Abstract
Background
A major portion of influenza disease burden during the 2009 pandemic was observed among young people.
Methods
We examined the effect of age on the transmission of influenza-like illness associated with the 2009 pandemic influenza A (H1N1) virus (pH1N1) for an April–May 2009 outbreak among youth-camp participants and household contacts in Washington State.
Results
An influenza-like illness attack rate of 51% was found among 96 camp participants. We observed a cabin secondary attack rate of 42% (95% confidence interval = 21%–66%) and a camp local reproductive number of 2.7 (1.7–4.1) for influenza-like illness among children (less than 18 years old). Among the 136 contacts in the 41 households with an influenza-like illness index case who attended the camp, the influenza-like illness secondary attack rate was 11% for children (5%–21%) and 4% for adults (2%–8%). The odds ratio for influenza-like illness among children versus adults was 3.1 (1.3–7.3).
Conclusions
The strong age effect, combined with the low number of susceptible children per household (1.2), plausibly explains the lower-than-expected household secondary attack rate for influenza-like illness, illustrating the importance of other venues where children congregate for sustaining community transmission. Quantifying the effects of age on pH1N1 transmission is important for informing effective intervention strategies.
Symptomatic infection with the 2009 pandemic influenza A (H1N1) virus (pH1N1) was first reported in Mexico City in late April 2009,1 and in the United States soon thereafter,2,3 with a pandemic declared in June 2009.4 A large proportion of seasonal influenza transmission typically occurs among school-aged children and young adults.5–7 Evidence suggests a similar pattern of age-specific transmission for pH1N1.8–10 Before July 2009, in the United States, the incidence of pH1N1 was 6 times greater among people 0–24 years of age than among those over 24 years.9 During the initial outbreak in Mexico, children younger than 15 years of age were at 2.1 times (95% confidence interval [CI] = 1.9–2.4) greater risk of clinical influenza than older persons.10 Differences in the incidence of pH1N1 by age are important for planning appropriately targeted intervention strategies in limited-resource settings.
Evidence points to households and schools as important venues for facilitating community transmission of seasonal6,11–13 and pandemic influenza viruses.8,14–17 Despite this evidence, recent estimates of the transmissibility of pH1N1 within households are not as high as observed during previous influenza epidemics and pandemics.17 Accumulating evidence suggests that the age structure of households may play a key role in influenza transmission within this venue.8,18 In contrast, estimates of the transmission potential of pH1N1 in medium-sized, single-age-group populations indicate that the pathogen transmits efficiently in settings where children are in frequent close contact, such as schools.15,17 A comparison of the transmissibility of pH1N1 within households and school-like settings serving the same population of individuals would help distinguish the relative importance of these venues in community transmission.
Investigations of disease outbreaks provide opportunities to observe transmission of a pathogen in closed populations, often in the absence of intervention. Outbreaks of pH1N1 in the US have been reported for a summer camp,19 households,2,3,8,20–24 and schools.15,17,23,25,26 The CDC provides guidelines for the management of outbreaks of pH1N1 in many settings, including camps.27 This report describes the transmission of pH1N1 among the participants of a youth camp and their household contacts, as well as the effect of age on susceptibility to symptomatic disease. The data were collected for a Public Health—Seattle and King County investigation of an April–May 2009 outbreak in Washington State, in collaboration with the US Centers for Disease Control and Prevention (CDC).
Methods
Study Population
From the 25th through the 30th of April, sixth-grade students from 4 local schools attended a youth wilderness education camp. Camp staff randomly assigned the sixth-grade students by sex, to sleep in 1 of 10 student cabins. High-school-aged counselors slept in student cabins as chaperones, and school faculty and staff lodged either in staff housing or off campus. By 7 May, a large proportion of campers and staff became ill with symptoms associated with pH1N1 infection (ie, fever, feverishness, sore throat, and cough). Five of these suspected pH1N1 cases were subsequently confirmed by reverse transcription polymerase chain reaction.
Data Collection
A retrospective survey of the study population was conducted to identify history of illness and potential risk factors for symptomatic pH1N1. The parents of the sixth-grade students received e-mail invitations from their child's school to complete a questionnaire either online or on paper. School faculty, camp staff, and high-school-aged counselors completed the surveys through in-person or phone interviews with the CDC field team. Survey forms and e-mail invitations were sent out to parents between May 18 and 20; data collection was completed by June 9.
Survey respondents (camp participants) were asked to provide their age; assigned sleeping cabin; and symptom histories, including onset dates, for fever, feverishness, cough, sore throat, diarrhea, difficulty breathing, runny nose, and vomiting. For respondents reporting an illness between 19 April and 15 May, we requested histories for the same symptoms for up to 8 additional household contacts, as well as the amount of time spent by each contact in the household during this period.
The CDC investigators entered the information from the paper survey forms into a digital format. Prior to analysis, all information that could identify the participants was removed. Data analysis was conducted using Stata v10× (Stata Corporation, College Station, TX) and TranStat (https://www.epimodels.org/midas/transtat.do).28 This activity was reviewed by the Institutional Review Boards (IRBs) of Washington State, the CDC, and the Fred Hutchinson Cancer Research Center and determined to be a public health response not requiring IRB approval. Participants indicated informed consent by completing paper and Internet-based survey forms and through verbal consent during in-person or telephone interviews.
Statistical Analysis
Symptomatic pH1N1
Individuals with an influenza-like symptom profile but no laboratory confirmation of pH1N1 infection were assumed to have been infected. To address uncertainty in the etiology of unconfirmed illnesses, we considered 6 case definitions (I-VI) for the influenza-like symptom profile (Table 1). The primary clinical outcome (case definition I), influenza-like illness, was defined as the appearance, within a 7-day period, of at least one member of each of the following pairs of symptoms: (1) fever or feverishness and (2) cough or sore throat. Case definition I is similar to the CDC-surveillance definition for influenza-like illness.29
Table 1. Six Case Definitions for Clinical 2009 Pandemic Influenza A (H1N1) Among the Participants of a Youth Camp and Their Household Contacts During an April–May 2009 Outbreak in Washington State, United States.
| Case Definition | Symptoms |
|---|---|
| Ia | Reported fever or feverishness and cough or sore throatb |
| II | At least one of the following symptoms: reported fever, feverishness, cough, sore throat, diarrhea, difficulty breathing, runny nose, or vomiting |
| III | Reported fever or feverishness |
| IV | Reported fever with measured temperature ≥100.4°F (38°C) |
| V | Reported fever and cough or sore throatb |
| VI | Reported fever with measured temperature ≥100.4°F (38°C) and cough or sore throatb |
Influenza-like illness, similar to the CDC-surveillance definition.29
The onset of fever or feverishness must occur within 7 days of the onset of cough or sore throat.
For each case definition, illness onset was defined as the earliest date on which an included symptom appeared. We considered camp participants with illness-onset dates prior to their second day at the camp as the camp index cases. Ill camp participants and household members with concurrent or earlier symptom-onset dates were considered the index cases for their households.
A case is assumed to become infectious at illness onset (ie, the incubation and latent periods are equal), and the length of the incubation period is assumed to follow a discrete uniform distribution ranging from 1 to 3 days, with a mean of 2 days. The length of a case's infectious period is assumed to follow a discrete uniform distribution and range from 3 to 7 days, with a mean of 5 days.28 Each person is assumed to be susceptible to symptomatic disease until onset of illness.
Outbreak Periods
The camp outbreak period (25 April–7 May) encompasses the illness onset dates of all camp participants. Participants are assumed to have returned home on the last day of reported attendance; the camp closed on 30 April. Each household outbreak covers a 10-day period (maximum length of the incubation + infectious periods) beginning with the illness onset date of the household index case or cases.
Person-to-person Transmission
We estimated the transmissibility of pH1N1 as a transmission probability, that is, the likelihood of becoming a case per potentially-infectious contact. One type of potentially-infectious contact is defined for each of the 3 groups (1): all camp participants during the daytime, (2) cabin mates during the nighttime, and (3) household members during each day, with the respective transmission probabilities defined as p1, p2 and p3 (Table 2). A potentially infectious contact is defined as a contact between a susceptible individual and an infectious case. We assume random mixing within each group.
Table 2. Transmission Probabilities Included in the Statistical Model With the Corresponding Epidemiologic Measures of Transmissibility.
| Type of Transmission | Transmission Group | Time of the Day | Time Perioda | Transmission Probability | Epidemiologic Measure |
|---|---|---|---|---|---|
| Person-to-Person | Camp participants | Day | Camp period | p1 | R |
| Cabin mates | Night | Camp period | p2 | Night cabin SAR | |
| Household members | Day and night | Household outbreak period | p3 | Household SAR | |
| Cabin mates | Day and night | Camp period | p4b | Cabin-mate SAR | |
| Community-to-personc | Camp participants | Day and night | Camp period | b1c | |
| Camp participants | Day and night | Postcamp period | b2 | ||
| Household members | Day and night | Household outbreak period | b3c |
The camp period was 25–30 April; the postcamp period was 1–7 May; and the household outbreak period extended 10 days from the date of illness onset of the index case.
1 − p4 = (1 − p1)(1 − p2).
b1 and b3 are assumed to be equal to 0 for the primary analysis.
R indicates camp local reproductive number; SAR, secondary attack rate.
A secondary attack rate is defined as the proportion of susceptible individuals who are exposed to an infectious case in a small group (eg, a household or cabin) and subsequently fall ill. We define the local reproductive number as the average number of cases expected to result from exposure to a typical case introduced into a group (eg, a camp). Both of these measures are specific to the types of venues where they are observed, and both capture the biologic characteristics of the virus as well as the nature of contact patterns within the venue. To provide epidemiologically relevant estimates of the transmissibility of pH1N1, the results of this analysis are presented as a local reproductive number and 2 secondary attack rates. Exposure is defined as participating in a potentially infectious contact. A camp local reproductive number, a nightly cabin secondary attack rate, and a household secondary attack rate are derived from p1, p2, and p3, respectively (see the eAppendix [http://links.lww.com/EDE/A445] for more detail). We calculated the combined probability of transmission due to contact between cabin mates within the camp during the daytime (p1) and the cabin during the nighttime (p2). A cabin-mate secondary attack rate was derived from the cabin-mate transmission probability, p4, a function of p1 and p2 (Table 2).
Community-to-person Transmission
Casual contact with people outside the camp or households is another potential source of exposure to infection. Because the dataset does not contain information concerning specific casual contacts, the analysis estimates average daily community-to-person transmission probabilities of disease due to exposure to unmeasured causal contacts (bx, where x differentiates separate bs; Table 2). For the estimated community-to-person transmission probabilities see the eAppendix (http://links.lww.com/EDE/A445).
While the camp was open, the camp population was assumed to be closed, meaning that exposure to disease from the outside community was assumed negligible (b1 = 0). Several camp participants fell ill after the camp closed, and these cases may have been exposed to other participants during the camp period. To avoid selection bias, these cases were included in the estimation of p1 and p2, and exposure to disease from the outside community during the 7-day postcamp period was assumed to be non-negligible (b2 ≥ 0). Because this outbreak occurred early in the pandemic and the household outbreak periods are short (10 days), we assumed that the community-to-person transmission probability for household contacts was negligible (b3 = 0).
Cabin Assignment
For camp participants who reported spending the night at the camp, but who did not report which cabin they slept in, we used a multiple-imputation approach30 to impute the missing cabin assignments, assuming that this information was missing at random.31 Six sixth-grade students (4 boys and 2 girls) did not report a cabin assignment, so they were each randomly assigned to 1 of the 5 student cabins allotted to others of the same sex. One female high-school-aged counselor did not report the cabin she slept in, so she was randomly assigned to 1 of 3 possible student cabins (available data excluded 2 cabins). We did not exclude these 7 camp participants from the analysis, because this could have led to a biased estimate of p2.
Age
The camp participants and the household contacts were categorized into 2 age groups: children (17 years or younger) and adults (18 years and older). Though the high-school-aged counselors were camp staff, the majority (7 of 8) was 17 or younger, slept in the same cabins as the sixth-grade students, and spent considerable time with the campers during the daytime. From a transmission standpoint, the high-school students spent the majority of their time with the sixth-grade students, and hence were placed into the same age group.
The effect of age group on susceptibility to symptomatic disease was estimated as an odds ratio comparing the transmission probability per contact among children versus adults. Due to the lack of adult index cases, we could not estimate the effect of age-group on infectiousness. If a person's age was unreported, the age group was assigned using the person's relationships with other members of the household (eg, father, mother, or sibling) and the ages of these members.
Statistical Model
To estimate the ps and bs, we used a maximum likelihood approach to fit a chain binomial model to the enumerated potentially-infectious contacts using a logit link function. Age group was the only covariate included in this model and was assumed to have the same effect on the susceptibility of both camp participants and household contacts, without regard to the source of exposure. Additional details are presented in the eAppendix (http://links.lww.com/EDE/A445) and elsewhere.28
Sensitivity Analysis
The sensitivity of the primary analysis (Scenario A) to several key assumptions was investigated through additional analytic scenarios (B-E). In Scenario B, we assumed no community-to-person exposure to disease for camp participants, both during and after the camp, and for household contacts (b1 = b2 = b3 = 0). In Scenario C, we assumed a common, nonzero level of community-to-person exposure to disease for camp participants during the 7 days after the camp period and for household contacts (b1 = 0, b2 = b3 ≥ 0). The sensitivity of results to assumptions about the infectious period was investigated by using a shorter, 2–7 day infectious period (mean of 3 days: Scenario D), and a longer, 5–8 day infectious period (mean of 6 days: Scenario E).
Results
Camp Transmission
A total of 111 sixth-grade students attended youth camp and 35 teachers and staff worked at the camp from 25–30 April. Of these, 65% (n = 72) of the sixth-grade students and 69% (n = 24) of the teachers and staff responded to the survey and were classified as camp participants (Table 3). Seventy-nine of the camp participants (82%) were 17 years or younger at the time of the survey. Table 3 provides further information about the camp participants.
Table 3. Description of an April–May 2009 Outbreak of 2009 Pandemic Influenza A (H1N1) Among the Participants of a Youth Camp and Their Household Contacts in Washington State, United States.
| Characteristic | Camp Participantsa (n = 96) | Household Contactsb (n = 136) |
|---|---|---|
| Type of respondent; no. (%) | ||
| Sixth-grade student | 72 (75) | |
| High school counselor | 8 (8) | |
| School faculty and camp staff | 16 (17) | |
| No. male: no. female | 38:58 | 63:64c |
| Age (years) | ||
| Mean (SD) | 16 (12) | 34d (18) |
| Median (minimum, maximum) | 12 (10, 59) | 41 (0.5, 74) |
| Age group | ||
| Children (≤17 years) | ||
| No. (% of all individuals) | 79e (82) | 48 (35) |
| Mean age in years (SD; minimum, maximum) | 12 (1; 10, 17) | 12 (4; 0.5, 17) |
| Adult (≥ 18 years) | ||
| No. (% of all individuals) | 17 (18) | 88 (65) |
| Mean age in years (SD; minimum, maximum) | 37 (17; 18, 59) | 46 (10; 18, 74) |
| No. cabins or households | 13f | 41 |
| No. of individuals per cabin or household; Mean (SD; minimum, maximum) | ||
| Total | 6.3 (2.8; 1, 10) | 3.3 (1.3; 1, 8) |
| Children | 7.2 (2.1; 4, 10) | 1.2g (0.8; 0, 3) |
| Adults | 3.0h (2.0; 1, 5) | 2.1 (0.7; 1, 5) |
Included are individuals who responded to the survey and were at the camp during the outbreak.
The contacts from the 41 households included in the primary analysis for case definition I are presented in this table.
Sex was not reported for 9 household contacts.
Two household contacts (1 adult and 1 child) were excluded because their ages were unreported.
Seven of the 8 high-school-aged counselors who responded to the survey were ≤17 years of age.
Three cabins housed only adults. Seven children and 7 adults are excluded here due to missing cabin assignments and not sleeping at the camp, respectively.
Eight households contain 0 child contacts.
One adult high-school-aged counselor is excluded, because this person slept in a cabin assigned to children.
SD indicates standard deviation.
Of the 72 responding sixth-grade students, 66 (92%) reported the cabin where they slept. Of the 24 responding staff, 17 (71%) reported spending nights at the camp; 7 of the 8 high-school-aged counselors reported in which student cabin they slept. The remaining 9 staff reported in which of 3 staff cabins they slept.
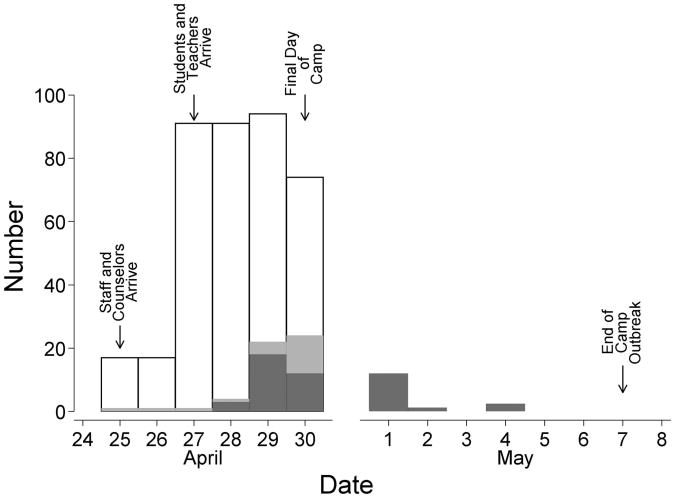
The number of participants at camp varied by day (Fig. 1). A total of 49 influenza-like illness cases were reported among the 96 camp participants, corresponding to an illness attack rate of 51% (Table 4). If the 50 camp participants who did not respond to the survey were all either noncases or cases of influenza-like illness, the lower and upper bounds of the attack rate would have been 34% and 68%, respectively.
Figure 1.
The number of students, teachers, and camp staff present (hollow bars) on each day of the camp period (25–30 April) during an April–May 2009 outbreak in Washington State, United States. The number of newly infectious influenza-ike illness cases (darker gray shaded bars) and influenza-like illness cases who are assumed to remain infectious (lighter gray shaded bars) among the participants. Because the camp closed on 30 April, only the number of newly infectious cases among camp participants is shown for the postcamp period (1–7 May).
Table 4. Clinical Cases of 2009 Pandemic Influenza A (H1N1) by Case Definition.
| Clinical Case Definition | Camp Outbreaka | Household Outbreakb | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Age | Age | ||||||
|
|
|
||||||
| Total (n = 96) AR (%)No. Cases | ≤17 years (n = 79) AR (%) No. Case s | ≥18 years (n = 17) AR (%) No. Cases | Total No. Cases/No. Contacts AR (%) | ≤17 years No. Cases/No. Contacts AR (%) | ≥18 years No. Cases/No. Contacts AR (%) | No. Households | |
| I | 49 (51) | 44 (56) | 5 (29) | 11/136 (8) | 7/48 (15) | 4/88 (5) | 41 |
| II | 66 (69) | 56 (71) | 10 (59) | 18/175 (10) | 10/65 (15) | 8/110 (7) | 54 |
| III | 57 (59) | 51 (65) | 6 (35) | 15/156 (10) | 10/56 (18) | 5/100 (5) | 48 |
| IV | 43 (45) | 40 (51) | 3 (18) | 9/132 (7) | 6/47 (13) | 3/85 (4) | 39 |
| V | 46 (48) | 42 (53) | 4 (24) | 11/127 (9) | 7/44 (16) | 4/83 (5) | 39 |
| VI | 36 (38) | 34 (43) | 2 (12) | 7/109 (6) | 5/38 (13) | 2/71 (3) | 32 |
There were 2 camp index cases for all case definitions except for case definition II for which there were 7.
Case definitions I, II, III, and V include 1 household with 2 camp participants who were both considered index cases.
AR indicates illness attack rate, expressed as a percent.
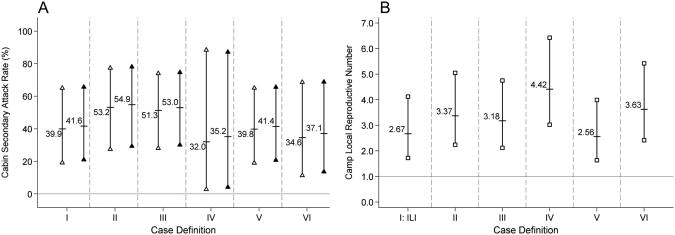
A nightly cabin secondary attack rate of 41% (95% CI = 22%–64%) was estimated for influenza-like illness (eTable 1, http://links.lww.com/EDE/A445), with an estimate of 40% (19%–65%) among the younger group (Fig. 2A). In the younger group, the cabin-mate secondary attack rate for influenza-like illness was estimated as 42% (21%–66%) (Fig. 2A). Estimates of the nightly cabin secondary attack rate for the other case definitions (II-VI) ranged from 40% to 51% (eTable 1). For case definitions I-VI, estimates of the nightly cabin secondary attack rate and cabin-mate secondary attack rate among those 17 or younger ranged from 32% to 53% and 35% to 55%, respectively (eTables 2 and 4, http://links.lww.com/EDE/A445).
Figure 2.
Among ≤17-year-old individuals, (A) the estimated nightly cabin (hollow triangle) and cabin mate (solid triangle) secondary attack rates and (B) the daily camp local reproductive number (hollow square). Estimates are presented for each case definition (Table 1). The labeled short horizontal lines denote the point estimate, and the solid vertical lines capped with symbols denote the 95% confidence intervals. The solid gray horizontal lines denote a null secondary attack rate of 0% and a null camp local reproductive number of 1.0.
The estimated camp local reproductive number for influenza-like illness was 2.2 (95% CI = 1.4–3.3) (eTable 5, http://links.lww.com/EDE/A445), with an estimate of 2.7 (1.7–4.1) among camp participants 17 or younger (Fig. 2B). Estimates for the camp local reproductive number ranged from 2.1 to 3.5 for the other case definitions (eTable 5) and from 2.6 to 4.4 among those 17 or younger (Fig. 2B). Because adults comprised a small proportion of camp participants, the camp local reproductive number, nightly cabin secondary attack rate, and cabin-mate secondary attack rate were not estimated for this age group.
Household Transmission
A total of 41 households contained 136 contacts of 42 influenza-like illness index cases (Table 3). The mean number of contacts per household was 3.3 (SD = 1.3). The age of household contacts ranged from 0.5 to 74 years. For the influenza-like illness case definition, illness developed in 7 of 48 susceptible household contacts 17 or younger, and 4 of 88 susceptible adult household contacts (Table 4).
For case definitions II-VI, the number of households, contacts, and cases are described in Table 4. The mean number of household contacts for case definitions II-VI ranged from 3.3 to 3.5. The mean age of household contacts ranged from 32 to 34 years (median = 38–41).
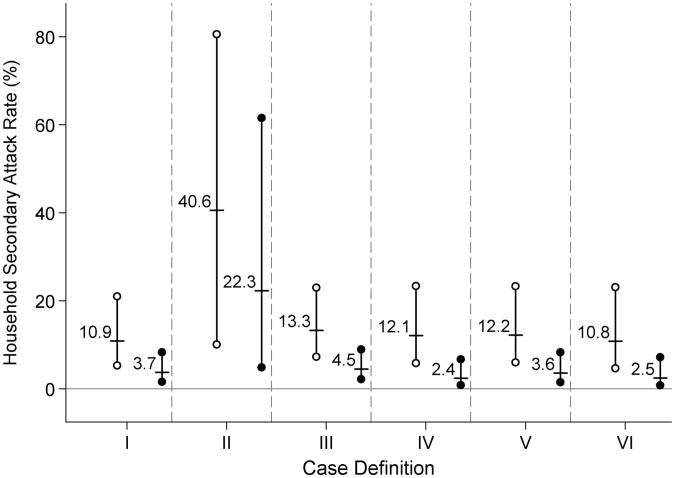
The household secondary attack rate for influenza-like illness was estimated to be 6% (3%–11%) (eTable 7, http://links.lww.com/EDE/A445). Among household contacts, the secondary attack rate was estimated to be 11% for children (5%–21%) and 4% for adults (2%–8%) (Fig. 3). For case definitions II-VI, estimates of the household secondary attack rate ranged from 5% to 32% (eTable 7). Among household contacts, household secondary-attack-rate estimates for case definitions II-VI ranged from 11% to 41% for children and 3%–22% for adults (Fig. 3).
Figure 3.
The estimated household secondary attack rates among ≤17- (hollow circle) and ≥18- (solid circle) year-old individuals for each case definition (Table 1). The labeled short horizontal lines denote the point estimate, and the solid vertical lines capped with symbols denote the 95% confidence intervals. The solid gray horizontal line denotes a null secondary attack rate of 0%.
Age-Group Effect
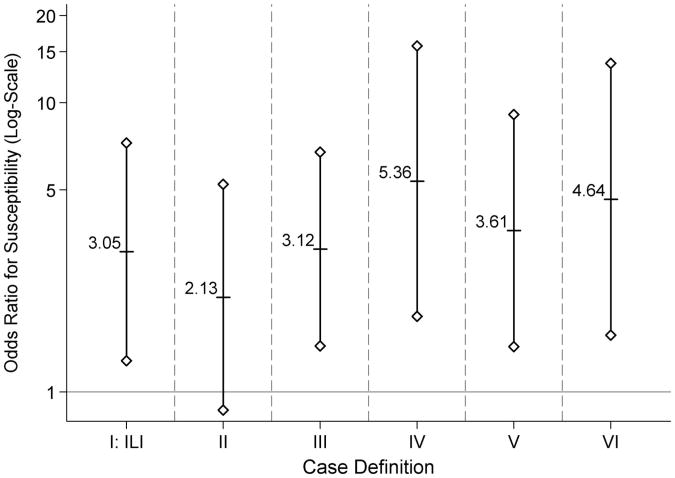
Among susceptible camp participants and household members, the odds ratio for susceptibility to influenza-like illness for children compared with adults was 3.1 (1.3–7.3) (Fig. 4). For the other case definitions, the age-group odds ratio for susceptibility ranged from 2.1 to.
Figure 4.
For each case definition (Table 1), the odds ratio for susceptibility to symptomatic disease for persons aged ≤17 versus ≥18 years during an April–May 2009 outbreak in Washington State, United States. The labeled short horizontal lines denote the point estimate, and the solid vertical lines capped with symbols (hollow diamond) denote the 95% confidence intervals. The solid gray horizontal line denotes a null odds ratio of 1.0.
Sensitivity Analysis
For all 6 case definitions, the household and nightly cabin secondary attack rates and the camp local reproductive number demonstrated little sensitivity to the alternate assumptions of no camp or household exposure to a community source of disease (Scenario B) and of a shorter (Scenario D) or longer (Scenario E) infectious period (eFig. 1, http://links.lww.com/EDE/A445). Including a common community-to-person probability of exposure to disease for the households and the camp participants during the postcamp period (Scenario C) had the expected effect of decreasing both the unadjusted and age-group-specific household secondary-attack-rate estimates, with little change to the values of the nightly cabin secondary attack rate or camp local reproductive number (eFig. 1). The estimates of the age-group odds ratio and the person-to-person transmission probabilities demonstrated a similar lack of sensitivity to the alternate assumptions in Scenarios B–E (eTables 10–21, http://links.lww.com/EDE/A445), with the exception being p3 under Scenario C (eTables 17–19, http://links.lww.com/EDE/A445).
Discussion
In the context of an outbreak of pH1N1, we report high levels of transmission of influenza-like illness in a camp setting, but low levels among the household contacts of camp-acquired cases. We also found a strong effect of age on susceptibility to influenza-like illness. The qualitative nature of these findings did not change when we considered alternate case definitions for clinical influenza. These findings illustrate the importance of collecting data from linked mixing groups (ie, a camp and associated households). To the best of our knowledge, this is the first detailed description of the transmission of pH1N1 associated influenza-like illness in a residential camp setting and of the household transmission of camp-acquired influenza-like illness.
The local reproductive number estimate for the camp is consistent with reported estimates for pH1N1 in schools. Three estimates of the school local reproductive number for pH1N1 range from 2.4 to 3.3.15,17,32 The similarity between these estimates and the camp local reproductive number estimated here suggests that the transmission intensity of this virus is similar in camps and schools.
The household secondary-attack-rate estimates presented here are low compared with previous estimates of 27% and 26% from early-pandemic outbreaks among US17 and Kenyan households,33 respectively, but are consistent with recently published estimates.8,15,18,22–24 A 7% secondary attack rate among household contacts not taking antiviral prophylaxis18 was reported for a May–June 2009 outbreak of pH1N1 in Kobe, Japan, although 92% of the index cases received antiviral treatment.18 Household secondary-attack-rate estimates were 9% for influenza-like illness in a spring 2009 outbreak in Texas24 and 6% for confirmed pH1N1 infection in an early summer 2009 outbreak in Hong Kong.23 Two analyses of influenza-like illness among the household contacts of children involved in an outbreak of pH1N1 in a New York City school estimated secondary attack rates of 18%15 and 11%,22 which fall within the 4%–23% range for US households.8
We found a strong effect of age group on susceptibility to influenza-like illness, with an odds ratio of 3.1 for children (age 17 years or younger) versus adults (age 18 or older). This age effect is consistent with estimated odds ratios of 2.0 for acute respiratory disease and 3.0 for influenza-like illness among 0–18 versus 19–50-year-old members of 216 US households.8 In the Kobe outbreak,18 siblings from the same household were at 7.8 times greater odds of becoming a secondary case of laboratory-confirmed pandemic influenza than their parents, which is qualitatively consistent with our results.
The 66% response rate to the survey suggests that selection bias may be present in the sample of camp participants included in the analysis. Symptomatic pH1N1 cases may have been more prone to respond to the survey than their nonill counterparts, leading to overestimation of the camp local reproductive number and nightly cabin secondary attack rate. The potential selection bias in the household analysis is partially addressed by including only the household contacts of ill camp participants.
Because most of the suspected pH1N1 cases in this outbreak were not confirmed by laboratory test, the true etiology of most symptomatic cases is uncertain. At the time of the outbreak, laboratory-based surveillance documented the circulation of parainfluenza, influenza B, respiratory syncytial virus, and metapneumovirus in the local population (unpublished data from Public Health—Seattle and King County). However, 5 of the camp cases were confirmed pH1N1 infections, suggesting that most of the cases with influenza-like illness were the result of this virus. Nevertheless, to address this uncertainty, we report results for a range of case definitions.
The combined impact of elevated susceptibility to disease among children and the low number of susceptible children per household (adults were twice as prevalent as children among household contacts) could together explain the lower transmission of pH1N1 in the households. In contrast, intense transmission was observed among camp participants. Considered together, these 2 results support the hypothesis that contact among children in settings such as schools and camps provides a venue for intense transmission of pH1N1, potentially providing a key locus for sustaining community transmission during outbreaks of pH1N1. Dampened levels of transmission within the household probably result from the depletion of the susceptible children due to their infection in other venues, such as schools.
There are other potential explanations (biologic, environmental, and sociological) for the observed differences between the transmissibility of pH1N1 in the camp versus the households, and these require further investigation. As demonstrated here, accurate estimation of the transmission potential of pH1N1 within community venues requires collecting data about the illness onset time, range of symptoms, and the persons present in each venue. These results support the need for community-based household studies, similar to the Tecumseh34 and the Seattle Virus Watch12 studies, in which virologic, serologic, and epidemiologic data are collected prospectively from enrolled households with children in schools. A better understanding of the age-effect and its impact on community transmission of pH1N1 will inform future prevention and control strategies. Intervention strategies, such as vaccination, will need to focus on children and the locations where they congregate, especially schools.
Acknowledgments
Supported by the National Institutes of Health: the National Institute of General Medical Sciences MIDAS grant U01-GM070749 and the National Institute of Allergy and Infectious Diseases grant R01-AI32042 (to J.D.S., Y.Y., M.E.H., I.M.L.); Public Health—Seattle and King County (to J.D.S.); United States Centers for Disease Control and Prevention (to N.N.B., M.L.T., L.J.S., G.E.F.); Washington State Department of Health (to M.L.T.).
Footnotes
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1–3. [PubMed] [Google Scholar]
- 2.Kansas Department of Health and Environment. Swine Influenza News Conference. Topeka, KS: Kansas Department of Health and Environment; 2009. [Accessed 29 April 2009]. Available at: http://www.dhe.state.ks.us/SwineFlu/swineflunewsconf.wmv. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:400–402. [PubMed] [Google Scholar]
- 4.Chan M. World now at start of 2009 influenza pandemic: statement to the press by WHO director-general. Geneva, Switzerland: World Health Organization; 2009. [Accessed 24 October 2009]. Available at: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/ [Google Scholar]
- 5.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med. 1978;298:587–592. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 6.Monto AS, Kioumehr F. The Tecumseh study of respiratory illness. IX: occurence of influenza in the community, 1966–1971. Am J Epidemiol. 1975;102:553–563. doi: 10.1093/oxfordjournals.aje.a112193. [DOI] [PubMed] [Google Scholar]
- 7.Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344:889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 8.Cauchemez S, Donnelly CA, Reed C, Ghani AC, Fraser C, Kent CK, Finelli L, Ferguson NM. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361:2619–2627. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiore A. Novel influenza A (H1N1) epidemiology update; Meeting of the Advisory Committee on Immunization Practices (ACIP); 2009; [Accessed 25 October 2009]. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jul09-flu/02-Flu-Fiore.pdf. [Google Scholar]
- 10.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brock C, Knowles M, Goh S. A school and community outbreak of influenza A. Commun Dis Rep CDR Rev. 1995;5:R177–R179. [PubMed] [Google Scholar]
- 12.Fox JP, Hall CE, Cooney MK, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. I: study design, methods and the occurrence of infections by time and age. Am J Epidemiol. 1982;116:212–227. doi: 10.1093/oxfordjournals.aje.a113407. [DOI] [PubMed] [Google Scholar]
- 13.Teare EL, Rawes JC, Chakraverty P, et al. Failure of influenza vaccine to prevent two successive outbreaks of influenza A H1N1 in a school community. Br J Gen Pract. 1990;40:10–12. [PMC free article] [PubMed] [Google Scholar]
- 14.Health Protection Agency West Midlands H1N1v Investigation Team. Preliminary descriptive epidemiology of a large school outbreak of influenza A(H1N1)v in the West Midlands, United Kingdom, May 2009. Euro Surveill. 2009;14.pii:19264. doi: 10.2807/ese.14.27.19264-en. [DOI] [PubMed] [Google Scholar]
- 15.Lessler J, Reich NG, Cummings DA, Nair HP, Jordan HT, Thompson N. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med. 2009;361:2628–2636. doi: 10.1056/NEJMoa0906089. [DOI] [PubMed] [Google Scholar]
- 16.Smith A, Coles S, Johnson S, Saldana L, Ihekweazu C, O'Moore E. An outbreak of influenza A(H1N1)v in a boarding school in South East England, May-June 2009. Euro Surveill. 2009;14(.pii):19263. doi: 10.2807/ese.14.27.19263-en. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Sugimoto JD, Halloran ME, et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science. 2009;326:729–733. doi: 10.1126/science.1177373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odaira F, Takahashi H, Toyokawa T, et al. Assessment of secondary attack rate and effectiveness of antiviral prophylaxis among household contacts in an influenza A(H1N1)v outbreak in Kobe, Japan, May–June 2009. Euro Surveill. 2009;14:1–5. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis—North Carolina, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:969–972. [PubMed] [Google Scholar]
- 20.Kansas Department of Health and Environment. KDHE reports 2 cases of swine flu in Kansas. Topeka, KS: Kansas Department of Health and Environment; 2009. [Accessed 5 May 2009]. Available at: http://www.kdheks.gov/news/web_archives/2009/04252009.htm. [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Update: swine influenza A (H1N1) infections—California and Texas, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:435–437. [PubMed] [Google Scholar]
- 22.France AM, Jackson M, Schrag S, et al. Household transmission of 2009 influenza A (H1N1) virus after a school-based outbreak in New York City, April–May 2009. J Infect Dis. 2010;201:984–992. doi: 10.1086/651145. [DOI] [PubMed] [Google Scholar]
- 23.Leung YH, Li MP, Chuang SK. A school outbreak of pandemic (H1N1) 2009 infection: assessment of secondary household transmission and the protective role of oseltamivir. Epidemiol Infect. 2010:1–4. doi: 10.1017/S0950268810001445. [DOI] [PubMed] [Google Scholar]
- 24.Morgan OW, Parks S, Shim T, et al. Household transmission of pandemic (H1N1) 2009, San Antonio, Texas, USA, April-May 2009. Emerg Infect Dis. 2010;16:631–637. doi: 10.3201/eid1604.091658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Swine-origin influenza A (H1N1) virus infection in a school—New York City, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:470–472. [PubMed] [Google Scholar]
- 26.Cauchemez S, Ferguson N, Wachtel C, et al. Closure of schools during an influenza pandemic. Lancet Infect Dis. 2009;9:473–481. doi: 10.1016/S1473-3099(09)70176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Interim CDC guidance on day and residential camps in response to human infections with the novel influenza A (H1N1) virus. Vol. 2010 Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Accessed 21 January 2010]. Available at: http://www.cdc.gov/H1N1flu/camp.htm. [Google Scholar]
- 28.Yang Y, Longini IM, Halloran ME. Design and evaluation of prophylactic interventions using infectious disease incidence data from close contact groups. Appl Stat. 2006;55:317–330. doi: 10.1111/j.1467-9876.2006.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Interim guidance for influenza surveillance: prioritizing RT-PCR testing in laboratories. Vol. 2009 Atlanta, GA: Centers for Disease Control and Preventio; 2009. [Accessed 25 October 2009]. Available at: http://www.cdc.gov/h1n1flu/screening.htm. [Google Scholar]
- 30.Schafer JL. Analysis of Incomplete Multivariate Data Monographs on Statistics and Applied Probability. New York: Chapman & Hall; 1997. [Google Scholar]
- 31.Little RJ, Rubin DB. Statistical Analysis with Missing Data. 2nd. New York: John Wiley; 2002. [Google Scholar]
- 32.Paterson B, Durrheim DN, Tuyl F. Influenza: H1N1 goes to school. Science. 2009;325:1071–1072. doi: 10.1126/science.325_1071b. author reply 1072–1073. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Introduction and transmission of 2009 pandemic influenza A (H1N1) virus—Kenya, June–July 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1143–1146. [PubMed] [Google Scholar]
- 34.Monto AS, Koopman JS, Longini IM., Jr Tecumseh study of illness. XIII: influenza infection and disease, 1976–1981. Am J Epidemiol. 1985;121:811–822. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]