relationship between the Poisson regression effect estimates and temperature; this substantiates the use of temperature as a surrogate for ventilation in the meta-regression. When temperatures were moderate (10°C–25°C) the estimated effect estimates for the association between nonaccidental mortality and PM2.5 were above the average effect, whereas at low and high temperatures outside of this range they were below average. The inverted U-shape of this curve shows that the use of a quadratic term for temperature was appropriate.
FIGURE 4.
Relationship between the PM2.5-mortality effect estimates and temperature (a surrogate for ventilation).
Results of effect modification by species-to-mass proportion (after controlling for temperature) are shown in Table 5. For single-species proportions, the association between PM2.5 and nonaccidental mortality was significantly (P ≤ 0.05) modified by aluminum, arsenic, sulfate, silicon, and nickel. For an IQR increase in the proportion of these chemicals, the increase in nonaccidental mortality associated with a 10-μg/m3 increase in PM2.5, was 0.58%, 0.55%, 0.51%, 0.41%, and 0.37%, respectively. Also, the effect of sulfate was similar in the west (IQR = 0.81% [P = 0.06]) and east (0.76% [0.18]). Including a combination of species proportions and performing a backward elimination resulted in aluminum, sulfate, and nickel remaining statistically significant (P ≤ 0.05) (Table 5). The IQR of both sulfate and aluminum were larger in the multispecies model. Replacing sulfate with arsenic in this multispecies meta-regression resulted in a statistically significant combination of species proportions; however, the IQR for arsenic was smaller than that of sulfate. The correlations between the species used in the multispecies model were low (eTables 3 and 4).
The heterogeneous variance component quantified the extent to which an effect modifier explained any residual variation in the PM2.5-mortality effect estimates over the 25 communities (Table 5). The quadratic function of temperature alone explained 10% of the residual heterogeneity. Sulfate alone explained 16% of the residual heterogeneity; sulfate in combination with temperature explained 33% of the residual heterogeneity. Of all species examined, aluminum and nickel explained the most residual heterogeneity in the effect estimates (22% alone and 45% in combination with temperature). After including temperature and at least one of the species proportions, the remaining heterogeneity was not statistically significant. In the multispecies meta-regression that included temperature, aluminum, nickel, and sulfate, 100% of the residual heterogeneity was explained. There was no distinct spatial pattern in the residuals from this model, indicating that the model predicted well in all regions. The residuals were highest in Erie, PA; Beaumont, TX; and Minneapolis, MN suggesting it did not fit as well in these specific locations.
The community-specific prevalence of central air conditioning (AC) did not affect the meta-regression. Of the socioeconomic parameters examined, only median household income had a significant meta-regression coefficient. However, it did not confound with the individual species; the effect modification of arsenic and sulfate was reduced slightly (from IQR = 0.55% to IQR = 0.48% for arsenic and from IQR = 0.51% to IQR = 0.41% for sulfate). When included in the multispecies meta-regression, it had no effect on the species proportions.
DISCUSSION
We examined the role of particle composition in the association between PM2.5 mass and mortality in a large population-based study of 25 communities across the United States, including over 1.3 million deaths. Single species and combinations of species proportions were used to explain the extent to which the PM2.5 mass-mortality association was modified based on the composition of the mass. The rationale for using species proportions as effect modifiers was that in the first stage of the analysis, the mortality risk was estimated per unit of the total PM2.5 mass. This encompassed all measured species, and therefore it would not be meaningful to use the species concentrations directly as the effect modifier.
We found proportions of aluminum, sulfate, and nickel alone and in combination to be major modifiers of the PM2.5 mass-mortality association. Arsenic and silicon also contributed individually to the modification of the estimated effect. Seasonal temperature, used as a surrogate for ambient particle penetration into the indoor environment, played an important role in explaining some of the between-community heterogeneity in the PM2.5-mortality effect estimates. The difference in the relationship between population exposure and outdoor concentrations needs to be taken into account when comparing risk across communities. For instance, the pooled PM2.5 mass effect was highest in spring and lowest in winter and fall. With ventilation likely peaking in the spring months, this result was expected. We also observed, despite higher PM2.5 mass concentrations, a lower effect estimate in the west than the east. Ventilation likely also contributed to this regional difference as homes are generally newer in the west and therefore allow less ambient air inside due to more tightly sealed windows and doors. Metrics such as community-specific AC prevalence have been used in previous studies.17,20 In this study, AC did not affect the meta-regression, suggesting that a quadratic function of seasonal average temperature was a superior surrogate. As AC is used only on days with high temperatures, it becomes irrelevant on the rest of the days of the year. In multicommunity studies, season alone may also be a poor surrogate for ventilation. For example, in cities with harsh winters and mild summers, ventilation is higher in summer than winter. The pattern is reversed for communities with hot summers and mild winters. Failure to control for these factors could result in confounding of the species proportions.
Single species are markers for more complex particle chemistry. One example is sulfate that is a secondary pollutant associated with multiple sources. Some studies have tackled the complexity of particle chemistry through the use of source apportionment; we chose not to use this approach. Sources identified by apportionment may not exhibit the same chemical profile in all communities and, thus, would not naturally allow for comparisons of health effects across communities. Source emission characteristics can vary widely depending on season and geographic location. It would be meaningless to determine effect modification by an exposure metric that did not hold the same meaning for all communities. Instead, we examined combinations of species and found that proportions of aluminum, sulfate, and nickel played the strongest role in modifying the PM2.5-mortality effect estimate. Notably, the effect modification of aluminum and sulfate was stronger in combination than as single species. To relate this combination to markers of specific sources, the proportion of sulfate was replaced by the proportion of arsenic, an element that has been used as a tracer of coal emissions. Arsenic was a significant modifier of the PM2.5-mortality association when examined alone and in the meta-regression with nickel and aluminum. This combination could represent 3 potential sources: coal combustion, oil combustion, and road dust. In the backwards elimination, which initially included the species proportions that were at least marginally statistically significant, nitrate dropped out as an explanatory factor. This suggests that the modification by the proportion of nitrate may be a surrogate for a low proportion of sulfate in PM2.5.
In examining the species proportions that had a greater impact on mortality, we found aluminum, originating mostly from windblown soil and road dust, was particularly high in arid climates and in summer and spring in communities in Texas (El Paso and Beaumont), and regions of California (Bakersfield, Fresno, and Sacramento). This corresponded with higher PM2.5 mass-mortality effects in Beaumont, Bakersfield, Fresno, and Sacramento. Silicon, from similar sources was highly correlated with aluminum and, although not as strong, was also a significant modifier of the PM2.5-mortality association. There is toxicologic evidence of a pulmonary biologic response in animals and humans for both aluminum and silicon.22,23
Sulfate has been associated with increases in mortality in previous epidemiologic studies,6,7 including the study by Mar et al,7 that found increased total and cardiovascular mortality associated with a regional sulfate factor in Phoenix. This suggests that the impact of sulfate is not only an East Coast phenomenon. Our data support this conclusion. Schlesinger24 noted that many toxicologic studies of sulfate have not found an important biologic response, although O’Neill et al25 found an association between sulfate and endothelial dysfunction, and Chuang et al26 found sulfate increased oxidative stress and coagulation in a panel study. The positive sulfate effects observed in epidemiologic studies (including ours) may be attributable to the greater complexity of ambient air than the sulfate used in toxicologic studies. For example, acid sulfate in the form of sulfuric acid or ammonium bisulfate can convert insoluble metal oxides to bioavailable sulfate salts. Another possibility is that fine-particle sulfate has a long life in the atmosphere and can coincide with high concentrations of photochemically produced pollutants from other sources, especially vehicular and biogenic emissions, which contain a large spectrum of potentially toxic organic compounds.
Previous epidemiologic evidence of a nickel-mortality association has been observed.6,27 Nickel is a marker of oil combustion emissions and a constituent of residual oil fly ash. Although this ash is frequently linked with occupational exposures, general population exposure to nickel is likely from industrial combustion sources that emit metal-rich particles (power plants, some smelters, oil based domestic heating, and ship emissions). Toxicologic studies indicate an inflammatory response in human lung cells28 and acute changes in heart rate and heart rate variability in atherosclerotic-prone mice associated with nickel particles.29
Data from the STN have been used in several studies of the chemistry and sources of PM2.530,31 but not in any large population-based studies of health effects associated with PM2.5 composition. The sparseness of speciation data due to STN sampling schedules greatly reduces the power to detect health effects associated with particle species concentrations. Had our analysis been limited to the days when speciation monitors were operating, the resultant ~75% reduction in the dataset would have substantially reduced our power to detect an association between PM2.5 mass and mortality, as well precluded the use of multiday exposure lags, which had the strongest association with mortality. Moreover, examining the direct effect of specific particle species is effectively examining an interaction of mass with composition, and interaction terms generally have much less power than main effects unless the interaction is extremely large. It was because of these limitations that we chose a hierarchical approach.
Although there may be some exceptions,32,33 PM2.5 mass, used in the first stage, is relatively homogeneous over the scale of a community. On the other hand, we recognize that certain species used in the meta-regression could have had more spatial variability than others. Those that are heterogeneous at a small spatial scale may exhibit exposure measurement error because of within-community variability, according to Ito et al,34 who found that correlations between daily concentrations of arsenic, elemental carbon, and nickel were moderate to low between closely located STN monitors in the New York City area. This spatial variability may play a role in the ability to characterize their health effects. In our study, limitations of the STN and availability of health data at the county level precluded assessment of within-community variability. We focused on examining the effects between communities and reduced the potential effects of measurement error by averaging the species proportions over each season and over multiple years. To address the possibility that outcomes might have been different had we used daily species proportions, we examined subseasonal temporal patterns and correlations between seasonally averaged and un-averaged species ratios. These analyses indicate that our approach adequately represented the predominant temporal variability of the species proportions and increased confidence that the seasonal average was an appropriate metric.
Another consideration is too little variability in the species proportions among communities (seasonally or spatially), making it difficult to detect effect modification of the PM2.5-mortality association. The proportions of elemental and organic carbon to PM2.5 mass had less variability across the 25 communities, and their small coefficients of variation could in part explain why no effect modification was seen.
In summary, this study has shown that certain chemical species significantly modify the association between PM2.5 and mortality. This illustrates that mass alone is not a sufficient metric when evaluating health effects of PM exposure. With knowledge of the differential health effects of particles, more targeted regulation and control are possible.
Supplementary Material
Acknowledgments
Supported by The US EPA Harvard University PM Center grant R832416-010 and NIEHS grant ES-00002.
Footnotes
Supplemental material for this article is available with the online version of the journal at www.epidem.com; click on “Article Plus.”
References
- 1.Dominici F, McDermott A, Daniels M, et al. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: mortality among residents of 90 cities. J Toxicol Environ Health A. 2005;68:1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- 2.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17:279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 3.Cifuentes L, Vega J, Kopfer K, et al. Effect of the fine fraction of particulate matter versus the coarse mass and other pollutants on daily mortality in Santiago, Chile. J Air Waste Manag Assoc. 2000;50:1287–1298. doi: 10.1080/10473289.2000.10464167. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc. 1996;46:927–939. [PubMed] [Google Scholar]
- 5.National Research Council. Continuing Research Progress. Washington DC: National Academy Press; 2004. Research Priorities for Airborne Particulate Matter. IV. [Google Scholar]
- 6.Laden F, Neas LM, Dockery DW, et al. Association of fine particulate matter from different sources with daily mortality in six US cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mar TF, Norris GA, Koenig JQ, et al. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostro B, Feng W-Y, Broadwin R, et al. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2007;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Air Quality System Technology Transfer Network [database online] Research Triangle Park, NC: US Environmental Protection Agency; 2007. Updated December 19, 2007. [Google Scholar]
- 10.National Climatic Data Center [database online] Asheville NC: National Oceanic and Atmospheric Administration; 2007. Updated May 2, 2007. [Google Scholar]
- 11.Schwartz J. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environ Health Perspect. 2000;108:563–568. doi: 10.1289/ehp.00108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braga A, Zanobetti A, Schwartz J. The lag structure between particulate air pollution and respiratory and cardiovascular deaths in 10 US cities. J Occup Environ Med. 2001;43:927–933. doi: 10.1097/00043764-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Zanobetti A, Schwartz J, Samoli E, et al. The temporal pattern of mortality responses to air pollution: a multi-city assessment of mortality displacement. Epidemiology. 2002;13:87–93. doi: 10.1097/00001648-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. Educ Behav Stat. 2005;30:261–293. [Google Scholar]
- 16.Koutrakis P, Suh HH, Sarnat JA, et al. Characterization of particulate and gas exposures of sensitive subpopulations living in Baltimore and Boston. Res Rep Health Eff Inst. 2005:1–65. discussion 67–75. [PubMed] [Google Scholar]
- 17.Janssen NAH, Schwartz J, Zanobetti A, et al. Air conditioning and source-specific particles as modifiers of the effect of PM10 on hospital admissions for heart and lung disease. Environ Health Perspect. 2002;110:43– 49. doi: 10.1289/ehp.0211043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Housing Survey [database online] Washington, DC: Department of Housing and Urban Development; 2007. Updated July 16, 2007. [Google Scholar]
- 19.Finkelstein MM, Jerrett M, DeLuca P, et al. Relation between income, air pollution and mortality: a cohort study. CMAJ. 2003;169:397– 402. [PMC free article] [PubMed] [Google Scholar]
- 20.Levy JI, Hammitt JK, Spengler JD. Estimating the mortality impacts of particulate matter: What can be learned from between-study variability? Environ Health Perspect. 2000;108:109–117. doi: 10.1289/ehp.00108109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.State and County Quickfacts [database online] Washington, DC: US Census Bureau; Updated October 22, 2007. [Google Scholar]
- 22.Clarke RW, Coull B, Reinisch U, et al. Inhaled concentrated ambient particles are associated with hematologic and bronchoalveolar lavage changes in canines. Environ Health Perspect. 2000;108:1179–1187. doi: 10.1289/ehp.001081179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker S, Dailey LA, Soukup J, et al. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Perspect. 2005;113:1032–1038. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlesinger RB. The health impact of common inorganic components of fine particulate matter (PM2.5) in ambient air: A critical review. Inhal Toxicol. 2007;19:811– 832. doi: 10.1080/08958370701402382. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 26.Chuang KJ, Chan CC, Su TC, et al. Urban air pollution on inflammation, oxidative stress, coagulation and autonomic dysfunction. Am J Respir Crit Care Med. 2007;176:370–377. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 27.Burnett RT, Brook J, Dann T, et al. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal Toxicol. 2000;12(suppl 4):15–39. doi: 10.1080/08958370050164851. [DOI] [PubMed] [Google Scholar]
- 28.Gao F, Barchowsky A, Nemec A, et al. Microbial stimulation by Mycoplasma fermentans synergistically amplifies IL-6 release by human lung fibroblasts in response to residual oil fly ash (ROFA) and nickel. Toxicol Sci. 2004;81:467– 479. doi: 10.1093/toxsci/kfh205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippmann M, Ito K, Hwang JS, et al. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu SH, Paisie JW, Jang BWL. PM data analysis–a comparison of two urban areas: Fresno and Atlanta. Atmos Environ. 2004;38:3155–3164. [Google Scholar]
- 31.Lee JH, Hopke PK. Apportioning sources of PM2.5 in St. Louis, MO using speciation trends network data. Atmos Environ. 2006;40:S360–S377. [Google Scholar]
- 32.Burton RM, Suh HH, Koutrakis P. Spatial variation in particulate concentrations within metropolitan Philadelphia. Environ Sci Technol. 1996;30:400– 407. [Google Scholar]
- 33.Jerrett M, Burnett RT, Ma RJ, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- 34.Ito K, Xue N, Thurston GD. Spatial variation of PM2.5 chemical species and source-apportioned mass concentrations in New York City. Atmos Environ. 2004;38:5269–5282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.