Abstract
The auditory system computes the location of a sound by detecting submillisecond time differences in the arrival of sound at the two ears. New in vivo and in vitro studies shed light on how this task is accomplished by the interaction of excitatory and inhibitory synapses.
Localizing sound sources is vital for the survival of predators,or to escape from them. Consequently, the auditory system has evolved macrocircuits and specialized synapsesthat precisely calculate the locus of sound sources (Fig. 1A; Ashida and Carr, 2011). The barn owl exemplifies an animal that has exquisite sound localization ability. Barn owls can determine the location of a mousein absolute darkness with a resolution of less than one degree (Payne, 1971). Because of this amazing accuracy, the barn owl has been a model system for understanding neural mechanisms of sound localization. Humans can also determine the location of a sound withhigh resolution (e.g. 1-2 degrees; Grothe et al., 2010). Understanding the neural mechanisms underlying this level of accuracy has been of considerable interest for many decades. Two papers in this issue (van der Heijen et al. and Roberts et al.) now provide new insights into the mechanisms ofmammalian sound localization.
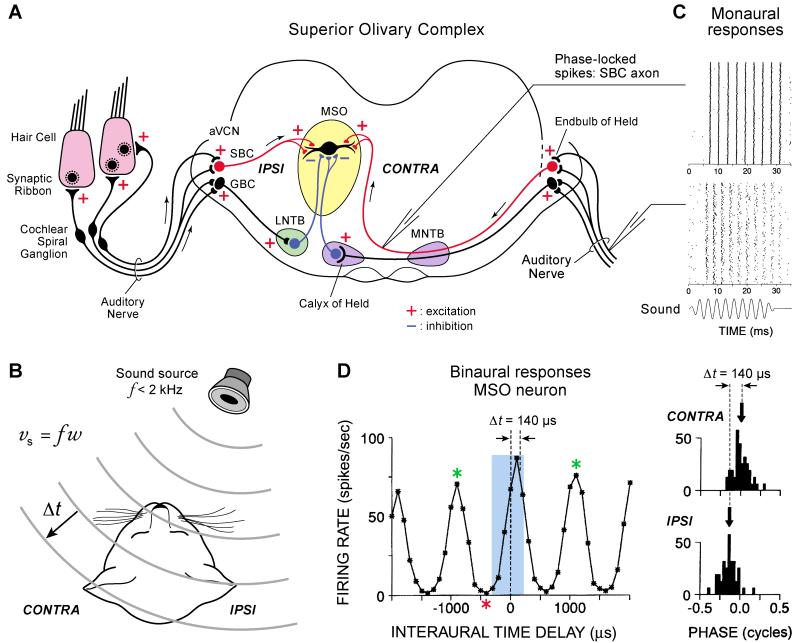
Figure 1.
The mammalian macrocircuit for the localization of low-frequency sounds.
A. Sound evoked signals are detected by inner hair cells in the cochlea and transmitted by specialized ribbon-type synapses to the afferent fibers (or dendrites) of spiral ganglion cells whose axons form the auditory nerve. Some of these axons terminate in the anterior ventral cochlear nucleus (aVCN) where they synapse onto spherical bushy cells (SBC), via large endbulbs of Held, and onto globular bushy cells (GBC). The ipsilateral axons of the spherical bushy cells then form excitatory synapses on the principal cells of the medial superior olive (MSO), which also receive inputs from the contralateral SBC. The MSO principal cell integrates the two excitatory inputs that originate from the two ears. They are thus coincidence detectors of binaural signals. The superior olivary complex also has two nuclei (the lateral and medial nucleus of the trapezoid body: LNTB and MNTB) that send inhibitory input to the MSO. This inhibitory input can arrive before the excitatory input and thus can shape the response of the MSO cells.
B. Low frequency sounds that are located at an angle to the midsagittal plane arrive in the ipsilateral (IPSI) ear before they reach the contralateral ear (CONTRA). Given the constant speed of sound (vs =fω; f is frequency and ω is wavelength) and a cat’s head size, this difference in time (Δt) varies from 0 to 400 μs for cats. The auditory system detects the Δt of sounds with f< 2 kHz and uses this Δt to localize sounds in the horizontal plane.
C. A pure tone sound of 350 Hz elicits spikes in the cat auditory nerve. The time of the sound stimulus is shown as well as timing of the spikes for 200 repetitions of stimulus. Note that the spikes occur in a periodic way that follows the stimulus period. The spike timing is thus phase-locked to the sound stimulus and the timing of the spikes can be used to determine the frequency of the sound. But note that spikes do not occur for every cycle of the sound stimulus and the precision and fidelity of the spikes is degraded towards the end of the stimulus. On top of the auditory nerve data, recordings from an SBC axon are shown for a 340 Hz sound stimulus. These display an even better phase locking than auditory nerve spikes. Furthermore, spikes are elicited at almost every cycle of the sound stimulus and the precision of the timing is well preserved during the stimulus. Spikes in the SBC axon are thus highly synchronous and display more precision and endurance than spikes in the auditory nerve (Modified from Joris et al., 1998).
D. Recordings of spikes in the cat MSO principal cells. The two right panels show monaural responses elicited by stimulation of just the CONTRA or IPSI ear with a sound of 1 kHz. Spikes are phase locked to a particular phase of the sound stimulus and the Δt between the two peaks in monaural evoked firing rate is 140 μs. The left panel shows the firing rates when the same 1 kHz sound is heard by the two ears (binaural responses). Note that the central firing peak occurs near to 140 s when both the CONTRA and IPSI excitatory signals coincide. This central peak determines the best delay (or best ITD, Interaural Time Difference). The worst delay or ITD occurs at the red asterisk when firing rates go to zero. The blue rectangle shows the region where the peak ITD and maximum slope ITD occur. The green asterisks indicate secondary peaks in ITD that occur when a pure tone is used as a stimulus. If a more natural broadband sound (with several frequencies) is used as a stimulus, the MSO cell response shows an enhanced central peak because sounds of different frequencies still have a best delay near 140 μs. However, the secondary peaks are reduced with broadband sound stimuli. This explains why more natural broadband sounds can be better localized than artificial pure tone sounds.(Modified from Yin and Chan, 1990).
In contrast to other sensory systems, such as vision and somatosensation, the sensory epithelium of the inner ear does not have an explicit representation of space. The inner hair cells are systematically arranged along the basilar membrane to create a place-code for sound frequency but not a code for auditory space. Consequently, the location of a sound source in space must be computed by the auditory system. There are two binaural cues that can be utilized to locate sounds in the horizontal plane; interaural timing differences (ITDs) and interaural level differences (ILDs). ITDs are employed in low frequency (< 2 kHz) localization tasks and ILDs are employed in high frequency localization tasks.When the wavelength of a sound is roughly equal to or shorter than the diameter of the head, an ILD is created because of a shadowing effect at the ear further from the sound source. Many mammals, including humans and cats, make use of both ITDs and ILDs for horizontal sound localization whereas some animals such as bats only use ILD because of their small head size and dependence on hearing ultra high frequency (e.g. 60-120 kHz) sounds for echolocation behaviors.Surprisingly, Mongolian gerbils use ITDs even with their relatively small head (Heffner and Heffner, 1988). This is thought to be because gerbilshave a need for long distance communication and thus have evolved low frequency hearing and use of low frequency vocalizations. As a result, cats and gerbils have been the animals of choice for understanding mechanisms of ITD coding, whereas many studies have used bats to understand mechanisms of ILD coding.
When sound sources are off the midsagittal plane, they generate differences in the arrival time of the stimulus at the two ears (onset ITD; Fig. 1B) and throughout the duration of the stimulus (ongoing ITD). Even at the most extreme horizontal sound position, the ITDs are extremely small; 700 μs in humans, 400 μs in cats and 135 μs in gerbils (Fig. 1B). Amazingly, however, humans can discriminate ITDs of 10-20 μs for low frequency sounds and they are capable of discriminating ILDs of 1-2 dB (Grothe et al., 2010). While discrimination ability for both ITDs and ILDs is impressive, the submillisecond resolution of the ITD cue is hard to comprehend considering the millisecond duration ofaction potentials in the auditory nerve. Thus, there has been considerable interest in the neural and biophysical mechanisms that support this exquisite temporal processing.
In mammals, the extraction of timing cues is performed by bipolar neurons in the medial superior olive (MSO). MSO neurons receive bilateral excitatory input from spherical bushy cells in the cochlear nucleus (Fig 1A). Ipsilateral inputs synapse onto lateral dendrites and contralateral inputs synapse onto the medial dendrites (Fig. 1A). Remarkably, these inputs are phase-locked to the stimulus waveform with a precision even greater than observed in auditory nerve fibers, due to a coincidence of synaptic input from the endbulb of Held synapses onto spherical bushy cells (Fig. 1C). MSO neurons also show phase-locked responses to monaural stimulation, however binaural stimulation at a best ITD generates a response that is greater than the sum of the monaural responses (Fig. 1D; Joris et al., 1998) and has a higher degree of phase-locking than at unfavorable ITDs (Yin and Chan, 1990). Thus, MSO neurons show sub-millisecond selectivity to ITDs. Note that the peak and the slope of the ITD function can be used to encode the location of the sound in cats (blue rectangle in Fig. 1D), whereas the peak ITD for gerbils may lie outside of the physiologically relevant time range (Grothe at al., 2010). Thus, there may be differences in the ITD coding mechanisms in cats and the small-headed gerbil.
A simple, but seminal model to describe coding of ITD was first proposed by Jeffress in 1948 (Grothe et al, 2010). According to this model, coincident detectors receive convergent input from the two ears, and fire maximally when the external delay (the time between the sound arriving at both ears; the ITD; Fig. 1B) is exactly compensated by an internal delay that is due to differences in the lengths of axons that converge onto the coincident detector neuron (Fig. 1A). The bushy cell inputs to the MSO phase-lock to low frequency sounds and MSO neurons fire maximally to coincident input (Fig. 1D; Yin and Chan, 1990). They are sensitive to positive ITDs, which means that they fire best to sounds that are on the contralateral side. This provides evidence for internal delay lines as it takes longer for the signal to travel from the contralateral ear compared to the ipsilateral ear.
While in birds there is both neurophysiological and anatomical support for the Jeffress model (Burger et al., 2011), it is more controversial in mammals. The controversies revolve around the origin of internal delays and the role of inhibition in shaping ITD tuning (Grothe et al., 2010). In contrast to the bird NL (Burger et al., 2011),MSO neurons receive feed-forward inhibitory input from the medial nucleus of the trapezoid body (MNTB) and the lateral nucleus of the trapezoid body (LNTB; Fig. 1A; Chirila et al., 2007). How these inputs interact to shape sensitivity to ITDs in MSO neurons is not entirely clear. Blocking glycinergic inhibition bilaterally in vivo by iontophoretic application of strychninebroadens ITD tuning and shifts peak tuning towards 0 μs (Brand et al., 2002; Pecka et al., 2008). Thus in mammals, glycinergic inhibition may function to actively shift the ITD selectivity of MSO neurons to preferentially respond to stimuli that lead in the contralateral ear. However, the elegant study by van der Heijen et al. in this issue provides compelling new evidence that ITD tuning in MSO neurons is determined by two simple features: the linear summation of the excitatory inputs from both ears and the nonlinear dependence of spike probability on the size of the EPSPs. Usingin vivo whole-cell and juxtacellular recordings, they found no evidence of inhibition in the MSO neurons they recorded during presentation of pure tone binaural sounds. The authors suggest that the glycinergic input to MSO neurons may improvethe dynamic range of the neurons as has been suggested in the NL in birds (Yamada et al., 2013) and in the SBCs (Kuenzel et al. 2011). It is also possible that inhibition plays a role in the localization of more natural broadband sounds composed of many frequencies (such as vocalizations), and for the cocktail effect (suppression of sounds in noisy environments).
Clearly, the question of the in vivorole of inhibition in the MSO has not been fully answered. The second study in this issue (Roberts et al.) provides new insight into the role that inhibition may play inthe MSO. Roberts and colleagues developed a new thick slice preparation that includes the whole macrocircuit shown in Figure 1A, except for the cochlea. They were thus able to stimulate the auditory nerve and obtain IPSC and EPSC recordings from the MSO cells.This is the first time that IPSCs evoked by auditory nerve stimulation have been obtained from MSO neurons in brain slices. They found that stimulating the inhibitory inputs from the MNTB and LNTB caused IPSPs in MSO neurons 300-400 μs prior to excitation.Surprisingly, inhibition from the contralateral side, which involves an extra synapse (the calyx of Held synapse), can arrive earlier than excitation. They suggest that all the inhibitory sources of input to the MSO provide feed-forward inhibition that restricts the MSO neuron from firing except when the binaural excitatory inputs provide the largest, most synchronous EPSPs. In contrast to the in vivo experiments that blocked inhibition (Brand et al. 2002; Pecka et al., 2008), Roberts and colleagues did not find that the presence of inhibition shifted the location of the ITD function. Thus, both studies in this issue provide new insight into the mechanisms of sounds localization coding in the MSO of mammals. There is no doubt that resolution of the remaining issues will be an exciting avenue of future research.
What are the biophysical mechanisms that allow coincidence detection a la Jeffress to occur? In the barn owls, recent tour-de-force in vivo patch clamp recordings have shown that nucleus laminaris(NL; the bird analog of the MSO) neurons have remarkable properties: 1) a very low input resistance and a passive soma that is devoid of Na+ channels, 2) insanely fast EPSCs (half-width of 100 μs; perhaps due to higher bird-brain temperatures of 40-41°C), and 3) hundreds of phase-locked synaptic inputs from the contra and ipsilateral afferent axons (analogs of the SBC axons shown in Fig. 1A; Köppl, 2012). This allows the bird’s NL neurons to function as leaky coincidence detectors that produce phase-locked spikes to sound frequencies of up to 8 kHz. In mammals, phase-locking can occur only for frequencies < 2-3 kHz. Like NL neurons, MSO neurons are very leaky (input resistance of 5-10 MΟ) and have small spikes (about 10-30 mV in amplitude), but unlike NL neurons they receive surprisingly few excitatory inputs from SBC axons (2-4 large excitatory fibers per dendrite) and do not appear to have ultrafast EPSCs (Couchman et al., 2010). The role of inhibition in these two circuits is also very different (see Roberts et al., 2013). Thus, the biophysical mechanisms for coding low frequency sounds appear to be very different in big-headed birds and small-headed mammals. Given that the tympanic ear evolved independently in frogs, birds and mammals, these differences may not be too surprising (Grothe et al., 2010). Apart from these differences, a common mechanism has emerged from studies of different species: leaky coincidence detectors integrate excitatory signals from specialized synapses to produce well-timed spikes that encode thehorizontal location of sound sources with amazing accuracy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashida G, Carr CE. Sound localization: Jeffress and beyond. Curr. Opin. Neurobiol. 2011;21:745–751. doi: 10.1016/j.conb.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Preciseinhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Burger RM, Fukui I, Ohmori H, Rubel EW. Inhibition in the balance: binaurally coupled inhibitory feedback in sound localization circuitry. J. Neurophysiol. 2011;106:4–14. doi: 10.1152/jn.00205.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirila FV, Rowland KC, Thompson JM, Spirou GA. Development of gerbil medial superior olive: integration of temporally delayed excitation and inhibition at physiological temperature. J. Physiol. 2007;584:167–190. doi: 10.1113/jphysiol.2007.137976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman K, Grothe B, Felmy F. Medial superior olivary neurons receive surprisingly few excitatory and inhibitory inputs with balanced strength and short-term dynamics. J. Neurosci. 2010;30:17111–17121. doi: 10.1523/JNEUROSCI.1760-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol. Rev. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Sound localization and use of binaural cues by the gerbil (Meriones unguiculatus) Behav. Neurosci. 1988;102:422–428. doi: 10.1037//0735-7044.102.3.422. [DOI] [PubMed] [Google Scholar]
- Joris PX, Smith PH, Yin TC. Coincidence detection in the auditory system: 50 years after Jeffress. Neuron. 1998;21:1235–1238. doi: 10.1016/s0896-6273(00)80643-1. [DOI] [PubMed] [Google Scholar]
- Köppl C. Auditory neuroscience: how to encode microsecond differences. Curr Biol. 2012;22:R56–58. doi: 10.1016/j.cub.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Kuenzel T, Borst JGG, van der Heijden M. Factors controlling the input/output relationship of spherical bushy cells in the gerbil cochlear nucleus. J. Neurosci. 2011;31:4260–4273. doi: 10.1523/JNEUROSCI.5433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RS. Acoustic location of prey by barn owls (Tyto alba) J. Exp. Biol. 1971;54:535–573. doi: 10.1242/jeb.54.3.535. [DOI] [PubMed] [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J. Neurosci. 2008;28:6914–6925. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MT, Seeman SC, Golding NL. A mechanistic understanding of the role of feedforward inhibition in the mammalian sound localization circuitry. Neuron. 2013 doi: 10.1016/j.neuron.2013.04.022. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden M, Lorteije JAM, Plauška A, Roberts MT, Golding NL, Borst JGG. Directional hearing by linear summation of binaural inputs at the medial superior olive. Neuron. 2013 doi: 10.1016/j.neuron.2013.04.028. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R, Okuda H, Kuba H, Nishino E, Ishii TM, Ohmori H. The cooperation of sustained and phasic inhibitions increases the contrast of ITD-tuning in lowfrequency neurons of the chick nucleus laminaris. J. Neurosci. 2013;33:3927, 3938. doi: 10.1523/JNEUROSCI.2377-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TCT, Chan JCK. Interaural time sensitivity in medial superior olive of cat. J. Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]