Abstract
Diffusion imaging has made significant inroads into the clinical diagnosis of a variety of diseases by inferring changes in microstructure, namely cell membranes, myelin sheath and other structures that inhibit water diffusion. This review discusses recent progress in the use of diffusion parameters in predicting functional outcome. Studies in the literature using only scalar parameters from diffusion measurements, such as apparent diffusion coefficient (ADC) and fractional anisotropy (FA), are summarized. Other more complex mathematical models and post-processing uses are also discussed briefly.
Introduction
Over the last decade, magnetic resonance imaging (MRI) has moved beyond traditional anatomic imaging methods evaluating qualitative imaging features into more quantitative methods of measuring relaxation constants and investigating functional changes. Diffusion imaging has made significant inroads into clinical diagnosis of a variety of diseases by inferring changes in the microstructure, namely cell membranes, myelin sheath, and other structures that inhibit water diffusion. Numerous studies have investigated not only assessing tissues’ current status but also on the predictive value of diffusion parameters. This review will discuss the progress that has been made in using diffusion parameters in predicting functional outcome. This review will summarize studies in the literature, where only scalar parameters from diffusion measurements, such as apparent diffusion coefficient (ADC) and fractional anisotropy (FA), are utilized. Other more complex mathematical models and post-processing uses will be briefly discussed at the end.
Methods
Diffusion is a naturally occurring phenomenon in which water molecules move due to thermal motion. Any cell membrane and structure will inhibit the free motion of water and cause a change in the measureable diffusion parameter. The most commonly used diffusion imaging acquisition is the spin-echo diffusion with echo planar (EPI) readout. The spin-echo diffusion preparation is done with diffusion sensitizing gradients around the 180° refocusing pulse. Due to diffusion and spin echo refocusing properties, the lost in signal is proportional to the amount of diffusion in the imaging slice. The signal equation is , where b is the gradient factor and D is the diffusion coefficient. b-value is chosen dependent on the optimal weighting to measure the amount of diffusion, i.e. in a premature brain with high water content, b-value would be lower (usually 600) in comparison to an adult (1000). The EPI readout ensures a fast acquisition, which combined with parallel imaging methods, a full brain diffusion imaging scan with multiple directions of diffusion encoding can be done within a few minutes on a state-of-the-art MRI scanner.
A decade ago when computers and electronic hardware were the limiting factors, 3-direction diffusions (with diffusion sensitizing gradients on three orthogonal axes) were done and the average diffusion values were presented as ADCs,. With improvements in computing power and hardware electronics, 6-direction diffusion (with non-colinear pair gradients) or diffusion tensor imaging soon became the norm and rotationally invariant diffusion measurements were introduced, and ADCs were also noted as Dav. symmetric (Dxy = Dyx, Dxz = Dzx, Dyz=Dzy), so only 6 elements are needed to compute the tensor. It is diagonalized to , where the eigenvector describe diffusion in the orthogonal directions. FA can be generated from the variance of directionality of the structure using eigenvalues (scalar component of the eigenvector) of the tensor. Soon, investigators began pushing for higher numbers of diffusion directions and multiple b-values to better characterize complex diffusion in detail, especially in areas with multiple fiber crossings. Higher order of diffusion encodes can be reduced to generate ADC and FA for comparison purposes. Clinically, ADC remains to be the dominant parameter evaluated, with considerations of FA. In some research studies, individual eigenvalues are investigated to explain the changes in FA and to speculate the underlying tissue changes.
Results
Diffusion imaging has been used in clinical diagnosis in the pediatric population since first reported by Rutherford et al [1], however, studies with outcome correlations remain limited due to a variety of factors such as difficulties in follow-up and standard data collection. Studies involving hypoxic ischemic (HIE) and traumatic brain injuries (TBI) are the most common and frequently studied with outcome measures. With a more recent focus on premature birth, an increasing number of studies and results are being reported. Other uses of diffusion in pediatric imaging also include stroke, infection, and white matter diseases.
HIE
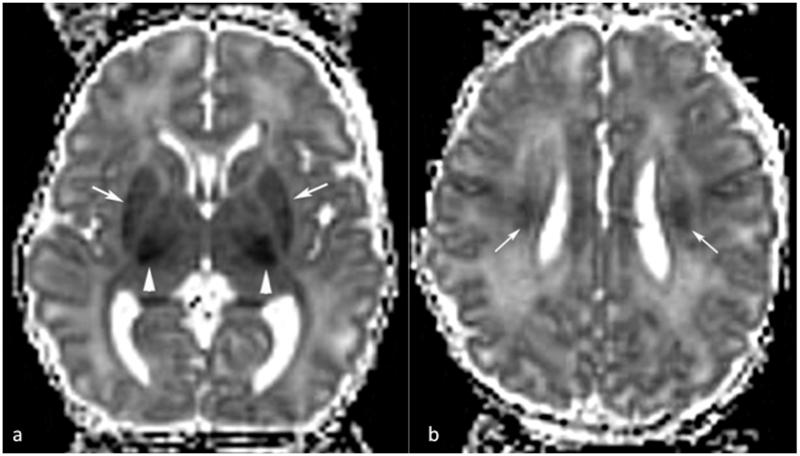
Approximately a decade ago, Barkovich et al reported the use of diffusion in assessing perinatal asphyxia [2]. Since then, numerous studies have investigated the use of diffusion, often together with standard anatomic and spectroscopic imaging, to better characterize the injury as well as the progression and subsequent changes [3-6]. HIE injuries are generally characterized by lower ADC that is seen within the first 24 hours after injury. Due to the complexity of the injury pattern and associated recovery, diffusion changes will vary depending on time of scan in respect to time of injury, with the ADC reaching a minimum value approximately 3 days after injury [7-10]. The majority of the studies show that low ADC is correlated with poor outcome. Figure 1 shows a typical case of HIE scanned at day 4 with low diffusivity. Areas of reduced diffusivity are shown in the lateral thalami/internal capsules (arrowheads in a), putamina (arrows in a), and corona radiata (arrows in b).
Figure 1.
Dav maps of a 4 day old neonate after hypoxia/ischemia. Areas of reduced diffusivity are shown in the lateral thalami/internal capsules (arrowheads in a), putamina (arrows in a), and corona radiata (arrows in b).
Hunt et al [4] found that reduced ADC in the posterior limb of the internal capsule (PLIC) is associated with poor neuromotor outcome at follow up (average of 12.9 months). Similarly, Vermenlen et al [11] found significantly lower ADC in the PLIC as well as other regions for their early imaging cohort (imaged at <4 days) and followed up at 2 years of age. Tekenouchi et al [12] also reported that ADC changes (imaged at day 7) in the splenium of the corpus callosum is associated with poor outcome classified by Mental Developmental Index of the Bayley Scales of Infant and Toddler Development, Third Edition at 18 months.
Using other classification methods, Alderliesten et al [13], in their 2011 study, classified their patients (imaged at median day 4, 1-14 days) into favorable and adverse using the Griffiths Developmental Quotient of less than 85 and found that patients with significantly lower ADC had adverse outcome at follow-up (average of 22 months). Boichot et al [14] reported lower ADC in the poor outcome group imaged at day 3 using the World Health Organization criteria of favorable outcome (Category 1 with no disability and Category 2 with mild to moderate disability) and poor outcome (Category 3 with severe disability and Category 4, death). The study of Liauw et al [15] indicated that lower ADC in the basal ganglia and brainstem is associated with adverse outcome in patients with HIE (imaged at mean 4.3 days, 1-9 days, grouped by Sarnat and Sarnat score [16]). However the presence of abnormal ADC in visibly abnormal regions did not predict outcome (classified by Van Wiechen examination, a Dutch child developmental assessment tool) during follow up at mean of 3.75 years.
In one of the few studies that did not find a correlation between ADC and outcome, Khong et al [17] reported no positive correlation between ADC and neuromotor outcome at 18 month. They noted that patients were imaged at different times following the injury (day 2-13 with mean of 6.7), which almost certainly contributed to the mixed results, as ADC values tend to normalize by 6-7 days after injury [8, 10].
TBI
Diffusion changes vary due to the severity and location of affected regions of the brain during a traumatic event [18]; therefore, correlations of diffusion with outcome are highly variable. Hergan et al [19] described three different appearances of diffusion changes and their suspected origins. The underlying mechanisms of injury, subsequent changes and time dependent imaging characteristics remain to be fully elucidated.
In 2008’s study by Galloway et al [20] demonstrated lower ADC across the brain associated with poor outcome determined by the Pediatric Cerebral Performance Category Scale (PCPCS) score. The children were imaged at 7 ± 4 days and followed up in 6-12 months. It was concluded that total average brain ADC predicted outcome in 83.8% of the cases with peripheral gray matter showing significant lower ADC in severely injured group with poor outcome. Galloway et al speculated that the higher ADC in the peripheral regions of patients with better outcome may indicate edema and injury repair mechanisms that remain to be investigated. It also suggested that the diffuse axonal injuries are likely to be hemorrhagic [21], in which the susceptibility effects may have contributed to the higher ADC values, which previous studies have reported [19]. Babikian et al [22] in 2009 also found that ADC values from the cortical regions, both gray and white matter, correlated with neurocognitive measures, while ADC from deep gray and white areas did not. In contrast, Oni et al [23] found increased ADC in frontal regions.
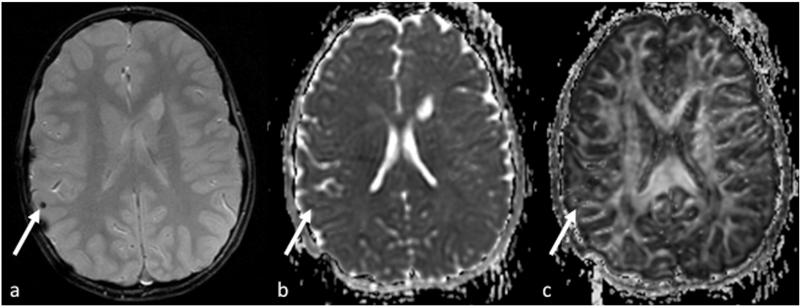
While ADC changes in TBI vary depending on the injury and time of scan [24-26], FA has shown to be reduced, reflected in the destruction of organization [27-29]. Figure 2 demonstrates a typical exam of TBI in an eight-year old patient with both low ADC and FA in the area of injury, also seen on the T2 image. Studies have further detailed regional FA changes and correlation with outcome, for example, Ewing-Cobbs et al in 2006 [30] and 2008 [31] correlated the decrease of FA in the corpus callosum. The corpus callosum changes in children with traumatic brain injury are of specific interest due to the complex nature of recovery from injury compounded by maturation through growth and myelination. Wu et al [32] performed a longitudinal evaluation of children with TBI at 3 and 18 months after injury, and demonstrated while reduced of FA was observed, those children did not perform differently on the arrow flanker test. It was suggested that at 3 months, adaptions were sufficient for the tasks and at 18 months, recovery through the development of other fiber pathways maybe possible.
Figure 2.
A typical exam of TBI in an eight-year old patient with both low ADC (b) and FA (c) in the area of injury, also seen on the T2 image (a).
Prematurity
With the continued increase in premature births, various ongoing studies are investigating the predictive value of diffusion with outcome. As the brain matures, ADC decreases and FA increases due to the development of structures and increased myelination [33-37] and they generally correlate with Bayley Scales of Infant Development [38]. With follow-ups at a late stage, Mullen et al [39] found positive correlation between language tests and FA in the uncinate fasciculi for children born premature and studied at age of 16. In comparison, FA was higher overall in those who were born at term. The investigators have also noted that no correlation was found in many other tracts, possibly due to early external intervention and different timing for pruning of white matter tracts.
A more recent development in evaluating preterm infant neuronal maturation is the focus on the cerebellum. A study by Tam et al [40] showed that increased diffusivity in the brain stem and cerebellum is associated with intraventricular hemorrhage (IVH) in prematurely born infants, and further demonstrated that IVH is associated with cerebellar hypoplasia [41]. Cerebellar involvement in motor learning [42] and memory [43] have been documented.
Stroke
Similar to imaging stroke for adult patients, the presentation of diffusion changes will vary with time of scan with respect to time of injury [44]. A study by Domi et al [45] demonstrated that acute diffusion weighted imaging (DWI) in the descending corticospinal tract can be used to predict motor outcome in childhood arterial ischemic stroke; the greater the reduction of the ADC, the more guarded the outcome. It is interesting to note that, similar to an earlier study [46], the investigators used the diffusion weighted images instead of ADC due to the variability of the latter [47]; one possible reason would be diffusion in specific orientations may be unevenly affected. The extent of hyperintensity on DWI had a strongly negative association with outcome measures. In the more recent studies, ADC values have been used to predict outcome. 2010’s study by Rosso et al showed that early ADC changes in motor areas of acute stroke predicted outcome better than DWI lesion volume. In 2011, van der Aa et al [48] demonstrated that diffusion values at 3 months of age in children who suffered neonatal stroke are, in fact, predictive of motor outcome.
Future Directions
The ability to create a brain connectivity network by the use of diffusion imaging and tractography is enabling the studies of the brain as a whole in children who have suffered ischemic or traumatic injury. This is highly advantageous due to the plasticity of the brain, which results in remapping some of its functionalities. Most recent interests in connectivity (connectome) will undoubtedly further utilize diffusion in predicting outcome. For example, Tymofiyeva et al described an association of increased characteristic path length and a decrease in clustering coefficient with increasing poor neuromotor score [49]. The predictive value of diffusion may be further improved when used in conjunction with other imaging methods such functional connectivity [50, 51], metabolic profile [52, 53], and perfusion.
References
- 1.Rutherford MA, Cowan FM, Manzur AY, et al. MR imaging of anisotropically restricted diffusion in the brain of neonates and infants. Journal of Computer Assisted Tomography. 1991;15(2):188–98. doi: 10.1097/00004728-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Westmark KD, Bedi HS, et al. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am J Neuroradiol. 2001;22(9):1786–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Boichot C, Walker PM, Durand C, et al. Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology. 2006;239(3):839–48. doi: 10.1148/radiol.2393050027. [DOI] [PubMed] [Google Scholar]
- 4.Hunt RW, Neil JJ, Coleman LT, et al. Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics. 2004;114(4):999–1003. doi: 10.1542/peds.2003-0935-L. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen RJ, Fetter WP, Hendrikx L, et al. Diffusion-weighted MRI in severe neonatal hypoxic ischaemia: the white cerebrum. Neuropediatrics. 2003;34(2):72–6. doi: 10.1055/s-2003-39599. [DOI] [PubMed] [Google Scholar]
- 6.Zarifi MK, Astrakas LG, Poussaint TY, et al. Prediction of adverse outcome with cerebral lactate level and apparent diffusion coefficient in infants with perinatal asphyxia. Radiology. 2002;225(3):859–70. doi: 10.1148/radiol.2253011797. [DOI] [PubMed] [Google Scholar]
- 7.Soul JS, Robertson RL, Tzika AA, et al. Time course of changes in diffusion-weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics. 2001;108(5):1211–4. doi: 10.1542/peds.108.5.1211. [DOI] [PubMed] [Google Scholar]
- 8.McKinstry RC, Miller JH, Snyder AZ, et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology. 2002;59(6):824–33. doi: 10.1212/wnl.59.6.824. [DOI] [PubMed] [Google Scholar]
- 9.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Archives of disease in childhood. Fetal and neonatal edition. 2010;95(3):F220–4. doi: 10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- 10.Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27(3):533–47. [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen RJ, van Schie PE, Hendrikx L, et al. Diffusion-weighted and conventional MR imaging in neonatal hypoxic ischemia: two-year follow-up study. Radiology. 2008;249(2):631–9. doi: 10.1148/radiol.2492071581. [DOI] [PubMed] [Google Scholar]
- 12.Takenouchi T, Heier LA, Engel M, et al. Restricted diffusion in the corpus callosum in hypoxic-ischemic encephalopathy. Pediatric neurology. 2010;43(3):190–6. doi: 10.1016/j.pediatrneurol.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Alderliesten T, de Vries LS, Benders MJ, et al. MR imaging and outcome of term neonates with perinatal asphyxia: value of diffusion-weighted MR imaging and (1)H MR spectroscopy. Radiology. 2011;261(1):235–42. doi: 10.1148/radiol.11110213. [DOI] [PubMed] [Google Scholar]
- 14.Boichot C, Mejean N, Gouyon JB, et al. Biphasic time course of brain water ADC observed during the first month of life in term neonates with severe perinatal asphyxia is indicative of poor outcome at 3 years. Magnetic resonance imaging. 2011;29(2):194–201. doi: 10.1016/j.mri.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Liauw L, van Wezel-Meijler G, Veen S, et al. Do apparent diffusion coefficient measurements predict outcome in children with neonatal hypoxic-ischemic encephalopathy? AJNR. American journal of neuroradiology. 2009;30(2):264–70. doi: 10.3174/ajnr.A1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of neurology. 1976;33(10):696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 17.Khong PL, Tse C, Wong IY, et al. Diffusion-weighted imaging and proton magnetic resonance spectroscopy in perinatal hypoxic-ischemic encephalopathy: association with neuromotor outcome at 18 months of age. Journal of child neurology. 2004;19(11):872–81. doi: 10.1177/08830738040190110501. [DOI] [PubMed] [Google Scholar]
- 18.Suskauer SJ, Huisman TA. Neuroimaging in pediatric traumatic brain injury: current and future predictors of functional outcome. Developmental disabilities research reviews. 2009;15(2):117–23. doi: 10.1002/ddrr.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hergan K, Schaefer PW, Sorensen AG, et al. Diffusion-weighted MRI in diffuse axonal injury of the brain. European radiology. 2002;12(10):2536–41. doi: 10.1007/s00330-002-1333-2. [DOI] [PubMed] [Google Scholar]
- 20.Galloway NR, Tong KA, Ashwal S, et al. Diffusion-weighted imaging improves outcome prediction in pediatric traumatic brain injury. Journal of neurotrauma. 2008;25(10):1153–62. doi: 10.1089/neu.2007.0494. [DOI] [PubMed] [Google Scholar]
- 21.Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227(2):332–9. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 22.Babikian T, Tong KA, Galloway NR, et al. Diffusion-weighted imaging predicts cognition in pediatric brain injury. Pediatric neurology. 2009;41(6):406–12. doi: 10.1016/j.pediatrneurol.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Oni MB, Wilde EA, Bigler ED, et al. Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. Journal of child neurology. 2010;25(8):976–84. doi: 10.1177/0883073809356034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of neurosurgery. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 25.Salmond CH, Menon DK, Chatfield DA, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29(1):117–24. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Tasker RC, Salmond CH, Westland AG, et al. Head circumference and brain and hippocampal volume after severe traumatic brain injury in childhood. Pediatric research. 2005;58(2):302–8. doi: 10.1203/01.PDR.0000169965.08854.25. [DOI] [PubMed] [Google Scholar]
- 27.Kurowski B, Wade SL, Cecil KM, et al. Correlation of diffusion tensor imaging with executive function measures after early childhood traumatic brain injury. Journal of pediatric rehabilitation medicine. 2009;2(4):273–83. doi: 10.3233/PRM-2009-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wozniak JR, Krach L, Ward E, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2007;22(5):555–68. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin HS, Wilde EA, Chu Z, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. The Journal of head trauma rehabilitation. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewing-Cobbs L, Hasan KM, Prasad MR, et al. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR. American journal of neuroradiology. 2006;27(4):879–81. [PMC free article] [PubMed] [Google Scholar]
- 31.Ewing-Cobbs L, Prasad MR, Swank P, et al. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42(4):1305–15. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu TC, Wilde EA, Bigler ED, et al. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Developmental neuroscience. 2010;32(5-6):361–73. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee P, Miller JH, Shimony JS, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221(2):349–58. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 34.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: Development in newborns with and without injury. Journal of Magnetic Resonance Imaging. 2002;16(6):621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 35.Neil J, Miller J, Mukherjee P, et al. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomed. 2002;15(7-8):543–52. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23(9):1445–56. [PMC free article] [PubMed] [Google Scholar]
- 37.Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurology. 2009;8(11):1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 38.Drobyshevsky A, Bregman J, Storey P, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Developmental neuroscience. 2007;29(4-5):289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 39.Mullen KM, Vohr BR, Katz KH, et al. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54(4):2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam EW, D M. Ferriero,, D Xu,, et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatric research. 2009;66(1):102–6. doi: 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam EW, Miller SP, Studholme C, et al. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. The Journal of pediatrics. 2011;158(3):366–71. doi: 10.1016/j.jpeds.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(12):4230–9. doi: 10.1523/JNEUROSCI.6353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Law N, Bouffet E, Laughlin S, et al. Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: impact on working memory. Neuroimage. 2011;56(4):2238–48. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 44.Kuker W, Mohrle S, Mader I, et al. MRI for the management of neonatal cerebral infarctions: importance of timing. Childs Nervous System. 2004;20(10):742–748. doi: 10.1007/s00381-004-0988-1. [DOI] [PubMed] [Google Scholar]
- 45.Domi T, deVeber G, Shroff M, et al. Corticospinal Tract Pre-Wallerian Degeneration A Novel Outcome Predictor for Pediatric Stroke on Acute MRI. Stroke. 2009;40(3):780–787. doi: 10.1161/STROKEAHA.108.529958. [DOI] [PubMed] [Google Scholar]
- 46.Kirton A, Shroff M, Visvanathan T, et al. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke. 2007;38(3):974–980. doi: 10.1161/01.STR.0000258101.67119.72. [DOI] [PubMed] [Google Scholar]
- 47.De Vries LS, Van der Grond J, Van Haastert IC, et al. Prediction of outcome in new-born infants with arterial ischaemic stroke using diffusion-weighted magnetic resonance imaging. Neuropediatrics. 2005;36(1):12–20. doi: 10.1055/s-2005-837544. [DOI] [PubMed] [Google Scholar]
- 48.van der Aa NE, Leemans A, Northington FJ, et al. Does Diffusion Tensor Imaging-Based Tractography at 3 Months of Age Contribute to the Prediction of Motor Outcome After Perinatal Arterial Ischemic Stroke? Stroke. 2011;42(12):3410–3414. doi: 10.1161/STROKEAHA.111.624858. [DOI] [PubMed] [Google Scholar]
- 49.Tymofiyeva O, Hess CP, Ziv E, et al. Towards the “baby connectome”: mapping the structural connectivity of the newborn brain. PLoS One. 2012;7(2):e31029. doi: 10.1371/journal.pone.0031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fransson P, Aden U, Blennow M, et al. The functional architecture of the infant brain as revealed by resting-state FMRI. Cereb Cortex. 2011;21(1):145–54. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- 51.Kalpakidou AK, Allin MP, Walshe M, et al. Neonatal Brain Injury and Neuroanatomy of Memory Processing following Very Preterm Birth in Adulthood: An fMRI Study. PLoS One. 2012;7(4):e34858. doi: 10.1371/journal.pone.0034858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 53.Xu D, Bonifacio SL, Charlton NN, et al. MR spectroscopy of normative premature newborns. J Magn Reson Imaging. 2011;33(2):306–11. doi: 10.1002/jmri.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]