This study investigated the prognostic significance of circulating tumor cells (CTCs) determined immediately before the second cycle of chemotherapy in patients with metastatic breast cancer (MBC). The data indicate that CTCs determined immediately before the second cycle of chemotherapy is an early and strong predictor of treatment outcome in MBC patients.
Keywords: Circulating tumor cells, Outcome prediction, Metastatic breast cancer
Learning Objectives
Explain the prognostic value of baseline CTCs.
Describe the predictive value of CTCs at day 21 after chemotherapy.
Explain the meaning of the change of CTCs from baseline to day 21 after chemotherapy.
Abstract
We investigated the prognostic significance of circulating tumor cells (CTCs) determined immediately before the second cycle of chemotherapy in patients with metastatic breast cancer (MBC). The CTC counts were taken at baseline, before the first cycle of chemotherapy (CTC-0), and on day 21 before commencing the second cycle of chemotherapy (CTC-21) in consecutive MBC patients. The study's primary objectives were to analyze relationships between CTC-21 count and overall survival (OS). Based on the current literature, the CTC measurements were dichotomized as 0–4 versus ≥5 CTCs. Of 117 patients recruited, 99 were evaluable. Patients with 0–4 CTCs on day 21 had a significantly better OS than those with ≥5 CTCs (median OS: 38.5 months vs. 8.7 months). They also had a significantly better progression-free survival (PFS; median: 9.4 months vs. 3.0 months) and clinical benefit rate (77% vs. 44%). The OS of patients whose baseline CTCs were ≥5 but dropped to <5 on day 21 was apparently similar to those who had <5 CTCs at baseline. In a Cox regression analysis, CTC-21 was the only independent variable significantly predicting OS and PFS. Our data indicate that CTCs determined immediately before the second cycle of chemotherapy is an early and strong predictor of treatment outcome in MBC patients.
Implications for Practice:
We investigate the prognostic significance of circulating tumor cells (CTCs) immediately before administering the second cycle of a chemotherapy regimen in patients with metastatic breast cancer. Patients with 0–4 CTCs on day 21 (regardless the baseline CTC count) had a significantly better PFS and OS than those with ≥5 CTCs. In a Cox regression analysis, CTC-21 was the only independent variable significantly predicting OS (p = .009) and PFS (p = .047). Our study suggests that CTCs count determined immediately before the second cycle of chemotherapy is an early, and strong, predictor of treatment outcome in MBC patients, since it can identify those patients who derive little benefit from the chemotherapy regimen and also have a poor prognosis with conventional treatment.
Introduction
The detection and enumeration of circulating tumor cells (CTCs) in patients with metastatic breast cancer (MBC) is currently undergoing intense investigation. Several studies have shown that the number of CTCs at baseline is an independent predictor of progression-free survival (PFS) and overall survival (OS) in MBC patients [1–4]. The U.S. Food and Drug Administration has approved a semiautomated immunomagnetic method, the CellSearch system (Veridex, LLC, Warren, NJ, https://www.cellsearchctc.com/), specifically for this purpose. CTC enumeration using the CellSearch system appears to be a reproducible method. In a previous study, we did not observe any significant intrapatient variability in CTCs in two consecutive determinations by the CellSearch system conducted 12 hours apart [5]. A potential use for CTC enumeration with the CellSearch system in MBC patients is the early discrimination of patients with good response from those with poor response to systemic chemotherapy. In this paper, we present the final results of a prospective study in which the prognostic value of CTC enumeration at baseline and after the first cycle of chemotherapy (on day 21) was determined.
Patients and Methods
Consecutive MBC patients scheduled to receive palliative chemotherapy in the Hospital Universitario San Carlos (Madrid, Spain) were eligible for the trial. The study was approved by the institutional review board. All patients signed an informed consent form before being enrolled in the trial. A complete staging workup, including body computed tomography (CT) scan and bone scan, had been carried out within the 2 weeks prior to recruitment into the study. Measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST 1.0) was required [6]. Other inclusion criteria were Eastern Cooperative Oncology Group performance status 0–1; life expectancy >3 months; no contraindications for chemotherapy treatment; no more than two lines of chemotherapy for metastatic disease; adequate kidney (serum creatinine <1.2 mg/dL), liver (aspartate transaminase, alanine transaminase <2.5 times the upper limit of normal, bilirubin <1.5 mg/dL), and medullar function (hemoglobin levels >10 mg/dL, absolute neutrophil count >1,500 cells per square millimeter, platelets >100,000 per cubic millimeter); and absence of known central nervous system involvement.
Study Objectives
The study hypothesis was that CTC enumeration on day 21 (CTC-21; immediately before the second cycle of chemotherapy) could prognosticate the outcome of MBC patients, with the patients with <5 CTCs having the best prognosis.
The primary objective was to evaluate correlations between CTC-21 enumeration (0–4 vs. ≥5 CTCs) and OS. OS was defined as the time lapse between day 0 (day of baseline CTC determination) and the patient's death.
Secondary objectives were to analyze the correlations (1) between CTC-0 enumeration (0–4 vs. ≥5 CTCs) and OS; (2) between CTC-21 enumeration (0–4 vs. ≥5 CTCs) and PFS (defined as the time lapse between day 0 [day of CTC determination] and the first sign of progressive disease or death, whichever occurred first); (3) between CTC-0 enumeration (0–4 vs. ≥5 CTCs) and PFS; (4) between CTC-0 enumeration (0–4 vs. ≥5 CTCs) and objective response rate according to RECIST 1.0 criteria; and (5) between CTC-21 enumeration (0–4 vs. ≥5 CTCs) and objective response rate according to RECIST 1.0 criteria.
Study Procedures
Patients were seen in the outpatient clinic every 3 weeks, prior to each chemotherapy cycle. All consecutive patients fulfilling the inclusion criteria were asked to participate in the trial. Complete blood cell counts and biochemistry tests (including liver enzymes and creatinine) were performed immediately prior to each chemotherapy administration. CT scans were repeated every three cycles (11–12 weeks) to evaluate response. Clinical response was evaluated according to RECIST 1.0 comparing pre- and postchemotherapy CT scans. In each individual patient, the best response recorded was considered as the final response (i.e., a partial response lasting for more than 1 month followed by disease progression was categorized as “partial response”). After disease progression while on the study chemotherapy line, patients received subsequent chemotherapy lines (with or without anti-HER-2 agents), usually until the performance status of the patient precluded the administration of further chemotherapy. The clinicians in charge of treating the patients enrolled in the study were blinded with respect to the CTC values.
Two samples (7.5 mL each) of peripheral venous blood were collected from the patients. The first sample was collected immediately before the first cycle of chemotherapy (CTC-0) and the second was collected 21 days later, usually at the end of the first cycle (i.e., before the administration of the second cycle of chemotherapy). The blood was processed (CellSearch system) and the CTCs isolated and enumerated according to the manufacturer's instructions (as described in [5]).
The interpretations of the results were independently confirmed by three trained specialists (M.-L.M.C., M.V.-L., V.O.) who were blinded with respect to the provenance of the samples.
Sample Size and Statistical Analyses
The median OS estimated for the entire patient population was 24 months, with 50% of patients alive at 2 years. For statistical purposes we assumed that the distribution of patients with 0–4 CTCs and those with ≥5 CTCs on day 21 would be evenly split. We estimated the percentage of patients alive at 2 years as being 35% for patients with ≥5 CTCs on day 21 versus 65% for patients with <5 CTCs on day 21, for a hazard ratio (HR) of 1.8. With these assumptions, 100 patients (50 in each arm) were necessary to demonstrate this difference in OS (bilateral α = .05, β = .80). Assuming a 10% dropout rate, 110 patients were planned for the study.
The associations between qualitative variables were determined by χ2 or Fisher's exact test. PFS and OS were estimated using the Kaplan-Meier method and stratified according to CTCs. Differences were tested using the log-rank test. Multivariate analysis was performed using the Cox regression analysis to estimate the HR for survival according to CTC count adjusted by age, HER-2 status, ER-estrogen receptor status, number of metastatic sites, visceral disease, and number of previous chemotherapy lines. All tests were two sided, and values of p < .05 were considered statistically significant. The outcome data were analyzed using the SPSS 18.0 software package (SPSS Inc., Chicago, IL, http://www.spss.com).
Results
Between May 2008 and July 2009, consecutive patients (n = 117) were initially registered as eligible (supplemental online Figure 1). Of these, 109 patients signed the informed consent and were enrolled in the trial. In 10 patients (8%), the results of CTC-21 were not available for the following reasons: toxic death after the first cycle of chemotherapy (1 patient), lack of blood sample on day 21 (2 patients), sample processing errors resulting in unreliable determinations on either CTC-0 or CTC-21 samples or on both (7 patients). The characteristics of the 99 patients included in the final analysis are shown in Table 1. Of these patients, 44% had HER-2-amplified tumors and were treated with trastuzumab (usually in combination with taxanes) or lapatinib (usually in combination with capecitabine) in addition to the standard chemotherapy.
Table 1.
Characteristics of the patients in the study

aPatients with HER-2-amplified tumors received trastuzumab or lapatinib in addition to chemotherapy.
CTC Enumeration
The numbers of patients with 0, 1–4, and ≥5 baseline CTCs (CTC-0) were 26, 26, and 47, respectively. The distributions of patients according to baseline CTCs were similar in patients with HER-2-positive and HER-2-negative tumors; 41% and 54%, respectively, had ≥5 CTCs. The same was true for estrogen receptor (ER)-positive and -negative tumors: 45% and 50% of patients with ER-positive and ER-negative disease, respectively, had ≥5 CTCs.
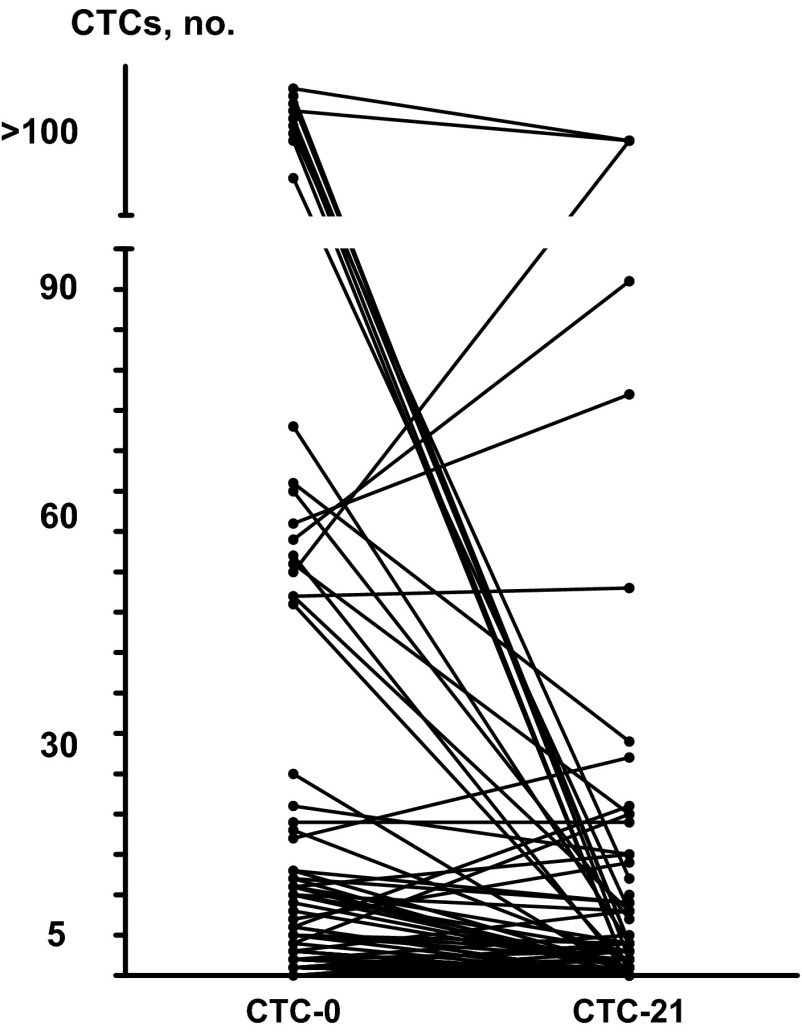
Figure 1 summarizes the changes in numbers of CTCs between baseline and day 21. Of 52 patients with 0–4 CTCs at baseline, 49 had 0–4 CTCs on day 21, whereas 3 had ≥5 CTCs. Of 47 patients with ≥5 CTCs at baseline, 22 had 0–4 cells on day 21, whereas 25 had ≥5 CTCs.
Figure 1.
CTC count change from day 0 to day 21.
Abbreviations: CTC, circulating tumor cell; CTC-0, circulating tumor cell count taken at baseline; CTC-21, circulating tumor cell count taken on day 21 before commencing a second cycle of chemotherapy.
Antitumor Activity
Clinical objective response rates (RECIST 1.0) to chemotherapy were as follows: 2 patients (2%) had complete response, 34 patients (34%) had partial response, 32 patients (32%) had stable disease, and 31 patients (31%) had disease progression.
Survival
Currently, 41 patients remain alive and 58 have died. The median follow-up of patients still alive is 35 months. The median PFS of the 99 patients was 8 months (95% confidence interval [CI], 6–11), and the median OS was 26.1 months (95% CI, 14–39).
Correlation of CTC-21 and CTC-0 Enumeration With OS
Patients with 0–4 CTCs on day 21 had a significantly better OS than those with ≥5 CTCs (primary endpoint of the study). The median OS was 39 months (95% CI, 26–50) for patients with 0–4 CTCs and 9 months (95% CI, 6–11) for patients with ≥5 CTCs (log-rank test, p < .001) (Fig. 2A).
Figure 2.
Overall survival according to CTC count taken on day 21 before commencing a second cycle of chemotherapy (A) and taken at baseline (B).
Abbreviations: CTC, circulating tumor cell; CTC-0, circulating tumor cell count taken at baseline; CTC-21, circulating tumor cell count taken on day 21 before commencing a second cycle of chemotherapy; OS, overall survival.
Conversely, the median OS in patients with baseline (CTC-0; prechemotherapy) of 0–4 CTCs was 33 months (95% CI, 22–45), whereas those with ≥5 CTCs had a median OS of 18 months (95% CI, 11–25). This difference was not statistically significant (log-rank test, p = .105) (Fig. 2B).
To ascertain the independent prognostic value of CTC-21 enumeration on OS, a Cox regression analysis was performed and included the following variables: CTC-21 (0–4 vs. ≥5 CTCs), CTC-0 (0–4 vs. ≥5 CTCs), age (continuous variable), HER-2 status (positive vs. negative), ER status (positive vs. negative), number of metastatic sites (1 vs. ≥2), visceral disease (yes vs. no), and number of previous chemotherapy lines (0 vs. 1 vs. 2) as independent variables and OS as the dependent variable (Table 2). The results indicated that CTC-21 was the only independent variable significantly predictive of OS (p = .009).
Table 2.
Cox regression analysis of overall survival
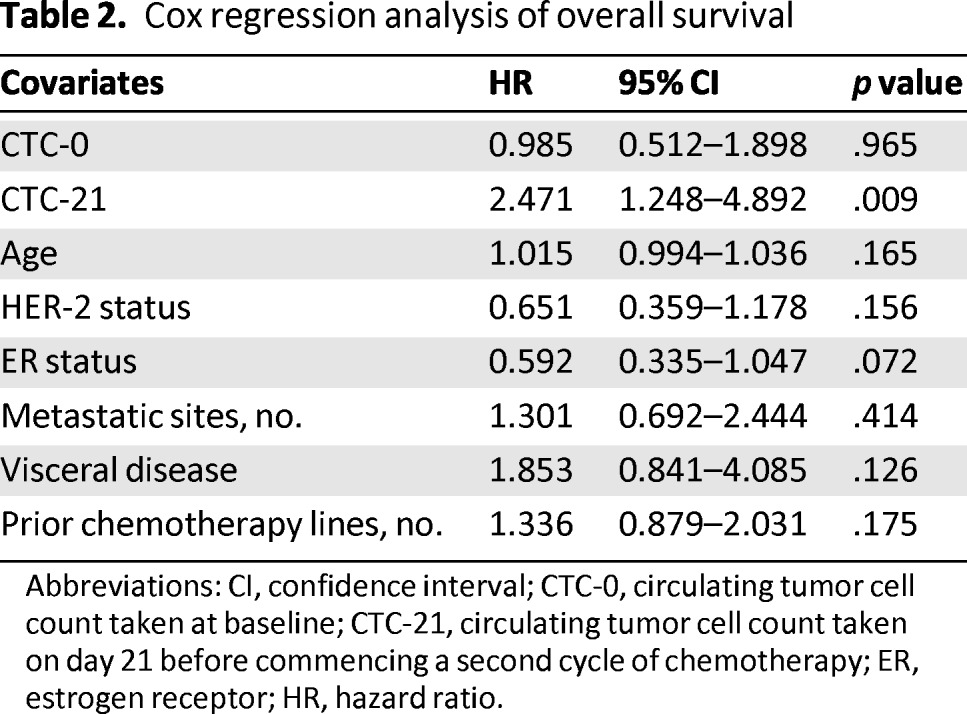
Abbreviations: CI, confidence interval; CTC-0, circulating tumor cell count taken at baseline; CTC-21, circulating tumor cell count taken on day 21 before commencing a second cycle of chemotherapy; ER, estrogen receptor; HR, hazard ratio.
Correlation of CTC-21 and CTC-0 Enumerations With PFS
The median PFS duration segregated according to CTC-21 enumeration was 9 months (95% CI, 8–11) for patients with 0–4 CTCs compared with 3 months (95% CI, 2–4) for patients with ≥5 CTCs (p = .001). With respect to CTC-0, the median PFS duration was 9 months (95% CI, 8–11) for patients with 0–4 CTCs compared with 3.5 months (95% CI, 0–7) for patients with ≥5 CTCs (p = .045) (supplemental online Fig. 2).
A Cox regression analysis was performed to assess the independent predictive value of CTC-21 enumeration on PFS. The independent variables included were CTC-21 (0–4 vs. ≥5 CTCs), CTC-0 (0–4 vs. ≥5 CTCs), age (continuous variable), HER-2 status (positive vs. negative), ER status (positive vs. negative), number of metastatic sites (0 vs. 1 vs. 2), visceral disease (yes vs. no), and number of previous chemotherapy lines (0 vs. ≥1). PFS was the dependent variable (supplemental online Table 1). The results indicated that CTC-21 was the only independent variable significantly predictive of PFS (p = .047).
Correlation of CTC-21 and CTC-0 Enumerations With Objective Response to Therapy
The distributions of objective responses according to CTC enumeration on day 21 (CTC-21) and day 0 (CTC-0) are shown in Table 3. There was a statistically significant correlation between CTC enumeration on day 21 (0–4 vs. ≥5 CTCs) and the distribution of response categories (p = .020) but not with the CTC values obtained on day 0. Specifically, the clinical benefit rate (complete response plus partial response plus stable disease) was 77% versus 44% for patients with <5 and ≥5 CTCs on day 21, respectively (Fisher's exact test, p = .0051).
Table 3.
Objective response according to CTC counts on day 21 and day 0

Abbreviations: CTC, circulating tumor cell; CTC-0, circulating tumor cell count taken at baseline; CTC-21, circulating tumor cell count taken on day 21 before commencing a second cycle of new chemotherapy; CTCs, circulating tumor cells.
Additional Analyses
The OS of patients who had ≥5 CTCs at baseline but dropped to <5 on day 21 was similar to those who had <5 CTCs at baseline (Fig. 3).
Figure 3.
Overall survival according to CTC change from day 0 to day 21. Blue curve: Patients with <5 CTCs on day 0 and day 21. Green curve: Patients with ≥5 CTCs on day 0 but <5 CTCs on day 21. Red curve: Patients with ≥5 CTCs on days 0 and 21. The curve of patients with <5 CTCs on day 0 and ≥5 CTCs on day 21 is not shown because this category contained only three patients.
Abbreviations: CTC, circulating tumor cell; CTC-0, circulating tumor cell count taken at baseline; CTC-21, circulating tumor cell count taken on day 21 before commencing a second cycle of chemotherapy; OS, overall survival.
The predictive value of CTC-21 was also studied in the subgroups of patients with and without HER-2 amplification (supplemental online Fig. 3). In patients with HER-2-positive tumors, the difference in survival was of borderline significance (median OS of 9 months [95% CI, 8–10] for patients with ≥5 CTCs compared with a median not reached for patients with 0–4 CTCs; log-rank test, p = .051). In patients with HER-2-negative tumors, the difference in OS was statistically significant (median OS of 5 months [95% CI, 0–11] for patients with ≥5 CTCs vs. median OS of 31 months [95% CI, 13–49] for patients with 0–4 CTCs; log-rank test, p = .001).
Discussion
The results of our study showed that the CTC counts on day 21 (i.e., immediately before the second cycle of chemotherapy in MBC) were strongly associated with the outcome of the patients. The prognostic value of CTC-21 was maintained in multivariate analysis in which the variables included were baseline CTCs and other classic prognostic factors. The analyses indicated that only CTC-21 was an independent predictive factor. Relative to patients with ≥5 CTCs on day 21, patients with <5 CTCs on that day had a significantly better clinical benefit rate (77% vs. 44%; p = .0051), a significantly longer PFS (9 months vs. 3 months; p = .001), and a significantly better OS (39 months vs. 9 months; p < .0001). Conversely, the CTC enumeration at baseline was weakly correlated with PFS and was not significantly correlated with objective response rate or OS; however, our study was not powered to show any significant correlations between these parameters, and we cannot not rule out that such a relationship exists, as that has been clearly shown in other trials [1–4]. Of considerable note is that the group of patients who had baseline ≥5 CTCs but had dropped to <5 at day 21 had outcomes similar to those with <5 CTCs on both the CTC-0 and CTC-21 determinations. This observation suggests that the determination of CTCs on day 21 is more useful for the prognostication of patient outcome than the baseline determination, that is, the influence of the therapy on the outcome is clearer.
The biological interpretation of these findings is subject to debate. Because CTC enumeration on day 21 is correlated with response rate and PFS, this determination appears to prognosticate the response to the initial chemotherapy; however, we also found a strong correlation between CTC count on day 21 and OS. Because patients received subsequent salvage chemotherapy regimens following the initial chemotherapy in which the CTC counts were determined, a low CTC count on day 21 is probably indicative of disease that is sensitive not only to the first chemotherapy line but also to subsequent lines.
Other published studies have addressed the prognostic and predictive value of CTC counts in MBC. In a seminal study, Cristofanilli et al. [2] reported the results of a prospective, multicenter study in 177 patients with measurable MBC. The CTCs were determined before starting a new line of treatment and at the first follow-up visit. The aim of the study was to evaluate the usefulness of CTC counts in predicting response to therapy, PFS, and OS in patients with MBC. Treatments included chemotherapy, immunotherapy, and hormonal therapy. In a multivariate Cox proportional hazard regression analysis, the numbers of circulating CTCs at baseline and at the first follow-up visit were the most significant predictors of PFS and OS. Contrary to our study, those authors did not find any predictive superiority of the CTC counts in the first follow-up visit (3–4 weeks after treatment initiation) compared with the baseline determination. Around 30% of patients in the study by Cristofanilli et al. [2] received hormonal therapy or immunotherapy, and we could speculate that those therapies possibly produced slower clearance of CTCs from the blood compared with chemotherapy. Pierga et al. [7] reported the results of the IC 2006–04 study, a multicenter study that addressed the predictive value of baseline CTC enumeration and CTC changes in 267 patients with MBC treated with first-line chemotherapy. In multivariate analysis, baseline CTC positivity (≥5 CTCs) was an independent prognostic factor for PFS and OS. When considering the determinations at baseline and after cycle 1, patients with persistent low (<5) and high (≥5) CTC counts had the best and worst outcomes, respectively, in terms of OS. Those patients with a high CTC count at baseline and a low CTC count after cycle 1 had an intermediate OS.
Liu et al. [8] correlated the number of CTCs with the response to chemotherapy or endocrine therapy in 68 patients with MBC. The odds of radiographic disease progression were sixfold for patients with ≥5 CTCs compared with the remaining patients.
The aims of our study were somewhat different from the above-mentioned trials; ours was designed specifically to address the predictive value of CTC counts on day 21 and probably was not powered to show other relationships. The practical implications of our results, which are concordant with the results from other trials, are essentially twofold. First, high CTCs on day 21 are an early indicator of poor outcome and can identify a group of patients with low sensitivity not only to the chemotherapy being administered but also to other conventional chemotherapies in general. This information could be of added value to the already reported ability of prechemotherapy CTC enumeration in the prognostication of outcomes. Second, CTC determination on day 21 could be used to select patients for experimental (new) therapeutic approaches.
Our study has several strengths as well as limitations. We focused on the predictive value of CTC counts on day 21. This was the principal objective and was reflected in the design of the study. The patient follow-up was long enough to allow for conclusions about long-term outcome: 59% of deaths and a median follow-up of 35 months for the patients still alive. However, the study was conducted in a single academic institution in which patients had access to successive experimental therapies. This could have had an impact on OS. Consequently, it is unclear whether our current findings could be extrapolated to a different population of breast cancer patients treated in the community setting. The sample size, although appropriate to answer the main study objective, was insufficient to identify other possible clinically relevant associations. The lack of significant correlation between baseline CTCs and OS in our study should be interpreted with caution because our study was not powered to show any such association. We observed a trend (albeit nonsignificant) in OS in favor of patients with 0–4 CTCs compared to those with ≥5 CTCs in baseline measurement.
Finally, the population of our study appears to contain a high proportion of patients with HER-2-amplified tumors (44% of the total). This over-representation might have resulted from many of our patients with metastatic HER-2-positive breast cancer being referred to our institution from other Spanish hospitals to participate in clinical trials, thus enriching our breast cancer population with HER-2-positive cases.
A recent report by Giordano et al. [9] confirmed that the number of baseline CTCs was predictive of OS in the overall population but reported that it was not predictive in the HER-2-positive population treated with anti-HER-2 therapies. In our study, we did not observe any relevant differences in the predictive value of CTC-21 in relation to HER-2 status. We did observe a similar trend toward a significantly better OS in those patients with ≥5 CTCs on day 21, regardless of HER-2 status.
Conclusion
CTC determination on day 21 of the first cycle of chemotherapy constitutes a simple and effective method of predicting the outcome of MBC patients. The measurement could also identify the population of patients with a high likelihood of poor outcome with conventional therapies.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This study was supported by funds from FEDER (RTICC-RD12/0036/0076), the Spanish National Network of Cancer Centers (RTICC, RD06/0020/0021), Accion Transversal del Cancer-Instituto Carlos III, and a grant from Fundación Mutua Madrileña. Editorial assistance was provided by Dr. Peter R. Turner of Tscimed.com.
Author Contributions
Conception/Design: Miguel Martín, Sara Custodio, Sara López-Tarruella, Maria-Luisa Maestro de las Casas, Eduardo Díaz-Rubio, José-Ángel García-Sáenz, Antonio Casado, Javier Sastre
Provision of study material or patients: Miguel Martín, Sara López-Tarruella, Yolanda Jerez, Iván Márquez-Rodas, Maria-Luisa Maestro de las Casas, Eduardo Díaz-Rubio, Marta Vidaurreta-Lazaro, Virginia de la Orden, José-Ángel García-Sáenz, Julio-César de la Torre, Antonio Casado, Javier Sastre
Collection and/or assembly of data: Miguel Martín, Sara Custodio, Sara López-Tarruella, Yolanda Jerez, Iván Márquez-Rodas, Jose-María Bellón-Cano, Maria-Luisa Maestro de las Casas, Eduardo Díaz-Rubio, Marta Vidaurreta-Lazaro, Virginia de la Orden, José-Ángel García-Sáenz, Julio-César de la Torre, Antonio Casado, Javier Sastre
Data analysis and interpretation: Miguel Martín, Sara Custodio, Sara López-Tarruella, Yolanda Jerez, Iván Márquez-Rodas, Jose-María Bellón-Cano, Maria-Luisa Maestro de las Casas, Eduardo Díaz-Rubio, Marta Vidaurreta-Lazaro, Virginia de la Orden, José-Ángel García-Sáenz, Julio-César de la Torre, Antonio Casado, Javier Sastre
Manuscript writing: Miguel Martín, Sara Custodio, Sara López-Tarruella, Yolanda Jerez, Iván Márquez-Rodas, Jose-María Bellón-Cano, Maria-Luisa Maestro de las Casas, Eduardo Díaz-Rubio, Marta Vidaurreta-Lazaro, Virginia de la Orden, José-Ángel García-Sáenz, Julio-César de la Torre, Antonio Casado, Javier Sastre
Final approval of manuscript: Miguel Martín, Sara Custodio, Sara López-Tarruella, Yolanda Jerez, Iván Márquez-Rodas, Jose-María Bellón-Cano, Maria-Luisa Maestro de las Casas, Eduardo Díaz-Rubio, Marta Vidaurreta-Lazaro, Virginia de la Orden, José-Ángel García-Sáenz, Julio-César de la Torre, Antonio Casado, Javier Sastre
Disclosures
The authors indicated no financial relationships.
Section editors: Gabriel Hortobágyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe (C/A); Novartis (RF); Taivex (O); board of directors for Citizen's Oncology Foundation; Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Sanofi, Ortho-Biotech, Amgen, and Bristol-Myers Squibb (C/A); (H)
Reviewer “A”: None
Reviewer “B”: None
Reviewer “C”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder;(SAB) Scientific advisory board
Reference
- 1.Pierga JY, Bonneton C, Vincent-Salomon A, et al. Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral and bone marrow breast cancer patients. Clin Cancer Res. 2004;10:1392–1400. doi: 10.1158/1078-0432.ccr-0102-03. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Budd GT, Ellis M, et al. Circulating tumor cells, disease progression and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 3.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging-predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M. Circulating tumor cells, disease progression and survival in metastatic breast cancer. Semin Oncol. 2006;33:S9–S14. doi: 10.1053/j.seminoncol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Martín M, García-Saenz JA, Maestro ML, et al. Circulating tumor cells in metastatic breast cancer: timing of blood extraction. Anticancer Res. 2009;29:4185–4187. [PubMed] [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.Pierga JY, Hajage D, Bachelot T, et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol. 2011;23:618–624. doi: 10.1093/annonc/mdr263. [DOI] [PubMed] [Google Scholar]
- 8.Liu MC, Shields PG, Warren RD, et al. Circulating tumor cells: A useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27:5153–5159. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giordano A, Giuliano M, De Laurentis M, et al. Circulating tumor cells in inmunohistochemical subtypes of metastatic breast cancer: Lack of prediction in HER-2-positive disease treated with targeted therapy. Ann Oncol. doi: 10.1093/annonc/mdr434. 10.1093/annonc/mdr434. [DOI] [PubMed] [Google Scholar]