BRCA1 or BRCA2 mutations predispose to cancer development and lead to defective DNA repair capacity, called “BRCAness.” Attempts have been made to exploit this differentially expressed feature between tumors and normal tissues by treatment with DNA-damaging chemotherapy agents. This review summarizes the data supporting differential chemotherapeutic sensitivity on the basis of defective DNA repair.
Keywords: BRCAness, Breast cancer, BRCA mutations, Differential chemosensitivity
Learning Objectives
Explain the concept of sporadic “BRCAness” in breast tumors.
Describe the current status, obstacles, and future direction for utility of sporadic “BRCAness” in breast tumors.
Abstract
BRCA1 or BRCA2 mutations predispose to cancer development, primarily through their loss of role in the repair of DNA double-strand breaks. They play a key role in homologous recombination repair, which is a conservative, error-free DNA repair mechanism. When mutated, other alternative, error-prone mechanisms for DNA repair take over, leading to genomic instability. Somatic mutations are rare in sporadic breast tumors, but expression of BRCA1 and BRCA2 genes can be downregulated in other mechanistic ways. These tumors have similar features in terms of their phenotypic and genotypic profiles, which are normally regulated by these genes, and mutations lead to defective DNA repair capacity, called “BRCAness.” Attempts have been made to exploit this differentially expressed feature between tumors and normal tissues by treatment with DNA-damaging chemotherapy agents. Cells with this functional BRCA deficiency should be selectively susceptible to DNA-damaging drugs. Preclinical and early clinical (primarily retrospective) evidence supports this approach. In contrast, there is emerging evidence of relative resistance of tumors containing BRCA1 or BRCA2 mutations (or BRCAness) to taxanes. In this review, we summarize the data supporting differential chemotherapeutic sensitivity on the basis of defective DNA repair. If confirmed with available, clinically applicable techniques, this differential chemosensitivity could lead to treatment choices in breast cancer that have a more individualized biologic basis.
Implications for Practice:
Women with germline BRCA mutations are more prone to develop breast, ovarian, and other cancers because of the inability to repair DNA damage effectively. These mutations cause a small minority of breast cancers, but studies have shown that such tumors respond better when treated with DNA-damaging chemotherapy agents. Evidence shows that nonmutated tumors also have defective DNA repair or “BRCAness” caused by other mechanisms and behave similarly to BRCA-mutated tumors. Some clinical data support that tumors with BRCAness respond better to DNA-damaging chemotherapy. Preliminary data suggest that tumors with intact BRCA1 respond better to treatment with antitubulin agents. In this review, we discuss BRCAness and the clinical data supporting preferential responses to different chemotherapy agents. No standardized test to detect BRCAness exists yet, and various techniques are being developed because this test could affect chemotherapy choice.
Introduction
Breast cancer is a heterogeneous disease with different clinicopathological features, responses to treatment, and prognoses. Progress in the development of targeted therapies (HER-2/neu targeting with monoclonal antibodies and small molecule inhibitors) has made a substantial difference in both response and survival. Despite significant clinical advances, there are still 40,000 women in the U.S. who die of breast cancer each year [1]. Consequently, there is a continuing need to search for other potential therapeutic strategies.
It is well known that women with germline mutations in BRCA1 or BRCA2 are at increased risk of developing breast and ovarian cancers [2]. In addition, there is a higher risk of pancreatic, prostate, and male breast cancer [2]. This risk is thought to be related to the roles of BRCA1 and BRCA2 genes in DNA repair. DNA damage activates cell-cycle check points and recruitment of DNA repair machinery. In cells deficient in BRCA1 or BRCA2, there is defective DNA repair of double-strand DNA breaks (DSB) through homologous recombination (HR), which is a conservative DNA repair mechanism with a high degree of fidelity. Alternative error-prone, potentially mutagenic DNA repair mechanisms like non-homologous end joining and single-stranded annealing compensate but lead to genomic instability [3]. The relative roles of BRCA1 and BRCA2 in repair of DNA DSB have been explored and better defined over the past two decades. BRCA1 is a critical organizing molecule that has been linked to a range of cellular processes beyond DNA repair, like transcriptional regulation and chromatin remodeling. BRCA2 function in HR is primarily via regulation of RAD51 activity [4]. BRCA2 regulates RAD51 recombinase, which is a critical step in strand invasion and homology-directed repair [4].
Germline mutation in one BRCA1 or BRCA2 allele is sufficient to predispose for cancer development [5]. There is a loss of heterozygosity, with loss of the normal allele while retaining the mutant allele, in the tumor tissue of susceptible individuals, suggesting that the genes play a normal role as tumor suppressors [6, 7]. Somatic mutations in BRCA1 or BRCA2 do not occur frequently in sporadic (or nonfamilial) breast cancer [8, 9], but potentially any (somatic) inactivation of the genes could result in their phenotypic repression [10]. A phenomenon called “BRCAness” (or, more properly, “BRCAlessness”) has been reported in sporadic cancers that do not have the germline mutations in BRCA1 or BRCA2 but that display similar inactivation of the BRCA-related genes and consequently have defective HR [3].
Inactivation of the BRCA1 gene leads to breast tumor histology that has a higher grade, that is more likely to be estrogen receptor (ER) negative, that has v-MYC avian myelocytomatosis viral oncogene homalog (CC-MYC) overexpression, and that lacks ERBB2 (HER-2/neu) amplification [11, 12]. BRCA1 gene mutation-associated breast cancers typically display a basal-like molecular subtype [13, 14] similar to that composing the majority of triple-negative breast cancers (TNBCs). This finding suggests that therapeutic approaches targeted to BRCA1 mutation-associated breast cancers might be applicable to sporadic TNBCs. Unlike the familial BRCA1 mutation-associated tumors, which have a characteristic phenotype, familial BRCA2 mutation-associated tumors do not have a consistent, distinct molecular phenotype. With currently available methods, it is hard to delineate histopathological characteristics that differentiate familial BRCA2 mutation-associated tumors from sporadic cancer, but BRCA2-associated tumors tend to be ER positive and HER2 negative [15].
The therapeutic implications of BRCA1 or BRCA2 mutations or of a sporadic BRCAness phenomenon remains unproven, but initial clinical evidence (see below in clinical data section) suggests that there could be higher activity of DNA damaging agents like alkylators, platinators, and anthracyclines in these groups. In addition, preliminary data suggest relative resistance of such tumors to agents that act by stabilizing microtubule polymers (e.g., the taxane class of drugs). This review addresses the possible relationship of the defective BRCA1 and/or BRCA2 function (in mutation carriers and sporadic breast cancers) in relation to sensitivity to different chemotherapeutic agents.
Etiology of Sporadic BRCAness
The BRCA1 gene is transcriptionally regulated by a CpG island promoter that is unmethylated in all normal human cell types [16]. The BRCA1 gene is inactivated by aberrant DNA methylation in approximately 15% of sporadic breast cancers overall, with a higher incidence of epigenetic inactivation in TNBC [17–19]. The frequency of epigenetic inactivation exceeds the frequency of genetic mutation of BRCA1 in breast cancer and is likely to be a primary force behind BRCAness. A second feature that can influence the BRCAness of a cell is gene dosage. The BRCA1 gene resides on chromosome 17 in a region frequently lost in breast cancer. Consequently, homozygous deletions of this region may lead to BRCA1 haploinsufficiency, reflecting a lesser but potentially relevant degree of BRCAness [20].
In contrast to BRCA1, hypermethylation of the BRCA2 gene promoter is not reported as a cause of its inactivation [21]. Instead, reports of chromosome 11 open reading frame 30 (C11orf30; also known as EMSY) gene amplification have been implicated in BRCA2 inactivation [22]. EMSY, when overexpressed, inhibits BRCA2 transcriptional activity by interacting with exon 3 of BRCA2. EMSY amplification has been reported in up to 13% of sporadic breast cancers with most of them having ER-positive tumor biology [3].
Unlike the familial BRCA1 mutation-associated tumors, which have a characteristic phenotype, familial BRCA2 mutation-associated tumors do not have a consistent, distinct molecular phenotype. With currently available methods, it is hard to delineate histopathological characteristics that differentiate familial BRCA2 mutation-associated tumors from sporadic cancer, but BRCA2-associated tumors tend to be ER positive and HER2 negative.
Detecting BRCAness
Several efforts to identify this signature have been reported using different methodologies. These efforts include array comparative genomic hybridization (aCGH), quantitative real-time polymerase chain reaction (qPCR), multiplex ligation-dependent probe amplification (MPLA), and immunohistochemistry (IHC). Techniques may be based on DNA, RNA, or proteins and have been reported using frozen or formalin-fixed tumor tissue. Each technique has its unique advantages and disadvantages, and those details are beyond the scope of this review. Currently no standard way of defining or detecting BRCAness has found its way to clinical application, but this is an area of intense investigation.
The recognized steps to incorporation of a predictive biomarker into clinical care involve analytic validation, then clinical validation, and finally assessment of clinical utility [23]. It is important to recognize at the outset that analytic validation of a standardized technique that can be applied to clinical pathological material has not yet been accomplished, as reflected by the multiplicity of methods currently in use; clinical validation and ultimate assessment of clinical utility could then be formally addressed. Other predictive markers with proven utility, including HER2 and the ER, went through a lengthy process on the way to achieving these goals for adoption into clinical practice, and we hope the same may ultimately prove to be true of a test for HR deficiency (HRD).
Preferential Effect of Chemotherapy in Relation to BRCA1 or BRCA2 Expression
The key roles that the BRCA1 and BRCA2 genes play in DNA repair is through HR, and their dysfunction affects this least error-prone repair mechanism. Consequently, these cells could be more sensitive to chemotherapy agents that produce DNA damage by causing strand breaks through failure to reseal cleavable complexes in strand passage or by intercalation with base pairs (e.g., anthracyclines) or through DNA adduct formation (e.g., alkylators and platinating agents) with subsequent intra- or interstrand DNA crosslinks and resultant DSB [24].
Preclinical data suggest that low levels of BRCA1 in cell lines correlate with resistance to taxanes and vinca alkaloids [24, 25]. This response may be dependent on tumor type, with breast cancer cell lines preferentially showing this effect [25]. These breast cancer cell line data have been replicated in vivo in mice, where docetaxel resistance was noted in spontaneous breast tumors that have deletion of BRCA1 [26]. The dominant mechanism by which BRCA1 is involved in taxane response is unknown, but several have been proposed. One is a differential apoptotic response, which is mediated by BRCA1 induction of Growth Arrest and DNA-Damage Inducible (GADD45A; also known as GADD45) transcription [27]. Another mechanistic hypothesis is a BRCA1-induced increase in c-Jun N-terminal kinase (JNK)/stress-activated protein kinase phosphorylation, with subsequent apoptosis in BRCA1-expressing cells treated with paclitaxel [28–30]. Taxanes disrupt mitotic spindle assembly by stabilizing microtubules and thereby triggering expression of a spindle check point. BRCA1 is important for transcriptional upregulation of spindle assembly checkpoint proteins, and loss of its function inhibits their critical disruption by taxanes [30]. Despite published clinical reports suggesting an analogous direct relationship between loss of BRCA2 expression and taxane resistance [31], a direct pathophysiological link between the BRCA2 gene and sensitivity to the taxane class of drugs has yet to be demonstrated.
Clinical Studies of BRCA1 or BRCA2 Mutation Carriers
The majority of the published clinical reports are retrospective (Table 1). Regimens used in those studies were reported as anthracycline-based or cyclophosphamide, methotrexate, and 5-flourouracil (CMF)-like regimens. Higher response rates were reported overall among patients with BRCA1 or BRCA2 mutations when treated with DNA-damaging regimens. The largest series reported responses in 121 BRCA1 or BRCA2 mutation carriers with metastatic breast cancer compared with matched sporadic breast cancer patients [32]. All patients in both groups received anthracycline-based or CMF or CMF-like regimens as treatment. Patients with BRCA2 mutations had a higher overall response rate (ORR), higher progression-free survival (PFS), and higher overall survival (OS) after the start of such chemotherapy in first-line treatment compared with their matched controls. In the same series, a nonsignificant trend for increased ORR and PFS was noted in BRCA1 mutation carriers. In this study, the cohorts were matched by age, year of primary cancer diagnosis, and year of metastatic breast cancer diagnosis but not for other predictive factors of known importance, such as ER and HER2, which could confound interpretation of the results.
Table 1.
Studies reporting chemotherapy efficacy in patients with BRCA1 or BRCA2 mutations
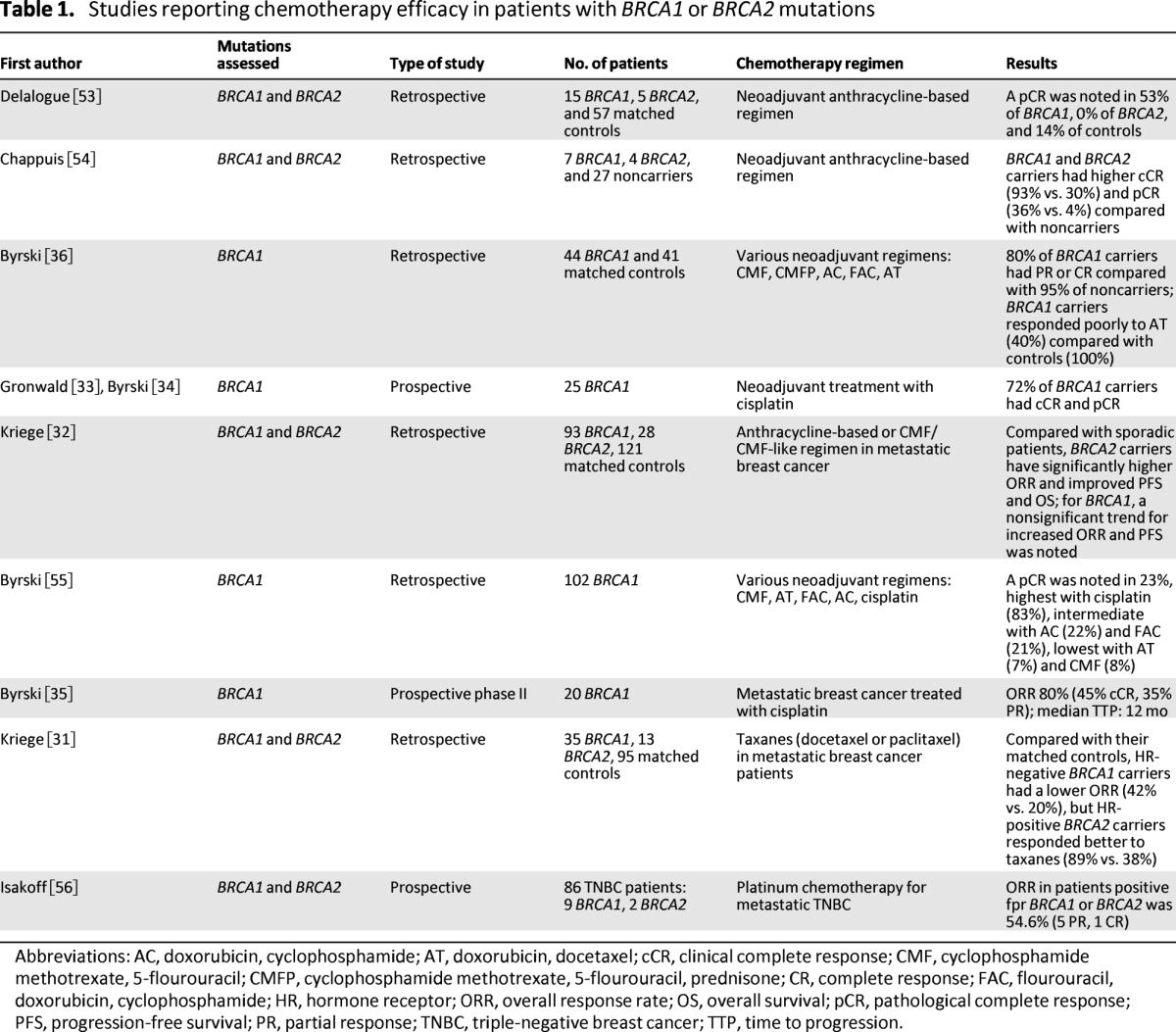
Abbreviations: AC, doxorubicin, cyclophosphamide; AT, doxorubicin, docetaxel; cCR, clinical complete response; CMF, cyclophosphamide methotrexate, 5-flourouracil; CMFP, cyclophosphamide methotrexate, 5-flourouracil, prednisone; CR, complete response; FAC, flourouracil, doxorubicin, cyclophosphamide; HR, hormone receptor; ORR, overall response rate; OS, overall survival; pCR, pathological complete response; PFS, progression-free survival; PR, partial response; TNBC, triple-negative breast cancer; TTP, time to progression.
Among various classes of DNA-damaging chemotherapeutic agents, cisplatin has been consistently reported to produce a higher ORR among BRCA1 mutation carriers. Two prospective clinical trials further assessed this hypothesis by administering cisplatin to patients with BRCA1 mutations. In a neoadjuvant trial of 25 BRCA1 mutation patients, there was a clinical complete response (cCR) and associated pathological complete response (pCR) (defined as no evidence of invasive cancer in breast and lymph nodes) in 18 patients after treatment with four cycles of cisplatin at 75 mg/m2 [33, 34]. In the metastatic BRCA1-associated breast cancer group, 20 patients were treated with cisplatin at similar doses for six cycles [35]. Response was observed in 16 patients, with 9 achieving complete response (CR) and 7 obtaining partial response (PR). These prospective clinical trials support the concept of exploiting defective HR in patients with BRCA1 (and BRCA2) mutations by treatment with platinum chemotherapy agents; however, this observation needs to be confirmed in larger, prospective clinical trials. Another caveat to any generalization about “platinators” is that experience in other cancer types indicates that drugs in the same family are not always equivalent (e.g., cisplatin is superior to carboplatin in the curative intent setting in testicular cancer), and this possibility exists in breast cancer as well, especially when carboplatin is given at an area under the curve of 2 rather than 6.
Efficacy of taxanes in patients with BRCA1 or BRCA2 mutations has been evaluated in two retrospective studies. One study reported a lower response rate (RR) in patients with BRCA1 mutations who received a neoadjuvant docetaxel-based combination regimen [36]. This study reported that 6 of 15 patients with BRCA1 mutations responded compared with 29 of 29 matched control patients (without the mutation). The cases were matched for age and treatment center. In addition to different tumor biology in the two cohorts (BRCA1 carriers were more likely to be triple negative), the regimen compared was a combination of docetaxel and doxorubicin and may confound the conclusions. Another study reported that taxanes were less effective (in terms of RR and PFS) in hormone receptor-negative BRCA1 mutation-associated metastatic breast cancers when matched to their sporadic breast cancer controls [31]. Again, appropriate control for other predictive markers for response was not done between the cohorts, and that significantly confounds the results. This is noted in the same study where 11 patients with hormone receptor-positive BRCA1 mutation-associated tumors have similar PFS as their sporadic counterparts. In addition, 10 patients with hormone receptor-positive BRCA2 mutation-associated breast cancer had a higher RR and similar PFS compared with sporadic breast cancer patients when treated with taxanes [31].
Studies Correlating BRCAness in Sporadic Tumors and Clinical Response
Table 2 lists the studies that report clinical correlation of a BRCA1- or BRCA2-like signature with administration of different chemotherapeutic agents. BRCA1- or BRCA2-like signature was determined with a variety of nonstandardized techniques (aCGH, MPLA, qPCR, or IHC). There is a higher incidence of BRCA1-like signature in tumors with triple-negative histology compared with BRCA2-like signature, which is reported to be higher in hormone receptor-positive breast tumors [18]. In addition to the different methodologies to identify BRCAness, information regarding other predictive markers for treatment outcome is not uniformly reported in all studies, a serious known shortcoming. Currently, there are no standardized ways to compare these techniques, making cross-study comparisons difficult.
Table 2.
Studies reporting chemotherapy efficacy in sporadic BRCAness
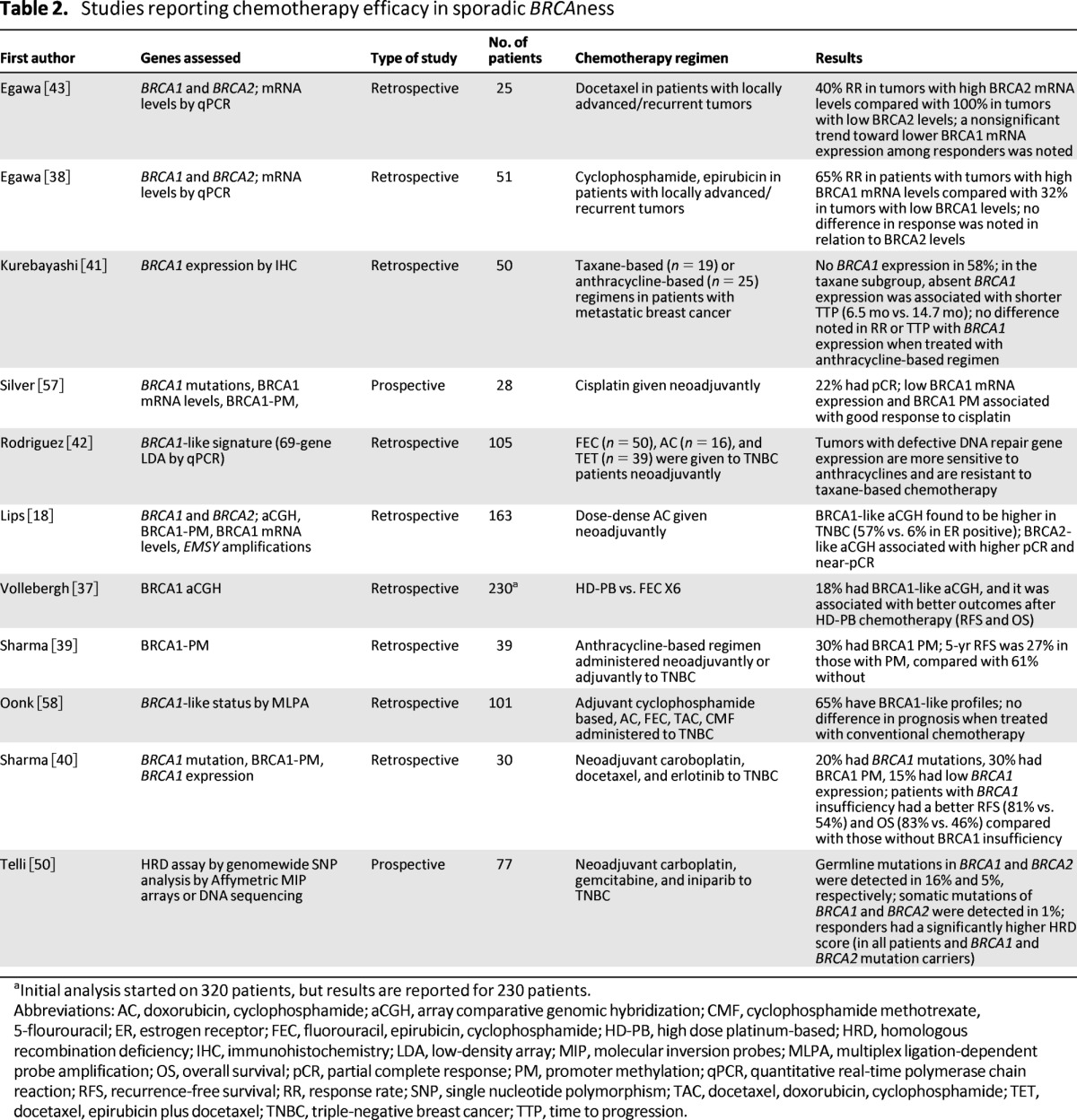
aInitial analysis started on 320 patients, but results are reported for 230 patients.
Abbreviations: AC, doxorubicin, cyclophosphamide; aCGH, array comparative genomic hybridization; CMF, cyclophosphamide methotrexate, 5-flourouracil; ER, estrogen receptor; FEC, fluorouracil, epirubicin, cyclophosphamide; HD-PB, high dose platinum-based; HRD, homologous recombination deficiency; IHC, immunohistochemistry; LDA, low-density array; MIP, molecular inversion probes; MLPA, multiplex ligation-dependent probe amplification; OS, overall survival; pCR, partial complete response; PM, promoter methylation; qPCR, quantitative real-time polymerase chain reaction; RFS, recurrence-free survival; RR, response rate; SNP, single nucleotide polymorphism; TAC, docetaxel, doxorubicin, cyclophosphamide; TET, docetaxel, epirubicin plus docetaxel; TNBC, triple-negative breast cancer; TTP, time to progression.
Tumors with BRCA1-like signature determined by aCGH are reported to be more sensitive to anthracycline-based or high-dose-platinum-based chemotherapy in two large retrospective studies [18, 37]. One of the studies reported that a BRCA2-like signature was surprisingly common among ER-positive patients, and such patients had a much higher rate of pathological response to neoadjuvant treatment with an anthracycline-based regimen [18]. The second large series found that 18% of all breast tumors had a BRCA1-like aCGH. A strength of this study was that specimens were assessed retrospectively from a randomized clinical trial comparing conventional chemotherapy with high-dose chemotherapy in stage II/III breast cancer patients. Among those patients with a BRCA1-like profile, significantly better recurrence-free survival (RFS) and OS was noted after treatment with the high-dose regimen, which contained platinator and alkylators at doses requiring autologous stem cell support for hematological recovery, versus the control arm [37].
Smaller studies have shown less consistent results. One study reported significantly higher BRCA1 messenger RNA (mRNA) levels in patients responding to treatment with cyclophosphamide and epirubicin [38]. The authors hypothesized that BRCA1 is needed for apoptosis, and an intact BRCA1 protein pushes the cell into apoptosis when DNA damage occurs. Preclinical and other clinical data reviewed suggest otherwise, and a further technical issue may involve intracellular localization of the RNA message being measured. Similarly, another report assessed BRCA1 promoter methylation in sporadic patients with TNBC and found decreased RFS at 5 years in patients whose tumors had BRCA1 promoter methylation, implying impaired, rather than augmented, effectiveness of an anthracycline-based approach in the setting of defective HR [39]. One possible difficulty with this observation is that it makes an assumption that BRCA1 promoter methylation accounts entirely for a BRCAness profile, whereas other causes (e.g., gene dosage) are known to exist. Moreover, the regimens used were reported as “anthracycline-based” adjuvant therapy, but frequently the standard approach involves administering an anthracycline and taxane, which may confound the drawn conclusion, especially if taxanes are less effective in tumors with defective repair. The same group recently reported clinical correlation of BRCA1 insufficiency (defined by either BRCA1 mutation or BRCA1 promoter methylation or decreased BRCA1 mRNA levels) with outcome in 30 TNBC patients who were treated with neoadjuvant combination of carboplatin, docetaxel, and erlotinib [40]. Patients with BRCA1 insufficiency had better RFS and OS when compared with those without BRCA1 insufficiency, but data for the mutation patients versus those with BRCAness were not provided.
Data are relatively scant with regard to taxanes and sporadic BRCAness. In a study of 50 patients with metastatic breast cancer, time to progression was shorter in patients with absent BRCA1 expression (as determined by IHC) when treated with taxanes [41]. This result is in concordance with preclinical data suggesting that intact BRCA1 is required for optimal taxane activity. In addition, it is supported by another small study of TNBC tumor patients in which a BRCA1-like signature was associated with relative resistance to taxane-based therapy [42]. In contrast, a study in 25 patients with locally advanced/recurrent breast tumors reported that a significantly lower BRCA2 mRNA level was noted among docetaxel responders compared with nonresponders [43]. Interpretation of this observation is complicated by the lack of a known mechanistic basis for specific effects of BRCA2 loss on taxane effectiveness. In the same study, there was a nonsignificant trend toward low BRCA1 mRNA levels in responders, whereas the preclinical data reviewed would support a relationship between intact BRCA1 function and taxane efficacy but in the opposite direction. All of these studies are retrospective, and the numbers of patients evaluated are small, precluding any definitive conclusions.
Less well known are data that intact BRCA1 function may be important for an optimal response to taxane-based therapy. Particularly among the patient subgroup with triple-negative disease, in which 30% to 50% may have BRCAness with loss of BRCA1 function, the presence of this feature may predict a better therapeutic index for DNA-damaging therapy (platinator, alkylator, anthracycline); it may predict for less benefit from antitubulin treatment with taxanes.
Discussion
BRCA1 and BRCA2 genes play a critical role in HR repair of DNA damage. Mutations in those genes or sporadic inactivation leading to BRCAness have been shown to have therapeutic implications, likely with an increased sensitivity to DNA-damaging agents. Less well known are data that intact BRCA1 function may be important for an optimal response to taxane-based therapy. Particularly among the patient subgroup with triple-negative disease, in which 30% to 50% may have BRCAness with loss of BRCA1 function [18], the presence of this feature may predict a better therapeutic index for DNA-damaging therapy (platinator, alkylator, anthracycline); it may predict for less benefit from antitubulin treatment with taxanes. Conversely, for the majority with intact BRCA1 function, taxane therapy may have the better therapeutic index. Because current standard of care chemotherapy for the triple-negative subset typically involves the combination of anthracycline/alkylator and taxane, it is important to determine the validity of these hypotheses; findings could result in superior outcomes based on more individualized choices. Currently, there are various techniques and methodologies to determine BRCAness, each with its own limitations. To mount appropriate prospective trials, there is a pressing need to develop reproducible, standardized techniques for the determination of BRCAness on formalin-fixed pathologic specimens.
Although BRCA2 is known to play an important role in HR repair, correlation of “BRCA2ness” with outcome to DNA damaging therapy is less well investigated, as is the frequency of this phenomenon. If the correlation is similar to that for BRCA1 downregulation and chemotherapy class response [18], and if the frequency is not rare, ER-positive patients may also benefit differentially from a choice of agent based on mechanism of action in the context of defective DNA repair. Again, the developmental of clinically applicable techniques to detect BRCA2ness will be critical to testing this hypothesis.
Currently, a randomized phase II/III trial is ongoing in Europe comparing responses of TNBC patients with defective HR, treated in the neoadjuvant setting with intensified alkylating chemotherapy (doxorubicin, cyclophosphamide followed by carboplatin, thiotepa, and cyclophosphamide) versus standard chemotherapy with dose-dense doxorubicin/cyclophosphamide or docetaxel/capecitabine (ClinicalTrials.gov identifier NCT01057069). In addition, another randomized phase II trial (ClinicalTrials.gov identifier NCT00861705) is evaluating the addition of carboplatin with and without bevacizumab to neoadjuvant weekly paclitaxel followed by dose-dense adiramycin-cyclophosphamide in TNBC. Blood and fresh frozen and fixed tumor tissue are being collected in this study for future biomarker analysis.
To date, published clinical data regarding differential chemosensitivity based on BRCA1 and BRCA2 mutation status seem strongest supporting platinum agents. The level of evidence is still preliminary, given the number of trials and patients evaluated. Data regarding taxanes in more preliminary and current evidence would not support withholding treatment with these agents in mutation carriers outside of a clinical trial. The concept of BRCAness in some sporadic tumors is provocative and, in our view, warrants further investigation. Studies have shown that sporadic BRCAness occurs in a reasonable proportion of patients, especially among those with TNBC. What needs to be developed is a standardized methodology to identify the signature, and larger trials are needed to evaluate chemosensitivity of such tumors to DNA-damaging agents. Only then can the assessment of BRCAness become part of clinical decision making outside of a clinical trial.
PARP1 and the Concept of “Synthetic Lethality”
Although related, it is important to distinguish between BRCAness in the context of DNA-damaging therapy and “synthetic lethality” in the context of concurrent inhibition of the PARP1 molecule. Synthetic lethality involved targeting of PARP1 in the setting of defective HR repair, which results in reciprocal increased dependence on upregulated PARP1 as a component of alternative repair pathways, such as base excision repair. When there was combined inhibition of both pathways in such a setting, it resulted in synthetic lethality and cell death in preclinical systems [44, 45]. The value of this approach has been demonstrated in breast and ovarian cancer patients with BRCA1 and BRCA2 mutations [46] but has not been demonstrated conclusively in the setting of sporadic BRCAness. Results of an initial promising phase II trial of the putative PARP inhibitor iniparib among sporadic metastatic breast cancer patients with TNBC have not been replicated in a larger phase III trial [47, 48]. Another phase II trial has failed to demonstrate any objective responses to the single-agent PARP inhibitor olaparib in TNBC patients [49]. In this study, however, no objective responses were noted, even among breast cancer patients with BRCA1 or BRCA2 mutations, which have been reported in other trials. A recent study of TNBC and BRCA1 and BRCA2 mutation-associated breast cancer reported that a higher HRD score predicts for pathological response after neoadjuvant platinum-based therapy in combination with iniparib [50]. Conflicting clinical data may be the result of the small sample size, of tumors from the patient populations under study that were variably enriched for BRCAness (not tested in the trials), and of the PARP inhibitor (iniparib) studied in some trials that has little actual activity against this target [51]. Several ongoing clinical trials of PARP inhibitors alone or in combination with chemotherapy (primarily platinum agents) [52] could provide more insight into the concept of synthetic lethality in sporadic breast tumors.
Conclusion
BRCA1 and BRCA2 germline mutations have an important role in DSB repair of DNA. Germline mutations or sporadic “BRCAness” cause defective BRCA1 or BRCA2 functions and subsequently impair DNA repair capacity. This feature makes these cells differentially more sensitive to DNA-damaging chemotherapeutic agents. There is emerging preclinical and some clinical evidence that such cells might be resistant to taxanes. Clinical studies (primarily retrospective and few prospective) have shown that this feature can be exploited for selecting chemotherapy agents, however, there is no standardized method to detect “BRCAness.” The concept of “BRCAness” is provocative and may bear fruit as a guide in future treatment selection. However, the test of its value awaits the validation of a simple, reproducible laboratory assay which can be applied to clinical material. Until then, we recommend further pursuit of this concept only in the context of clinical trials.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Pavani Chalasani, Robert Livingston
Provision of study material or patients: Pavani Chalasani, Robert Livingston
Collection and/or assembly of data: Pavani Chalasani, Robert Livingston
Data analysis and interpretation: Pavani Chalasani, Robert Livingston
Manuscript writing: Pavani Chalasani, Robert Livingston
Final approval of manuscript: Pavani Chalasani, Robert Livingston
Disclosures
The authors indicated no financial relationships.
Section editors: Gabriel Hortobágyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe (C/A); Novartis (RF); Taivex (O); board of directors for Citizen's Oncology Foundation; Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Sanofi, Ortho-Biotech, Amgen, and Bristol-Myers Squibb (C/A); (H)
Reviewer “A”: None
Reviewer “B”: Arno Therapeutics, Eisai, Genentech, GlaxoSmithKline, Johnson & Johnson, Roche (C/A)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348:2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- 3.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 4.Turner N, Tutt A, Ashworth A. Targeting the DNA repair defect of BRCA tumours. Curr Opin Pharmacol. 2005;5:388–393. doi: 10.1016/j.coph.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Venkitaraman AR. Functions of BRCA1 and BRCA2 in the biological response to DNA damage. J Cell Sci. 2001;114:3591–3598. doi: 10.1242/jcs.114.20.3591. [DOI] [PubMed] [Google Scholar]
- 6.Collins N, McManus R, Wooster R, et al. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12–13. Oncogene. 1995;10:1673–1675. [PubMed] [Google Scholar]
- 7.Cornelis RS, Neuhausen SL, Johansson O, et al. High allele loss rates at 17q12–q21 in breast and ovarian tumors from BRCAl-linked families. The Breast Cancer Linkage Consortium. Genes Chromosomes Cancer. 1995;13:203–210. doi: 10.1002/gcc.2870130310. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman N, Stratton MR. The genetics of breast cancer susceptibility. Annu Rev Genet. 1998;32:95–121. doi: 10.1146/annurev.genet.32.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Rhiem K, Todt U, Wappenschmidt B, et al. Sporadic breast carcinomas with somatic BRCA1 gene deletions share genotype/phenotype features with familial breast carcinomas. Anticancer Res. 2010;30:3445–3449. [PubMed] [Google Scholar]
- 11.Catteau A, Harris WH, Xu CF, et al. Methylation of the BRCA1 promoter region in sporadic breast and ovarian cancer: Correlation with disease characteristics. Oncogene. 1999;18:1957–1965. doi: 10.1038/sj.onc.1202509. [DOI] [PubMed] [Google Scholar]
- 12.Grushko TA, Dignam JJ, Das S, et al. MYC is amplified in BRCA1-associated breast cancers. Clin Cancer Res. 2004;10:499–507. doi: 10.1158/1078-0432.ccr-0976-03. [DOI] [PubMed] [Google Scholar]
- 13.Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 14.Lakhani SR, Reis-Filho JS, Fulford L, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 15.Lakhani SR, Van De Vijver MJ, Jacquemier J, et al. The pathology of familial breast cancer: Predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20:2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Rice JC, Massey-Brown KS, Futscher BW. Aberrant methylation of the BRCA1 CpG island promoter is associated with decreased BRCA1 mRNA in sporadic breast cancer cells. Oncogene. 1998;17:1807–1812. doi: 10.1038/sj.onc.1202086. [DOI] [PubMed] [Google Scholar]
- 17.Rice JC, Ozcelik H, Maxeiner P, et al. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000;21:1761–1765. doi: 10.1093/carcin/21.9.1761. [DOI] [PubMed] [Google Scholar]
- 18.Lips EH, Mulder L, Hannemann J, et al. Indicators of homologous recombination deficiency in breast cancer and association with response to neoadjuvant chemotherapy. Ann Oncol. 2011;22:870–876. doi: 10.1093/annonc/mdq468. [DOI] [PubMed] [Google Scholar]
- 19.Esteller M, Silva JM, Dominguez G, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 20.Staff S, Isola J, Tanner M. Haplo-insufficiency of BRCA1 in sporadic breast cancer. Cancer Res. 2003;63:4978–4983. [PubMed] [Google Scholar]
- 21.Bosviel R, Durif J, Guo J, et al. BRCA2 promoter hypermethylation in sporadic breast cancer. OMICS. 2012;16:707–710. doi: 10.1089/omi.2012.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes-Davies L, Huntsman D, Ruas M, et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115:523–535. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- 23.Hayes DF, Bast RC, Desch CE, et al. Tumor marker utility grading system: A framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996;88:1456–1466. doi: 10.1093/jnci/88.20.1456. [DOI] [PubMed] [Google Scholar]
- 24.Quinn JE, Kennedy RD, Mullan PB, et al. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003;63:6221–6228. [PubMed] [Google Scholar]
- 25.De Ligio JT, Velkova A, Zorio DA, et al. Can the status of the breast and ovarian cancer susceptibility gene 1 product (BRCA1) predict response to taxane-based cancer therapy? Anticancer Agents Med Chem. 2009;9:543–549. doi: 10.2174/187152009788451798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rottenberg S, Nygren AO, Pajic M, et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci U S A. 2007;104:12117–12122. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullan PB, Quinn JE, Gilmore PM, et al. BRCA1 and GADD45 mediated G2/M cell cycle arrest in response to antimicrotubule agents. Oncogene. 2001;20:6123–6131. doi: 10.1038/sj.onc.1204712. [DOI] [PubMed] [Google Scholar]
- 28.Lafarge S, Sylvain V, Ferrara M, et al. Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene. 2001;20:6597–6606. doi: 10.1038/sj.onc.1204812. [DOI] [PubMed] [Google Scholar]
- 29.Chabalier C, Lamare C, Racca C, et al. BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle. 2006;5:1001–1007. doi: 10.4161/cc.5.9.2726. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore PM, McCabe N, Quinn JE, et al. BRCA1 interacts with and is required for paclitaxel-induced activation of mitogen-activated protein kinase kinase kinase 3. Cancer Res. 2004;64:4148–4154. doi: 10.1158/0008-5472.CAN-03-4080. [DOI] [PubMed] [Google Scholar]
- 31.Kriege M, Jager A, Hooning MJ, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. 2012;118:899–907. doi: 10.1002/cncr.26351. [DOI] [PubMed] [Google Scholar]
- 32.Kriege M, Seynaeve C, Meijers-Heijboer H, et al. Sensitivity to first-line chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:3764–3771. doi: 10.1200/JCO.2008.19.9067. [DOI] [PubMed] [Google Scholar]
- 33.Gronwald J, Byrski T, Huzarski T, et al. Neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients [abstract 502] J Clin Oncol. 2009;27(suppl):15s. doi: 10.1007/s10549-008-0128-9. [DOI] [PubMed] [Google Scholar]
- 34.Byrski T, Huzarski T, Dent R, et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2009;115:359–363. doi: 10.1007/s10549-008-0128-9. [DOI] [PubMed] [Google Scholar]
- 35.Byrski T, Dent R, Blecharz P, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012;14:R110. doi: 10.1186/bcr3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrski T, Gronwald J, Huzarski T, et al. Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat. 2008;108:289–296. doi: 10.1007/s10549-007-9600-1. [DOI] [PubMed] [Google Scholar]
- 37.Vollebergh MA, Lips EH, Nederlof PM, et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2011;22:1561–1570. doi: 10.1093/annonc/mdq624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egawa C, Motomura K, Miyoshi Y, et al. Increased expression of BRCA1 mRNA predicts favorable response to anthracycline-containing chemotherapy in breast cancers. Breast Cancer Res Treat. 2003;78:45–50. doi: 10.1023/a:1022101310500. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Kimler BF, Park YA, et al. Association of BRCA1 promoter methylation in triple-negative breast cancer (TNBC) with resistance to standard anthracycline-based adjuvant chemotherapy. J Clin Oncol. 2011;29(suppl) abstract 1123. [Google Scholar]
- 40.Sharma P, Stecklein S, Kimler BF, et al. BRCA1 insufficiency is predictive of superior survival in patients with triple negative breast cancer treated with platinum based chemotherapy. Cancer Res. 2012;72(suppl) abstract PD09–02. [Google Scholar]
- 41.Kurebayashi J, Yamamoto Y, Kurosumi M, et al. Loss of BRCA1 expression may predict shorter time-to-progression in metastatic breast cancer patients treated with taxanes. Anticancer Res. 2006;26:695–701. [PubMed] [Google Scholar]
- 42.Rodriguez AA, Makris A, Wu MF, et al. DNA repair signature is associated with anthracycline response in triple negative breast cancer patients. Breast Cancer Res Treat. 2010;123:189–196. doi: 10.1007/s10549-010-0983-z. [DOI] [PubMed] [Google Scholar]
- 43.Egawa C, Miyoshi Y, Takamura Y, et al. Decreased expression of BRCA2 mRNA predicts favorable response to docetaxel in breast cancer. Int J Cancer. 2001;95:255–259. doi: 10.1002/1097-0215(20010720)95:4<255::aid-ijc1043>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Alli E, Sharma VB, Sunderesakumar P, et al. Defective repair of oxidative dna damage in triple-negative breast cancer confers sensitivity to inhibition of poly(ADP-ribose) polymerase. Cancer Res. 2009;69:3589–3596. doi: 10.1158/0008-5472.CAN-08-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iglehart JD, Silver DP. Synthetic lethality—new direction in cancer-drug development. N Engl J Med. 2009;361:189–191. doi: 10.1056/NEJMe0903044. [DOI] [PubMed] [Google Scholar]
- 46.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 47.O'Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 48.O'Shaughnessy J, Schwartzberg LS, Danso MA, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC) J Clin Oncol. 2011;29(suppl) abstract 1007. [Google Scholar]
- 49.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 50.Telli ML, Jensen KC, Abkevich V, et al. Homologous recombination deficiency (HRD) score predicts pathologic response following neoadjuvant platinum-based therapy in triple-negative and BRCA1/2 mutation-associated breast cancer (BC) Cancer Res. 2012;72(suppl) abstract PD09–04. [Google Scholar]
- 51.Jiuping J, Lee MP, Kadota M, et al. Pharmacodynamic and pathway analysis of three presumed inhibitors of poly (ADP-ribose) polymerase: ABT-888, AZD2281 and BSI201. Cancer Res. 2011;71(suppl) abstract 4527. [Google Scholar]
- 52.Chionh F, Mitchell G, Lindeman GJ, et al. The role of poly adenosine diphosphate ribose polymerase inhibitors in breast and ovarian cancer: Current status and future directions. Asia Pac J Clin Oncol. 2011;7:197–211. doi: 10.1111/j.1743-7563.2011.01430.x. [DOI] [PubMed] [Google Scholar]
- 53.Delaloge S, Pelissier P, Kloos I, et al. BRCA1-linked breast cancer (BC) is highly more chemosensitive than its BRCA2-linked or sporadic counterparts. Paper presented at: 27th Congress of the European Society of Medical Oncology. [Google Scholar]
- 54.Chappuis PO, Goffin J, Wong N, et al. A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. J Med Genet. 2002;39:608–610. doi: 10.1136/jmg.39.8.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 56.Isakoff SJ, Goss PE, Mayer EL, et al. Impact of BRCA1/2 mutation status in TBCRC009: A muticenter phase II study of cisplatin or carboplatin for metastatic triple negative breast cancer. Cancer Res. 2012;72(suppl) abstract PD09–03. [Google Scholar]
- 57.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oonk AM, van Rijn C, Smits MM, et al. Clinical correlates of ‘BRCAness’ in triple-negative breast cancer of patients receiving adjuvant chemotherapy. Ann Oncol. 2012;23:2301–2305. doi: 10.1093/annonc/mdr621. [DOI] [PubMed] [Google Scholar]


