Abstract
Background
A multiple myeloma (MM) vaccine has been developed whereby patient derived tumor cells are fused with autologous dendritic cells (DCs), creating a hybridoma that stimulates a broad anti-tumor response. We report on the results of a phase II trial in which patients underwent vaccination following autologous stem cell transplantation (ASCT) to target minimal residual disease.
Methods
Twenty-four patients received serial vaccinations with DC/myeloma fusion cells following post-transplant hematopoietic recovery. A second cohort of 12 patients received a pre-transplant vaccine followed by post-transplant vaccinations. DCs generated from adherent mononuclear cells cultured with GM-CSF, IL-4 and TNFα were fused with autologous bone marrow-derived MM cells using polyethylene glycol (PEG). Fusion cells were quantified by determining the percentage of cells that co-express DC and MM antigens.
Findings
The post-transplant period was associated with reduction in general measures of cellular immunity; however, an increase in CD4 and CD8+ myeloma specific T cells was observed after ASCT that was significantly expanded following post-transplant vaccination. Seventy-eight percent of patients achieved a best response of CR+VGPR and 47% achieved a CR/nCR. Remarkably, 24% of patients who achieved a partial response following transplant were converted to CR/nCR after vaccination and at over 3 months post-transplant, consistent with a vaccine-mediated effect on residual disease.
Interpretation
The post-transplant period for patients with multiple myeloma provides a unique platform for cellular immunotherapy in which vaccination with DC/MM fusions resulted in the marked expansion of myeloma specific T cells and cytoreduction of minimal residual disease.
Introduction
Advances in biologically based therapy with agents such as bortezomib and lenalidomide have resulted in high rates of disease response and improved long term outcomes for patients with multiple myeloma (MM)1. However, patients uniformly experience progression due to the persistence of resistant disease. The unique efficacy of cellular immunotherapy is supported by the observation that allogeneic hematopoietic stem cell transplantation is curative for a subset of patients due to the graft versus disease effect mediated by allo-reactive lymphocytes2–7. Conversely, allogeneic transplantation is associated with significant morbidity and mortality secondary to the lack of specificity of the allo-reactive response, which results in graft versus host disease. A major area of investigation is focused on developing strategies to elicit myeloma specific immune responses that selectively eliminate malignant cells.
We have developed a tumor vaccine in which patient derived myeloma cells are fused with autologous dendritic cells (DCs) such that a broad array of tumor antigens are presented in the context of the antigen presenting machinery of the DC fusion partner8. DC/tumor fusions uniquely stimulate both helper and cytotoxic T cell responses9. In animal tumor models including MM, vaccination with DC/tumor fusions results in eradication of established disease10–13. In a phase I trial of patients with myeloma, we demonstrated that vaccination with DC/tumor fusions was well tolerated and induced potent anti-tumor immune responses and disease stabilization in a majority of patients14.
A fundamental challenge to developing effective cellular immunotherapy is reversing the immunosuppressive milieu found in patients with myeloma. Animal models have demonstrated a paradoxical increase in response to tumor vaccines during the period of post-transplant lymphopoietic reconstitution associated with the transient reversal of tumor mediated tolerance15,16. We hypothesized that autologous transplantation would provide an ideal platform for the fusion vaccine due to the enhanced immunologic environment resulting from tumor cytoreduction and regulatory T cell depletion. In this study, patients with multiple myeloma underwent ASCT followed by vaccination with DC/MM fusions in the early post-transplant period (cohort 1) or a single pre-transplant vaccination followed by post-transplant boosting (cohort 2).
Methods
Patient Characteristics
Patients considered candidates for autologous transplantation were potentially eligible. A minimum of 20% plasma cells in the bone marrow was required to facilitate vaccine generation. Patients with a history of clinically significant autoimmune disease or organ dysfunction were excluded. Prior to initiating post-transplant vaccination, patients were required to have evidence of hematopoietic recovery (WBC> 2.0 K/µl and platelets > 50 K/µl) and resolution of grade III or greater transplant associated toxicity.
Reagents for Vaccine Characterization and Immunologic Assays
Purified mouse anti-human monoclonal antibodies (mAbs) against HLA-DR, CD80, CD86, CD40, CD83, CD38, and CD138; phycoerythrin (PE)-conjugated mouse anti-human mAbs against CD4; fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (RPA-T4, IgG1), CD8 (RPA-T8, IgG1) and FITC-, PE- conjugated matching isotype IgG1, IgG2a, IgG2b controls; and purified mouse monoclonal IgG1 (MOPC-21) isotype control were purchased from BD PharMingen (San Diego, CA). Monoclonal antibody DF3 (anti-MUC1 N-ter) has been described previously17. Anti-human CD4 TC-conjugated, matching isotype control (IgG2a) PE-conjugated anti-human mAbs against IFN-γ (mouse IgG1-B27) and PE-conjugated matching isotype controls (rat IgG1-PE and mouse IgG1-PE) were purchased from Invitrogen (Carlsbad, CA). FITC-conjugated goat anti-mouse (IgG1) was purchased from Chemicon International (Temecula, CA).
Vaccine Generation
Bone marrow mononuclear cells were isolated from 20–30 cc of bone marrow aspirate by ficoll density gradient centrifugation and cultured in RPMI 1640 culture media containing 2 mmol/l L-glutamine (Lonza, Walkersville, MD), heat-inactivated 10% autologous serum, and 10 µg/ml gentamicin (Baxter). In some cases, myeloma cells were cryopreserved in 10% DMSO/90% autologous plasma, and later thawed at the time of fusion generation. An aliquot of myeloma cells was cryopreserved for subsequent assessment of myeloma specific immunity.
DCs were generated from adherent mononuclear cells isolated from a leukapheresis collection and cultured with 1000U/mL GM-CSF (Berlex Wayne/Montville, NJ) and 500 IU/ml IL-4 (Cellgenix USA, Antioch, IL) for 5–7 days and matured in the presence of 25 ng/ml TNFα (Cellgenix) for 2–3 days. DC/tumor fusions were generated using polyethylene glycol (PEG) as previously described14,18. DC/tumor fusions were quantified by determining the percentage of cells that co-express DC (CD80, CD86, and CD83) and tumor associated (CD38 and CD138) antigens by immunohistochemical analysis. The cell product was cryopreserved without further manipulation in autologous plasma (90%) and DMSO (10%) in single dose vial of 5×105–5×106 fusion cells (depending on cell yields). The sterility of the product was confirmed by mycoplasma, endotoxin, and sterility assays. At time of vaccine administration, the fused cells were thawed, assessed for viability and sterility, and irradiated with 30cGy.
The capacity of the fusion cell preparation to stimulate allogeneic T cell proliferation was assessed by co-culturing 1×105 T cells obtained from leukopak collections with MM cells, DCs, or fusion cells at a ratio of 10:1 for 5 days. T cell proliferation was determined by measuring incorporation of [3H]thymidine following overnight pulsing (1µCi/well) of triplicate samples.
Study Schema
Patients received up to 1 year of primary induction therapy. Stem cell mobilization was accomplished with cyclophosphamide (2.5 or 3 grams/m2) followed by G-CSF. A minimum of 2×106 CD34+ cells/kg was required to proceed with high dose chemotherapy (melphalan 200mg/m2).
Vaccine Administration
Patients received three post transplant vaccinations given at 4 week intervals. Patients in the second cohort received an additional vaccination prior to stem cell mobilization. GM-CSF (100 µg) was administered as a subcutaneous injection at the vaccine site on the day of vaccination and for 3 days thereafter. Patients were evaluated weekly during the period of vaccination and then monthly for 6 months after the completion of vaccination.
Assessment of myeloma specific immunity
PBMCs were collected prior to stem cell mobilization and pre-transplant vaccination (cohort 2), prior to each post-transplant vaccination, and at 1, 3, and 6 months post-vaccination. At the completion of the study, 1 × 106 cells were cultured for 5 days with autologous myeloma lysate generated by repeated freeze thaw cycles of 1 ×105 autologous myeloma cells. Cells were restimulated with tumor lysate for 6 hours and cultured overnight with 1 ug/ml GolgiStop. Intracellular expression of IFNγ by CD4+ or CD8+ T cells was determined by FACS analysis. In HLA-A2.1 patients, the number of CD8+ T cells binding the MUC1 tetramer was determined by bidimensional FACS analysis using CD8-FITC and MUC1 tetramer-PE antibody. As measures of general immunity, PBMCs were cocultured with tetanus toxoid (10 µg/ml) for 5 days or the mitogen, PHA, (2 ug/ml) for 3 days and the proliferative response was quantified by measuring incorporation of [3H]thymidine following overnight pulsing (1µCi/well). Levels of regulatory T cells were quantified by determining the percentage of CD4/CD25high T cells using bidimensional FACS analysis. Expression of FOXP3 by CD4/CD25 cells was measured using intracellular FACS analysis.
Clinical Disease Assessment
Disease status was evaluated by serum and urine protein electrophoresis, serum light chain quantification, skeletal survey, and bone marrow aspiration and biopsy. Disease response was assessed according to the international myeloma working group uniform response criteria19.
Statistical analysis
For analysis of immune response to vaccination, Wilcoxon signed rank test and Wilcoxon rank sum test were used to compare paired and independent samples, respectively. Progression-free survival (PFS), defined as the time from the date of transplantation to the date of disease progression or death from any cause, is estimated using the Kaplan-Meier approach. Statistical analysis was performed using SAS/STAT software, Version 9.2 of the SAS System for Windows Copyright © 2002–2008 SAS Institute Inc.
Results
Patient Characteristics
Twenty-four and 12 patients were vaccinated in the first and second cohorts, respectively. 26 patients were enrolled into the first cohort, of which 24 were vaccinated; nineteen patients were enrolled into the second cohort, of which 12 were vaccinated. 9 patients were removed from study prior to receiving vaccination for the following reasons: Two patients developed an intercurrent illness during pre-transplant therapy and did not undergo an autologous transplant; one patient was found to have amyloidosis and was removed from study; two patients chose not to undergo autologous transplantation; one patient did not respond to pre-transplant therapy and underwent an allogeneic rather than an autologous transplantation, and two patients withdrew consent and chose to be treated with standard of care autologous transplantation without vaccination. In one instance, vaccination could not be made according to specification and the patient was removed from study.
Patient characteristics are summarized in Table 1. Patients received a median of 2 regimens prior to initiating stem cell mobilization with the goal of achieving maximal cytoreduction prior to transplantation. Therapy consisted of thalidomide, lenalidomide, and bortezomib based regimens in 11 (30.5%), 7 (19%), and 24 (67%) patients, respectively. Eleven patients (30.5%) received lenalidomide, bortezomib and dexamethasone (RVD) as part of their pre-transplant therapy.
Table 1.
Patient Characteristics
| Male, female (%) | 73, 27 |
| Median age years (range) | 57.5 (35–70 years) |
| Median % plasma cells in bone marrow at enrollment | 55 |
| Median # of regimens prior to stem cell collection (range) | 2 (1–5) |
| Pre-transplant therapy: | |
| Velcade based regimen (%) | 67 |
| Lenalidomide based regimen (%) | 19 |
| Thalidomide based regimen (%) | 30.5 |
| Lenalidomide, Velcade, dexamethasone (RVD) (%) | 30.5 |
| Median time from study enrollment to transplant | 6.8 months |
| Median time from transplant to vaccination (months) | 1.3 |
Vaccine Characteristics
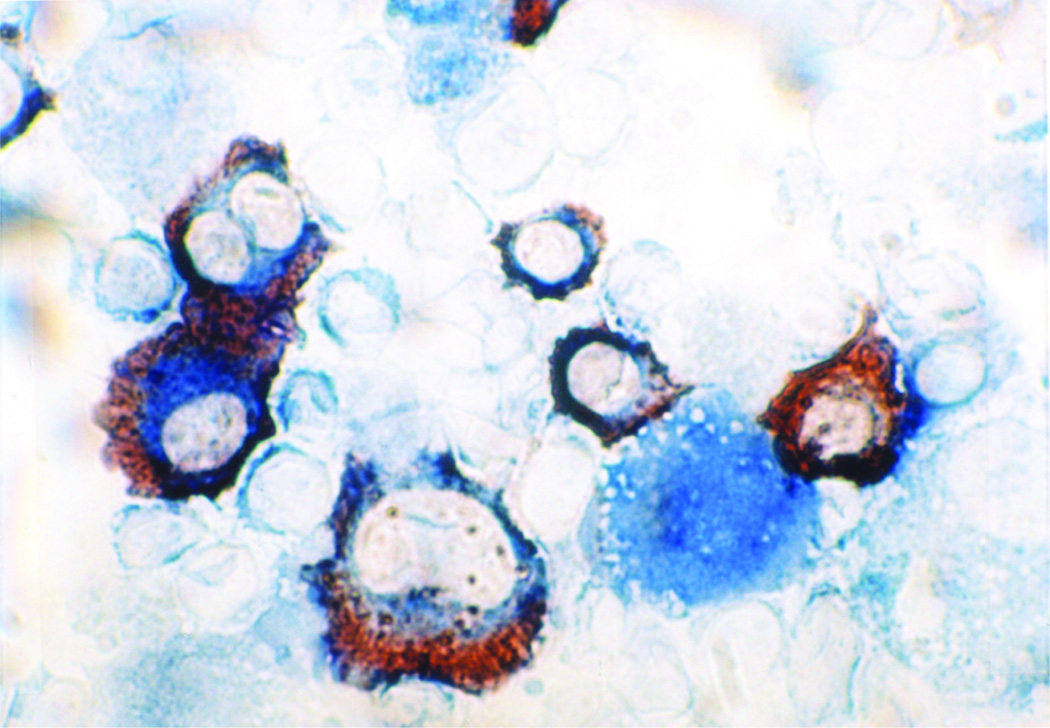
Mean yield of the DC and MM cells was 1.74 × 108 and 6.5 × 107 cells, respectively. Mean fusion efficiency as determined by the percentage of cells that co-expressed unique DC (CD80, CD86, and/or CD83) and MM (CD38 and/or CD138) antigens was 38% (Figure 1). The mean dose administered was 3.6 × 106 fusion cells. Mean viability of the DC, myeloma, and fusion preparations was 87%, 87%, 79%, respectively. The DC/MM fusion preparations exhibited potent antigen presenting capacity as evidenced by their stimulation of allogeneic T cell proliferation with a mean stimulation index of 29.9, similar to that observed with the DC preparation prior to fusion (mean stimulation index of 49.8). In contrast, the unfused myeloma cells demonstrated minimal capacity for T cell stimulation (mean stimulation index of 12.7).
Figure 1. Immunohistochemical staining of DC/myeloma fusion cells.
Fusion cells were generated by co-culture of DCs and myeloma cells in the presence of PEG. Fusion cell preparations were analyzed for co-expression of the DC derived costimulatory molecule CD86 (blue) and tumor associated antigen CD38 (red). Image is shown at 100× magnification.
Adverse Events
Toxicities judged to be possibly related to vaccination are summarized in Table 2. All vaccine associated toxicities were of grade I–II intensity. The most common toxicity was erythema and induration at the vaccine injection site associated with T cell infiltration on biopsy. The other common side effects were transient pruritis, rash, fatigue, fever, and myalgias. One patient had a transient elevation in the ANA level without clinical evidence of autoimmunity. One patient developed transient grade 2 leukopenia, but no evidence of graft compromise was observed following vaccination.
Table 2.
Related Adverse events
| Adverse event | Grade | Number of episodes |
|---|---|---|
| Vaccine site reaction (erythema, itching) | 1 | 38 |
| 2 | 6 | |
| Myalgia | 1 | 9 |
| Arthralgia | 1 | 2 |
| Pruritis | 1 | 5 |
| 2 | 1 | |
| Leukopenia | 1 | 3 |
| 2 | 2 | |
| Headache | 1 | 3 |
| Diarrhea | 1 | 1 |
| Fatigue | 1 | 2 |
| 2 | 1 | |
| Fever | 1 | 3 |
| Chills | 1 | 2 |
| Elevated TSH | 1 | 1 |
| Edema | 1 | 1 |
| Decreased appetite | 1 | |
| Rash | 1 | 4 |
| 2 | 2 | |
| ANA positivity | 1 | 1 |
| Periorbital edema | 1 | 1 |
Post-transplant Immune Reconstitution
Consistent with prior reports, the post-transplant period was associated with the relative suppression of general measures of cellular immunity. Decreased levels of CD4+ T cells and the inversion of the CD4/CD8 ratio for greater than 6 months post-transplant was seen (data not shown). T cell proliferation in response to in vitro exposure to the T cell mitogen, PHA, or the recall antigen, tetanus toxoid, was markedly depressed in the early post-transplant period and did not fully recover by 6 months post-transplant (Figure 2).
Figure 2. Depression of general measures of immunity following autologous transplant.
PBMC were collected at the indicated time points and incubated with 2 ug/ml of PHA (A) or tetanus toxoid (B) at 10 ug/ml. Proliferation was measured by incorporation of tritiated thymidine. Values are presented as a stimulation index (proliferation of stimulated/unstimulated cells). The results are presented as mean values +/− SEM from 33 patients.
Impact of Vaccination on Myeloma Specific Immunity
Vaccination resulted in the marked expansion of myeloma reactive lymphocytes, as determined by percentage of CD4 and CD8+ T cells expressing IFNγ in response to ex vivo exposure to autologous tumor lysate (Figure 3 A,B,C,D). All evaluable patients demonstrated at least a twofold expansion of myeloma specific CD4+ and/or CD8+ T cells. The median percentage of myeloma specific CD8+ T cells prior to transplant was 0.64, which reached a peak of 6.0 post-vaccination (p<0.05). The median percentage of myeloma specific CD4+ T cells was 0.43 and 4.0 pre-transplant and post-vaccination, respectively (p<0.05). Vaccination resulted in a mean log10 fold increase or 8.32 (95% CI 4.68;15.14) and 9.55 (95% CI 5.37;16.98) in CD8 and CD4+ myeloma specific T cells, respectively. Vaccination was associated with the expansion of T cells targeting the myeloma specific antigen, MUC1. In a cohort of patients who were HLA-A2, the median percentage of MUC1 specific T cells was 0.12 pre-transplant, and increased to 1.84 at 3 months after completion of vaccination (p<0.05), representing a median fold increase of 17.5 (Figure 4). Concomitant with the expansion of tumor reactive lymphocytes, regulatory T cells remained at low levels in the first 6 months post-transplant (Figure 5).
Figure 3.
A and B. Myeloma specific CD4 and CD8 T cells were significantly increased post-transplant and further expanded following post-transplant vaccination. Intracellular expression of IFNγ by CD4+ (A) and CD8+ (B) populations. PBMCs isolated prior to transplant, prior to each vaccination, and at serial time points post vaccination, were-cocultured with autologous tumor lysate. Cells were pulsed with Golgi Stop, labeled with FITC-conjugated CD4 or CD8 antibodies, and then permeabilized by incubation in Cytofix/Cytoperm plus™ (PharMingen). Cells were then incubated with PE-conjugated anti-interferon gamma or a matched isotype control antibody, and fixed in 2% paraformaldehyde. Labeled cells were analyzed by flow cytometry. The percentage of CD4+ (A) and CD8+ (B) T cells expressing IFNγ for individual study patients prior to stem cell mobilization (white), prior to first post-transplant vaccination (grey) and peak response following vaccination (black) is shown.
C and D. Mean levels of myeloma specific T cells pre-transplant, post-transplant, and peak post-vaccination. Mean percentage of CD4+ (C) and CD8+ (D) T cells expressing interferon gamma following ex-vivo exposure to autologous tumor lyste is shown. Percentage of tumor reactive T cells is shown prior to stem cell mobilization, prior to the first post-transplant vaccination, and at the peak time post-vaccination. The results are presented as mean values +/− SEM from 23 patients.
Figure 4. Expansion of MUC1 tetramer positive cells following vaccination.
CD8+ T cells binding the MUC1 tetramer were quantified at serial time points (prior to each vaccimation and at 1, 3, 6 months post vaccination) in patients who are HLA-A2.1. Binding to a control tetramer was quantified in parallel and the control value was subtracted from that obtained for the MUC1 tetramer. Values of a representative patient (A) and mean values of 5 patients (B) are presented demonstrating a marked increase in MUC1 tetramer+ cells following vaccination.
Figure 5. Circulating regulatory T cells decline following autologous transplantation.
PBMCs were collected prior to transplant, prior to each vaccination, and at 1, 3, and 6 months post-vaccination. CD4/CD25high cells were quantified by FACS analysis. Red lines represent mean values at each timepoint.
A rise in tumor reactive T cells was observed following autologous transplantation prior to vaccination (cohort 1), which was further boosted following vaccination with DC/MM fusions. The mean log10 fold increase in myeloma specific CD8+ T cells from pre-mobilization to post-transplant and from pre- mobilization to peak post-vaccination was 6.76 (95% CI 3.02;15.49) and 11.48 (95% CI 4.17;32.36), respectively. Similarly, CD4+ T cells pre-mobilization and at peak post-vaccination were 3.55 (95% CI 1.58;8.13) and 9.55 (95% CI 5.37;16.98), respectively. Of note, no difference in peak levels of CD4+ or CD8+ circulating myeloma specific T cells was observed between the cohort receiving a pre-transplant vaccine and that undergoing post-transplant vaccination alone (p=0.185 and p=0.689, respectively).
Clinical Response
Seventy-eight percent of patients achieved a CR or VGPR (47% CR/nCR; 31% VGPR). Thirty-one percent achieved a CR/nCR in the early post-transplant period, whereas an additional 17% (6 patients: 4 from VGPR, 2 from PR) achieved CR/nCR as best response only after day 100 post-transplant after undergoing vaccination. The presence of late responses several months after ASCT is consistent with an impact of vaccine therapy on post-transplant residual disease. This response is illustrated by minimal residual disease analysis in a representative patient for whom transplant resulted in morphologic remission, but persistence of plasma cell kappa restriction. Following completion of vaccination, plasma cells were found to be polyclonal without evidence of light chain restriction (Figure 6). At a median follow-up from transplant of 45.6 months, the two year progression free survival was 57% (90% CI 41.5,69.8).
Figure 6. Eradication of post-transplant residual disease following vaccination.
Bone marrow biopsies were performed at diagnosis, prior to stem cell collections, prior to initiation of post-transplant vaccination, and following completion of post-transplant vaccinations. Bone marrow core biopsies were stained with hematoxylin and eosin (H+E) and immunohistochemistry for CD138 was performed to assess for plasma cell involvement. To assess for plasma cell clonality, immunohistochemistry for kappa and lambda light chain restriction was performed. An example (patient 18) demonstrating a representative pattern of disease response is shown. Following pre-transplant therapy, the patient achieved a PR with reduction in bone marrow plasmacytosis. Following transplant, less than 5% clonal plasma cells were detected in the marrow, which were kappa light chain restricted. Elimination of post-transplant residual disease was observed following completion of vaccination, where polyclonal plasma cells were observed without evidence of light chain restriction.
Discussion
We report on the use of a patient specific myeloma vaccine to elicit anti-myeloma immunity and target minimal residual disease post-transplant. The vaccine consists of a hybridoma of patient derived myeloma cells and autologous DCs with the potential to evoke a polyclonal response directed against multiple antigens, including those unique to a particular patient. In pre-clinical studies, fusion cells have been shown to more potently stimulate anti-tumor immunity than single antigen peptide vaccines20, and have demonstrated the ability to eradicate established metastatic disease21. Fusion cells are unique in eliciting a broad CD4 and CD8 mediated response and in targeting tumor heterogeneity. In a phase I study of DC/MM fusions, vaccination was well tolerated, induced anti-myeloma immunity in late stage patients and resulted in prolonged disease stability14.
ASCT in patients with MM results in high rates of disease response and a prolonged period of remission compared to standard chemotherapy, but is not curative22. The period of post-transplant lymphopoietic reconstitution is associated with enhanced responsiveness to cancer vaccines due to the depletion of inhibitory elements such as regulatory T cells that mediate tumor associated tolerance. In the present study, we demonstrated that vaccination with DC/MM fusions following ASCT was feasible, well tolerated, associated with the induction of anti-myeloma immunity and conversion of patients from PR to CR/nCR in the late post-transplant period. An approximately 10 fold expansion of myeloma specific CD4 and CD8+ T cells was observed as manifested by the percentage of T cells expressing IFNγ following ex vivo exposure to autologous tumor lysate. We also demonstrated a 15 fold increase in MUC1+ T cells as evidence for the vaccine mediated expansion of T cells with specificity for myeloma associated antigens. As predicted in pre-clinical models, the early post-transplant period was characterized by the suppression of general measures of cellular immunity, but the paradoxical moderate expansion of myeloma reactive T cells in the context of low levels of regulatory T cells. This provided an ideal platform for vaccination, which resulted in the further expansion of myeloma specific T cells.
To date, myeloma vaccines have focused on the targeting of individual myeloma associated antigens including idiotype, WT1, survivin, hTERT, and NY-ESO23–25. While vaccination has resulted in antigen specific immunity particularly in patients with low volume disease, clinical efficacy has been uncertain. In the present study, 47% of patients achieved a CR as best response and 78% of patients achieved at least a VGPR, comparing favorably to other studies combining bortezomib and/or lenalidomide induction with ASCT26. Of note, nearly 35% of CRs occurred greater than 100 days post-transplant, after undergoing vaccination. While delayed effects of chemotherapy may be observed, the significant number of late responses in the absence of maintenance therapy is strongly suggestive of a vaccine mediated effect.
While there is strong rationale for the use of patient specific vaccines that express multiple antigens, there is potential concern for the feasibility of this approach and its applicability across different medical centers. In patients with multiple myeloma, there is ready availability of autologous tumor cells via bone marrow aspiration. Vaccine production was successful in 98% of patients and the process of hybridoma generation is feasible for centers with experience in cell manipulation. Another concern is the reestablishment of tumor tolerance over time facilitating disease progression. The negative check point co-stimulatory molecules embodied in the PD-1/PDL-1 pathway are critical mediators of tumor anergy27. In an ongoing study, we are evaluating the effect of combining vaccination with DC/myeloma fusion cells and PD-1 blockade following autologous transplantation.
Patients in this study did not receive maintenance therapy with lenalidomide. Recent randomized studies have demonstrated that lenalidomide maintenance therapy following autologous transplantation results in improved progression free and overall survival28,29. In vitro studies demonstrate that lenalidomide augments immune response to vaccines30, suggesting a potential for synergy between lenalidomide and vaccines in the post-autologous transplant setting. Combining vaccination with maintenance lenalidomide following autologous transplantation is an area worthy of study. In summary, this study demonstrates that potent anti-tumor immune responses and elimination of post-transplant residual disease can be achieved with an autologous tumor vaccine administered in the early post-transplant period. A randomized trial is planned to examine the impact of post-transplant vaccination on disease free and overall survival.
Supplementary Material
Statement of Translational relevance.
The manuscript details results of a clinical study of patients with multiple myeloma who undergo autologous transplantation in conjunction with vaccination with a patient-specific myeloma vaccine created by the fusion of myeloma cells with autologous dendritic cells. Vaccination in the post-transplant period results in the expansion of myeloma specific T cells. Most significantly, despite the lack of post-transplant maintenance therapy, 47% and 78% of patients achieved a best response of complete response or >90% regression, respectively. Nearly half of those patients only achieved complete response greater than 100 days post-transplant after the period of vaccination, suggesting a role of post-transplant immunotherapy. These data suggest that vaccination post-transplant may impact post-transplant residual disease potentially changing the paradigm of myeloma therapy. It sets the platform for studying the role of vaccination in conjunction with maintenance therapy in a randomized trial.
Acknowledgements
This work was funded in part by the Leukemia and Lymphoma Society (LLS grant #R6133-05 to DA), the Multiple Myeloma Research Foundation (MMRF Senior Research Award to DA) and the National Institute of Health (NIH grant # 5 PO1 CA078378-10 to KA). KA is an American Cancer Society Clinical Research Professor. Dr. Glen Dranoff critically reviewed the manuscript.
Footnotes
Authorship Contributions:
Dr. Jacalyn Rosenblatt was involved in research design, patient accrual and assessments, analyzing data, and manuscript preparation. Dr. Irit Avivi was involved with study design, patient accrual and assessments, and manuscript preparation. Dr. Baldev Vasir was involved in research design, performance of immunologic correlative studies, data analysis, and manuscript preparation. Drs. Lynne Uhl and Tami Katz supervised vaccine preparation. Poorvi Somaiya and Lina Bisharat were responsible for vaccine generation. Heidi Mills was responsible for vaccine characterization. Drs. Robin Joyce, James D. Levine, Nikhil Munshi Paul Richardson, Bimalangshu Dey, Dimitrios Tzachanis, Jacob Laubach, Noopur Raje, Vassiliki Boussiotis, were involved in patient accrual and assessments. Yan Emily Yuan and Viki Held were involved in data collection. Dr. Edie Weller and Federico Campigotto were involved in data analysis. Dr. Kenneth Anderson was involved in patient accrual, study design, and manuscript preparation. Dr. Jacob Rowe was involved in study design and manuscript preparation. Dr. Donald Kufe was involved in study design, data analysis and manuscript preparation. Dr. David Avigan is the principal investigator of the clinical trial, and was involved in study design, data analysis, and manuscript preparation.
Conflict of Interest Disclosures: There are no conflicts of interest to disclose
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Bellucci R, Wu CJ, Chiaretti S, et al. Complete response to donor lymphocyte infusion in multiple myeloma is associated with antibody responses to highly expressed antigens. Blood. 2004;103:656–663. doi: 10.1182/blood-2003-07-2559. [DOI] [PubMed] [Google Scholar]
- 3.Crawley C, Iacobelli S, Bjorkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109:3588–3594. doi: 10.1182/blood-2006-07-036848. [DOI] [PubMed] [Google Scholar]
- 4.Gahrton G, Svensson H, Cavo M, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983--93 and 1994--8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–216. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 5.Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 6.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkstrand BB, Ljungman P, Svensson H, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood. 1996;88:4711–4718. [PubMed] [Google Scholar]
- 8.Vasir B, Borges V, Wu Z, et al. Fusion of dendritic cells with multiple myeloma cells results in maturation and enhanced antigen presentation. Br J Haematol. 2005;129:687–700. doi: 10.1111/j.1365-2141.2005.05507.x. [DOI] [PubMed] [Google Scholar]
- 9.Parkhurst MR, DePan C, Riley JP, Rosenberg SA, Shu S. Hybrids of dendritic cells and tumor cells generated by electrofusion simultaneously present immunodominant epitopes from multiple human tumor-associated antigens in the context of MHC class I and class II molecules. J Immunol. 2003;170:5317–5325. doi: 10.4049/jimmunol.170.10.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong J, Avigan D, Chen D, et al. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci U S A. 2000;97:2715–2718. doi: 10.1073/pnas.050587197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 12.Gong J, Chen D, Kashiwaba M, et al. Reversal of tolerance to human MUC1 antigen in MUC1 transgenic mice immunized with fusions of dendritic and carcinoma cells. Proc Natl Acad Sci U S A. 1998;95:6279–6283. doi: 10.1073/pnas.95.11.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J, Koido S, Chen D, et al. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002;99:2512–2517. doi: 10.1182/blood.v99.7.2512. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt J, Vasir B, Uhl L, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–3019. [PubMed] [Google Scholar]
- 16.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 18.Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002;51:299–310. doi: 10.1007/s00262-002-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 22.Moreau P, Avet-Loiseau H, Harousseau JL, Attal M. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J Clin Oncol. 29:1898–1906. doi: 10.1200/JCO.2010.32.5878. [DOI] [PubMed] [Google Scholar]
- 23.Szmania S, Tricot G, van Rhee F. NY-ESO-1 immunotherapy for multiple myeloma. Leuk Lymphoma. 2006;47:2037–2048. doi: 10.1080/10428190600742292. [DOI] [PubMed] [Google Scholar]
- 24.Bergenbrant S, Yi Q, Osterborg A, et al. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996;92:840–846. doi: 10.1046/j.1365-2141.1996.419959.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsuboi A, Oka Y, Nakajima H, et al. Wilms tumor gene WT1 peptide-based immunotherapy induced a minimal response in a patient with advanced therapy-resistant multiple myeloma. Int J Hematol. 2007;86:414–417. doi: 10.1007/BF02983998. [DOI] [PubMed] [Google Scholar]
- 26.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005–01 phase III trial. J Clin Oncol. 28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 27.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 30.Luptakova KGB, Mills H, Stroopinsky D, Vasir B, Rosenblatt J, Kufe D, et al. Lenalidomide Decreases PD-1 Expression, Depletes Regulatory T-Cells and Improves Cellular Response to a Multiple Myeloma/ Dendritic Cell Fusion Vaccine In Vitro. Blood (ASH Annual Meeting Abstracts) 2010;116:492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.