Abstract
Peripheral chemoreceptors in the carotid body regulate respiration, sympathetic outflow, and blood pressure in response to hypoxia. The carotid bodies play a role in the counterregulatory response to hypoglycemia in animal models and may interact with the arterial baroreflex. We hypothesized that desensitization of the carotid bodies by hyperoxia in humans would blunt hypoglycemic counterregulation and baroreflex control of blood pressure during hypoglycemia. Seven healthy adults (age 26.7 ±0.39, BMI 25 ± 0.32, M/4, F/3) underwent two 180 min hyperinsulinemic (2 mU/kg FFM/min), hypoglycemic (3.33 mmol/L) clamps one week apart, randomized to either normoxia (arterial PO2 (PaO2) 111 ± 6.3 mmHg) or hyperoxia (PaO2 345 ± 80.6 mmHg) (p<0.05). Plasma glucose concentrations were similar during normoxia and hyperoxia at baseline (5.52 ± 0.15 vs. 5.55 ± 0.13 umol/ml) and during the clamp (3.4 ± 0.05 vs. 3.3 ± 0.05 umol/ml). The glucose infusion rate was 44.2 ± 3.5 % higher (p< 0.01) during hyperoxia than normoxia during the clamp (28.2 ± 0.15 vs 42.7 ± 0.65 umol/kg FFM/min; p<0.01). Area under the curve values (expressed as % normoxia response) for counterregulatory hormones during hypoglycemia were significantly suppressed by hyperoxia (norepinephrine 50.7 ± 5.2%, epinephrine 62.6 ± 3.3%, cortisol 63.2 ± 2.1%, growth hormone 53.1 ± 2.7%, glucagon 48.6 ± 2.1%, all p<0.05 vs normoxia). In addition, mean blood pressure during hypoglycemia was significantly lower with hyperoxia than with normoxia (delta reduction from baseline: −5.4 ± 3.4 mmHg normoxia vs. −13.8 ± 1.9 mmHg hyperoxia, p<0.05). The typical baroreflex-mediated rise in heart rate and sympathetic activity with lower blood pressure did not occur when the CB were silenced. These data support the idea that the carotid bodies play a role in the counterregulatory response to hypoglycemia and in baroreflex control of blood pressure in humans.
Introduction
Peripheral chemoreceptors in the carotid bodies regulate respiration, sympathetic outflow, and blood pressure in response to hypoxia. In addition, cellular and animal studies suggest that the carotid bodies sense and respond to low glucose [1, 2]. Given the developing understanding of the carotid bodies are polymodal sensors and that they have a strong influence on sympathetic nervous system, we tested the hypothesis that the carotid bodes play an integrative role in mediating both the counterregulatory hormone response to hypoglycemia as well as the baroreflex control of blood pressure during hypoglycemia. We have recently published a comprehensive report on the potential influence of the carotid chemoreceptors on hypoglycemic counterregulation [3]. In the current paper we highlight our earlier findings and also report novel data on potential chemoreflex/baroreflex interactions and blood pressure regulation that were generated during our original study [3].
We sought to test the role of the carotid bodies in the systemic counterregulatory response to hypoglycemia and blood pressure control in humans. One approach to study this topic in humans is to use hyperoxia to acutely desensitize the carotid bodies. In humans hyperoxia can depress minute ventilation [4], suppresses afferent nerve traffic from the carotid bodies in animals [5], and inhalation of 100% oxygen is thought to substantially reduce peripheral chemoreceptor activity [6]. On separate days we performed paired hyperinsulinemic hypoglycemic clamps in healthy humans exposed to normoxia or hyperoxia. We hypothesized that the glucose infusion rate would be augmented and neuro-hormonal counterregulation blunted during hypoglycemia when the carotid bodies were desensitized by hyperoxia. In addition, we tested the role of the carotid bodies in blood pressure regulation in hypoglycemia under the same experimental conditions and hypothesized that desensitizing the carotid bodies would attenuate baroreflex control of blood pressure.
METHODS
Subjects and monitoring
Subjects were healthy non-obese (BMI < 28 kg/m2) persons between 21 and 35 years old.
Prior to starting the glucose clamp, a 20-gauge catheter for blood sampling and blood pressure monitoring was placed in a brachial artery under ultrasound guidance after local anesthesia. Two intravenous catheters were placed in the arm opposite the brachial arterial catheter for infusions. For patient safety, heart rate was monitored with a 5-lead electrocardiogram, respirations via a pneumobelt, and arterial oxygen saturation by a pulse oximeter.
Hypoglycemic Clamps
Intravenous insulin (Novolin®, Novo Nordisk Inc., Princeton NJ) was infused at a constant rate of 2.0 mU/kg FFM/min from protocol time (T) 0 to T180 minutes, and exogenous glucose (50% Dextrose solution (Hospira, Inc, Lake Forest, IL)) was infused in amounts sufficient to maintain glucose concentrations at hypoglycemic levels (~ 3.3 mmol/L (60 mg/dl)) [7]. From T0 until the end of the study, subjects breathed either normoxic or hyperoxic gas via a face mask as described below. Plasma glucose was measured every 5–10 minutes at the bedside using a glucose oxidase method (Analox Instruments USA Inc., Lunenberg, Massachusetts).
Hyperoxia and Normoxia
During hyperoxia trials subjects breathed 100% oxygen through a face mask connected to a non-rebreathing valve and large meteorological balloon which served as a volume reservoir. On the normoxia day, the identical set up was used except the bag was filled with air (21% oxygen). Normoxia and hyperoxia trials occurred on different days separated by one week in random order. Arterial blood gases were measured at baseline and every hour during the clamp.
Analytical Methods
Arterial blood was drawn for glucose and hormone measurements (insulin, c-peptide, glucagon, growth hormone, cortisol, epinephrine, dopamine, and norepinephrine) at T -120, -30, -20, -10, 0 and during the glucose clamp at 60, 120, 150, 160, 170, and 180 minutes.
Data Analysis and Statistics
Each subject was assessed under both experimental conditions (normoxia and hyperoxia). The time-by-group interaction term was included in the model and supplemental analyses were performed to compare groups at each time period using the paired t-test. For hormones, an area under the curve above baseline was calculated for each hormone in both conditions over time and expressed as percentage of normoxia response. Where relevant, baseline values were calculated as an average of T-30,-20,-10 and 0 and clamp values as an average of T150,160,170,180. P-values of <0.05 were considered statistically significant. Sigma Stat 2.03 software was used for analysis.
RESULTS
Blood gases
The average PaO2 during normoxia was 111 ± 6.3 mmHg and significantly elevated during hyperoxia to 345 ± 80.6 mmHg.
Plasma glucose, insulin, and c-peptide
Plasma glucose concentrations were similar during normoxia and hyperoxia at baseline (5.52 ± 0.15 vs. 5.55 ± 0.13 umol/ml) and during the clamp (3.4 ± 0.05 vs. 3.3 ± 0.05 umol/ml). Baseline and clamp insulin concentrations were no different between normoxia and hyperoxia study days (baseline: 36.4 ± 6.7 vs 32.3 ± 4.9 pmol/L and clamp: 944.2 ± 86.8 vs 916.1 ± 75.9 pmol/L).
Glucose infusion rate
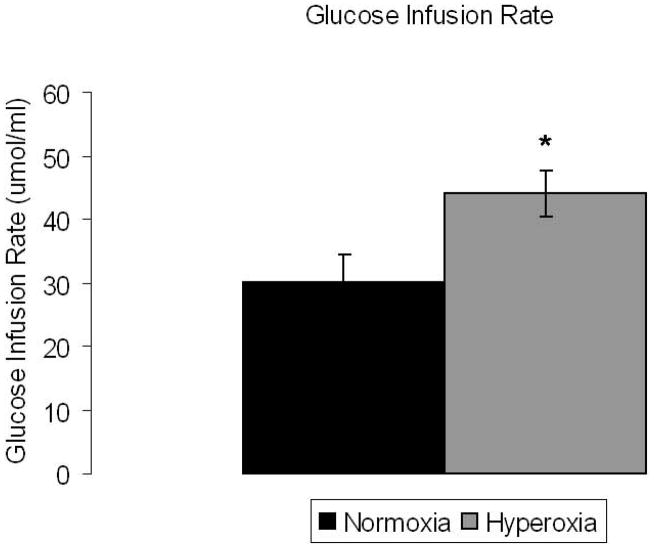
Despite similar plasma glucose concentrations during normoxia and hyperoxia, the glucose infusion rate required to maintain hypoglycemia was significantly higher during hyperoxia compared to normoxia (42.7 ± 0.65 vs 28.2 ± 0.15 umol/kg FFM/min; p<0.01) by 44.2 ± 3.5 % (Figure 1). The higher glucose infusion rate on the hyperoxia study day was observed in all subjects.
Figure 1. Glucose infusion rate is significantly higher under hyperoxic conditions.
Glucose infusion rate (umol/ml) under normoxia and hyperoxia at steady state hypoglycemia. Normoxia: black bar, hyperoxia: gray bar, Significance denoted as * p<0.05. N=7.
Counterregulatory hormones
Table 1 summarizes the data for norepinephrine, epinephrine, cortisol, glucagon, and growth hormone concentrations in response to hypoglycemia. All catecholamines and hormones were significantly lower (p<0.05) during hyperoxia vs. normoxia.
Table 1. Plasma levels of key counterregulatory hormones in hypoglycemia.
Data is shown as percent reduction in catecholamine and hormone levels in hyperoxic hypoglycemia vs normoxic conditions.
| Catecholamines and Counterregulatory Hormones | Percent Reduction in Hyperoxia From Normoxia Plasma Levels (%) |
|---|---|
| Norepinephrine | −50.7 ± 5.2%* |
| Epinephrine | −62.6 ± 3.3%* |
| Glucagon | −48.6 ± 2.1%* |
| Growth Hormone | −53.1 ± 2.7%* |
| Cortisol | −63.2 ± 2.1%* |
p<0.05. N=7.
Blood pressure
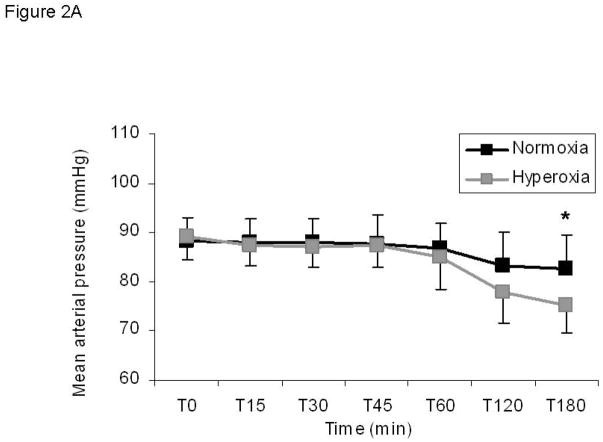
Mean arterial pressure during steady state hypoglycemia fell from baseline in both normoxia and hyperoxia groups and was significantly lower with hyperoxia than with normoxia (Figure 2; delta from baseline: −5.4 ± 3.4 mmHg normoxia vs. −13.8 ± 1.9 mmHg hyperoxia, p<0.05). Systolic and diastolic blood pressures were similarly lower when the carotid bodies were inactivated with hyperoxia compared to normoxia but did not reach statistical significance with these preliminary findings (Data not shown; systolic delta from baseline: 12.0 ± 8.7 mmHg normoxia vs. −3.8 ± 5.8 mmHg, p=0.1; diastolic delta from baseline: −8.6 ± 2.0 mmHg vs. −15.3 ± 1.5 mmHg, p=0.3). Despite the reduction in all measures of arterial pressure during hyperoxia, the typical baroreflex-mediated rise in heart rate and sympathetic activity that normally occurs with lowered blood pressure did not occur when the CB were silenced. This is evidenced by a similar heart rate under both conditions at steady state clamp conditions (Figure 2B; normoxia delta from baseline 12.2 ± 3.2 beats per minute vs hyperoxia delta from baseline 7.9 ± 3.3 beats per minute, p>0.05) and by a paradoxical reduction in plasma catecholamines under conditions that should induce a rise in sympathetic activity as described above.
Figure 2. Blood pressure is reduced during hypoglycemia under hyperoxic conditions without a corresponding baroreflex increase in heart rate.
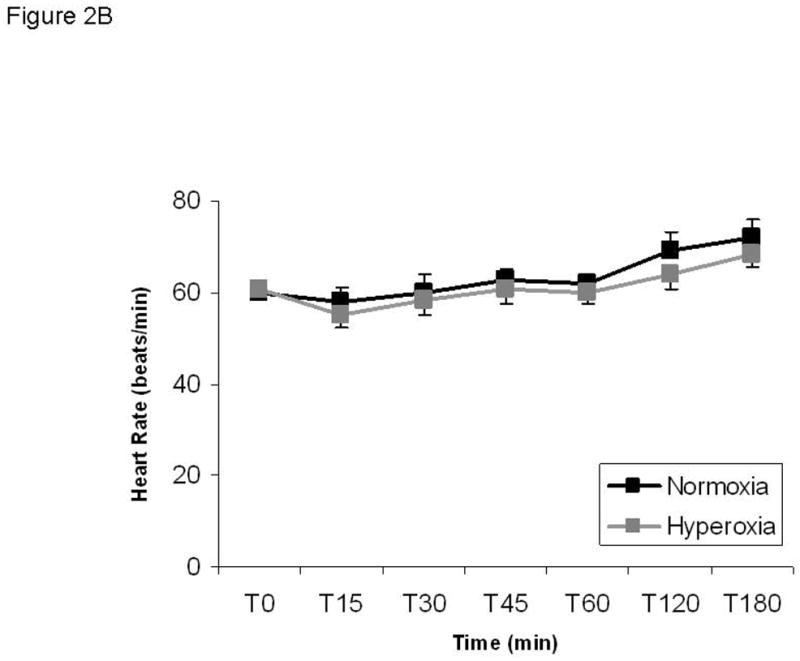
A) Mean arterial pressure. Normoxia: black squares; hyperoxia: gray squares. B) Heart rate is not different between conditions. Significance denoted as * p<0.05 for differences between normoxia and hyperoxia. N=5.
DISCUSSION
The carotid bodies were “inactivated” in humans by exposure to hyperoxia in conjunction with a hypoglycemic clamp. Our study provides evidence that the carotid bodies play an important role in the regulation of integrative physiology including blood glucose homeostasis and baroreflex control of blood pressure in humans: 1) the exogenous glucose infusion rate during the hyperinsulinemic hypoglycemic clamps was ~50% higher during desensitization of the carotid bodies with hyperoxia and hyperoxia during hypoglycemia resulted in a marked blunting of the counterregulatory hormone responses. 2) Sympathetic baroreflex adjustments to reduced arterial pressure during hypoglycemia are blunted during carotid body desensitization. Our data are also consistent with animal data showing the carotid bodies play an integral role in blood glucose homeostasis in vivo [2, 8, 9] and reveal for the first time that the carotid bodies are vital in blood pressure maintenance during hypoglycemia in humans.
The present report highlights our previously published findings [3] showing that exposure to hyperoxia during hypoglycemia blunted the release of numerous counterregulatory hormones, including the primary counterregulatory hormones to acute hypoglycemia glucagon and epinephrine [10]. Consequently, hyperoxia resulted in an increase in glucose infusion rate during the clamp. These findings are consistent with studies in carotid body resected dogs [2, 9]. In addition, we present novel findings in this report demonstrating for the first time that the carotid bodies are integral to the blood pressure responses to hypoglycemia. With the carotid bodies desensitized with hyperoxia, there is a fall in arterial pressure below normoxic conditions and this is not countered by the typical baroreflex response of elevation of heart rate and sympathetic outflow. This supports the notion that the peripheral chemoreceptor afferents integrate in a meaningful way with the arterial baroreflex or that there are barosensitive characteristics in the carotid body organ per se.
Mechanisms
Although we did not directly test the mechanisms by which hypoglycemia interacts with the carotid body glomus cells in humans, a discussion of potential mechanisms is appropriate. Isolated Type I glomus cells from the carotid bodies release neurotransmitter in response to both decreased extracellular glucose and hypoxia [1]. In addition, hypoglycemia has been shown to activate a standing sodium current that depolarizes glomus cells in a dose dependent manner [11]. These studies provide a powerful rationale for our overall hypothesis, our specific study design, and the interpretation of our data. In this context, the proposed mechanism for our findings is that hyperoxia desensitized the carotid bodies limiting the ability of the carotid chemoreceptor cells to release neurotransmitter in response to low glucose in vivo. Therefore, carotid sinus nerve afferent activation under hyperoxic conditions was reduced with an associated attenuation of activity at the primary central synapse of these sensory fibers in the nucleus tractus solitarii (NTS) [12]. This sequence of events then leads to blunted counterregulatory hormonal responses.
The involvement of the NTS as the first synapse of the carotid body afferent fibers is important in that, in addition to mediating counterregulatory responses, it is a key site for integration of the arterial baroreflex. In addition, there is evidence that the peripheral chemoreflex and arterial baroreflex are highly integrated. Taken together, it is of interest to understand the role that carotid body afferents play in mediating the central integration of the baroreflex. In this study we determined that the baroreflex control of blood pressure during hypoglycemia was attenuated as evidenced by a significant fall in blood pressure under hyperoxia conditions. This drop is arterial pressure would typically be countered by a rise in heart rate and sympathetic activity when the baroreflex pathways are intact; however, in this study we show that there is no increase in heart rate and there is a paradoxical decrease in plasma catecholamines suggesting that the sympathetic outflow is inhibited by the desensitization of the carotid bodies.
Conclusion
We have provided evidence consistent with the idea that the carotid bodies play a role in the counterregulatory responses to hypoglycemia and in baroreflex control of blood pressure during hypoglycemia in humans. Additionally, it is tempting to speculate that altered carotid body function might have implications for clinical conditions associated with altered regulation of blood glucose and blood pressure including sleep apnea, Type II diabetes and hypertension.
References
- 1.Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5(3):197–8. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- 2.Koyama Y, et al. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49(9):1434–42. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- 3.Wehrwein EA, et al. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol. 2010;588(Pt 22):4593–601. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downes JJ, Lambertsen CJ. Dynamic characteristics of ventilatory depression in man on abrupt administration of O. J Appl Physiol. 1966;21(2):447–53. doi: 10.1152/jappl.1966.21.2.447. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald R, Lahiri S. In: Handbook of Physiology-The Respiratory System II: Chapter 10, Reflex responses to chemoreceptor stimulation. Fishman A, editor. Vol. 2. Bethesda: American Physiological Society; 1986. pp. 313–362. Vol. Section 3. [Google Scholar]
- 6.Lahiri S, DeLaney RG. Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol. 1975;24(3):267–86. doi: 10.1016/0034-5687(75)90018-3. [DOI] [PubMed] [Google Scholar]
- 7.Lecavalier L, et al. Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am J Physiol. 1989;256(6 Pt 1):E844–51. doi: 10.1152/ajpendo.1989.256.6.E844. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Buylla R, et al. Pituitary and adrenals are required for hyperglycemic reflex initiated by stimulation of CBR with cyanide. Am J Physiol. 1997;272(1 Pt 2):R392–9. doi: 10.1152/ajpregu.1997.272.1.R392. [DOI] [PubMed] [Google Scholar]
- 9.Koyama Y, et al. Role of carotid bodies in control of the neuroendocrine response to exercise. American Journal of Physiology - Endocrinology & Metabolism. 2001;281(4):E742–8. doi: 10.1152/ajpendo.2001.281.4.E742. [DOI] [PubMed] [Google Scholar]
- 10.Gerich J, et al. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979;236(4):E380–5. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Fernandez M, et al. Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes. 2007;56(12):2893–900. doi: 10.2337/db07-0122. [DOI] [PubMed] [Google Scholar]
- 12.de Campos Cruz J, et al. Fos expression in the NTS in response to peripheral chemoreflex activation in awake rats. Auton Neurosci. 2010;152(1–2):27–34. doi: 10.1016/j.autneu.2009.08.016. [DOI] [PubMed] [Google Scholar]