Neoadjuvant cisplatin-based combination chemotherapy for muscle-invasive bladder cancer (MIBC) has been shown to confer a survival advantage, but utilization remains dismal with nearly one-half of patients ineligible for cisplatin-based therapy because of renal dysfunction, impaired performance status, and/or coexisting medical problems. This situation highlights the need for the development of novel therapies for the management of MIBC. This review is focused on the neoadjuvant paradigm for accelerated drug development using bladder cancer as the ideal model.
Keywords: Bladder cancer, Neoadjuvant, Urothelial cancer
Learning Objectives
Describe how pathological complete response predicts for improved outcome in patients with MIBC.
Explain the biological rationale for neoadjuvant chemotherapy in MIBC related to the dysregulation in PI3K/AKT, RAF/MEK/ERK signaling pathways, and ERBb family.
Abstract
Neoadjuvant cisplatin-based combination chemotherapy for muscle-invasive bladder cancer (MIBC) has been shown to confer a survival advantage in two randomized clinical trials and a meta-analysis. Despite level 1 evidence supporting its benefit, utilization remains dismal with nearly one-half of patients ineligible for cisplatin-based therapy because of renal dysfunction, impaired performance status, and/or coexisting medical problems. This situation highlights the need for the development of novel therapies for the management of MIBC, a disease with a lethal phenotype. The neoadjuvant paradigm in bladder cancer offers many advantages for accelerated drug development. First, there is a greater likelihood of successful therapy at an earlier disease state that may be characterized by less genomic instability compared with the metastatic setting, with an early readout of activity with results determined in months rather than years. Second, pre- and post-treatment tumor tissue collection in patients with MIBC is performed as the standard of care without the need for research-directed biopsies, allowing for the ability to perform important correlative studies and to monitor tumor response to therapy in “real time.” Third, pathological complete response (pT0) predicts for improved outcome in patients with MIBC. Fourth, there is a strong biological rationale with rapidly accumulating evidence for actionable targets in bladder cancer. This review focuses on the neoadjuvant paradigm for accelerated drug development using bladder cancer as the ideal model.
Implications for Practice:
Recent recommendations to use the neoadjuvant setting in breast cancer as an accelerated drug development pathway make a similar approach in bladder cancer very appealing. The current article will review the rationale for consideration of bladder cancer as the ideal neoadjuvant model for accelerated drug development. Several factors including the ease of bladder tumor tissue collection performed as standard of care, the use of pathologic response as an intermediate marker for overall outcome, and a richer understanding of the important molecular pathways involved in bladder cancer development and progression make the neoadjuvant paradigm particularly relevant. The ability to conduct clinical trials that require fewer patients and efficiently explore disease biology will undoubtedly lead to the development of novel therapies and have a profound effect on every day medical practice.
Introduction
The National Cancer Institute (NCI) clinical trials design task force statement supports trial designs that improve efficiency and shorten development time [1]. Overall survival (OS) remains the gold standard and the most dependable endpoint in cancer clinical trials to support drug approval by the U.S. Food and Drug Administration (FDA); however, surrogate endpoints, such as disease-free survival (DFS), are also acceptable for accelerated approval with a sponsor commitment to provide confirmatory evidence in subsequent trials [2]. OS is precise, free from bias, and easy to measure, yet it requires randomized controlled studies with large patient populations for adequate power and may be difficult to achieve with subsequent therapeutic interventions that affect outcome. From December 11, 1992, to July 1, 2010, the FDA granted accelerated approval to 35 oncology products for 47 new indications [3]. Confirmatory trials were performed for 26 of the 47 new indications, resulting in regular approval. Accelerated approval of oncology products has resulted in time savings in terms of earlier availability of drugs for cancer patients (median time between accelerated approval and regular approval of oncology products was 3.9 years; mean: 4.7 years) [3]. Anastrozole, for example, was granted accelerated approval on September 5, 2002, for adjuvant therapy for postmenopausal hormone receptor-positive breast cancer based on the surrogate endpoint of DFS, with regular approval occurring nearly 3 years later [4].
The neoadjuvant chemotherapy paradigm is an effective clinical platform in patients with various solid tumor malignancies [5–8]. In a large breast cancer study (B18) by the National Surgical Adjuvant Breast and Bowel Project (NSABP), 1,523 women were randomly assigned to preoperative or postoperative doxorubicin and cyclophosphamide [9]. The primary study endpoint included clinical tumor response to preoperative therapy. Nearly 15 years ago, this study established that neoadjuvant chemotherapy was as effective as adjuvant chemotherapy, permitted more lumpectomies in early stage disease, and simultaneously allowed for further evaluation of breast cancer biology. Tumor response in this setting correlated with outcome and could serve as a surrogate marker for chemotherapy effect on micrometastases. Patient-reported outcomes like health-related quality of life measures are actively being pursued and have resulted in several cancer-drug approvals [10]. Ongoing neoadjuvant chemotherapy studies in breast cancer, such as the Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis 2 (I-SPY2), have evolved from this NSABP model and incorporate the exploitation of actionable biological targets using histological subtypes as well as human epidermal growth factor receptor 2 (Her-2) positivity [11, 12–15]. In a New England Journal of Medicine editorial, Prowell and Pazdur discuss a bolder step for accelerated drug development using pathological complete response as a surrogate for OS or DFS for accelerated drug approval in early breast cancer [16]. Much like the neoadjuvant trials in breast cancer, pathological response after neoadjuvant therapy represents a marker for outcome in bladder cancer [17]. This review focuses on bladder cancer as the ideal model for utilization of the neoadjuvant paradigm for accelerated drug development.
Bladder Cancer
In the United States, bladder cancer is a common malignancy with an estimated 73,510 new cases and 14,880 deaths for the year 2012 [18]. Bladder cancer is predominantly a disease of older persons with an average age of 73 years. Although the majority of patients are diagnosed with noninvasive disease, nearly 20%–30% will progress to the lethal phenotype of muscle-invasive bladder cancer (MIBC) and approximately 20%–30% of patients will have MIBC at the time of initial diagnosis. Despite an aggressive surgical approach with radical cystectomy (RC) with bilateral pelvic lymph node dissection for MIBC, >50% of these patients will develop recurrent or metastatic disease and succumb to complications related to bladder cancer. To improve on the poor outcome for many patients with MIBC, new targeted therapeutics and novel approaches to drug development are desperately needed.
Adjuvant Therapy in MIBC: Poor Accrual, Early Closure
Many of the adjuvant chemotherapy trials in bladder cancer have been problematic and underpowered, and a definitive survival benefit has been difficult to demonstrate [19]. Several trials were undertaken but closed prematurely due to poor accrual. The European Organization for Research and Treatment of Cancer's EORTC 30994, for example, a randomized phase III trial comparing immediate versus deferred chemotherapy after RC in patients with pT3-pT4, and/or N+M0 transitional cell carcinoma of the bladder, was closed after 7 years with 278 patients enrolled of a planned 340 patients (ClinicalTrials.gov identifier NCT00028756).
SOGUG 99/01, the Spanish Oncology Genitourinary Group-sponsored randomized phase III adjuvant trial using paclitaxel, cisplatin, and gemcitabine, was prematurely closed after 7 years due to poor recruitment and failure to meet its planned accrual goal of 340 patients [20]. A phase III study sponsored by the Italian National Research Council using adjuvant cisplatin-gemcitabine versus observation after RC in patients with high-risk bladder cancer was closed after 6 years with 194 patients of a planned accrual of 610 patients [21]. The study was underpowered to demonstrate a survival difference in patients receiving four cycles of adjuvant cisplatin-gemcitabine (p = .24; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.84–1.99). With the failure of the adjuvant chemotherapy studies to date, neoadjuvant chemotherapy represents an alternative with more promising data to support its use.
Neoadjuvant Therapy in MIBC: Survival Benefit
U.S. Intergroup Trial
Neoadjuvant cisplatin-based combination chemotherapy for MIBC has been shown to improve survival in two randomized clinical trials and a large meta-analysis (Table 1) [22]. Grossman et al. enrolled 317 patients with MIBC over an 11-year period in an intergroup study from 126 institutions affiliated with the Southwest Oncology Group (SWOG), the Eastern Cooperative Oncology Group (ECOG), and Cancer and Leukemia Group B [22]. The patients were randomly assigned to RC alone or to three cycles of methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) followed by RC. Median survival among patients assigned to surgery alone was 46 months, compared with 77 months among patients assigned to combination therapy (unstratified: p = .05; stratified according to age and tumor grade: p = .06). The p value stratified according to age and tumor grade of .06 remains valid in the context of the other supporting data and based on a one-sided trial design that tested the hypothesis that patients improved with M-VAC only. In both groups, improved survival was associated with pathological complete response (pT0). More patients in the group that had neoadjuvant M-VAC followed by RC than in the RC-alone group achieved pT0 (38% vs. 15%; p < .001), and those achieving pT0 had an 85% 5-year survival rate.
Table 1.
Major neoadjuvant studies in muscle-invasive bladder cancer

ap = .06 by a stratified log-rank test.
bp = .048; hazard ratio, 0.85 (95% confidence interval, 0.72, 1.00).
Abbreviations: CMV, cisplatin, methotrexate, vinblastine; M-VAC, methotrexate, vinblastine, doxorubicin, cisplatin.
International Collaboration of Trialists Trial
A larger international phase III randomized trial supports the results seen in the U.S. Intergroup trial [23]. This trial investigated the use of neoadjuvant cisplatin, methotrexate, and vinblastine (CMV) chemotherapy in MIBC treated with cystectomy and/or radiotherapy. In total, 976 patients were enrolled from 106 institutions in 20 countries by seven different national or international clinical groups. Patients were randomly assigned to receive neoadjuvant CMV versus no CMV. Neoadjuvant CMV prior to cystectomy, radiotherapy, or both resulted in a 16% reduction in the risk of death (HR, 0.84; 95% CI, 0.72–0.99; p = .037), equivalent to increases in 3-year survival from 50% to 56%, in 10-year survival from 30% to 36%, and in median survival time of 7 months (from 37 months to 44 months). The pT0 rate in patients receiving chemotherapy was 33%, compared with 12% among those undergoing cystectomy, radiation, or both without chemotherapy [24].
Advanced Bladder Cancer Meta-Analysis Collaboration
Updated results from the Advanced Bladder Cancer Meta-analysis Collaboration on neoadjuvant chemotherapy in 3,005 patients with MIBC treated in 11 randomized clinical trials demonstrated a significant survival benefit associated with platinum-based combination chemotherapy (HR, 0.86; 95% CI, 0.77–0.95; p = .003) [25]. This translates to a benefit in OS from 45% to 50%, equivalent to a 5% absolute improvement in survival at 5 years. This benefit was consistent across all patient subgroups. There was also a significant DFS benefit (HR, 0.78; 95% CI, 0.71–0.86; p < .0001), which is equivalent to a 9% absolute improvement at 5 years.
Neoadjuvant Therapy in MIBC: M-VAC or Gemcitabine and Cisplatin?
In the study of patients with metastatic bladder cancer by von der Maase et al., the combination of gemcitabine and cisplatin (GC) was shown to be similar in efficacy to M-VAC, with less toxicity associated with the GC doublet [26]. Neoadjuvant chemotherapy with GC has also been evaluated in two retrospective analyses with endpoints including pathologic response and response rate. Dash et al. retrospectively evaluated MIBC patients who received neoadjuvant therapy with M-VAC or GC before RC between November 2000 and December 2006 at Memorial Sloan-Kettering Cancer Center (MSKCC) [27]. The endpoints included post-therapy pathological downstaging, chemotherapy delivery, and DFS. In 39 patients who completed four cycles of neoadjuvant GC, the proportion of patients with no residual muscle-invasive disease (lower than pT2) was 36%; in the 54 M-VAC-treated patients, the rate was 35%. All 15 patients who received neoadjuvant GC who achieved a category lower than pT2 remained disease-free at the median follow-up of 30 months. The pT0 rates for the GC- and M-VAC-treated patients were 26% and 28%, respectively.
The results from a retrospective study by Fairey et al. are consistent with the MSKCC experience [28]. The study analyzed 116 patients with MIBC, 58 in the neoadjuvant GC group and 58 in the neoadjuvant M-VAC group. The outcomes measured were complete response rate (CRR; pT0N0), partial response rate (PRR; pTaN0, pT1N0, or pTisN0), overall mortality (OM), and recurrence. There was no statistically significant difference between the GC and M-VAC groups in terms of CRR (27.3% vs. 17.1%; p = .419) or PRR (45.5% vs. 37.1%; p = .498). In addition, there was no difference between the predicted 5-year rates of freedom from OM (p = .634) and recurrence (p = .891). A major limitation of both studies is the retrospective study design, thus we are unable to definitively conclude that GC and M-VAC are equivalent in this setting. Phase II clinical trials evaluating dose-dense GC as neoadjuvant therapy in MIBC (ClinicalTrials.gov identifier NCT01589094) and in patients with high-grade upper tract urothelial carcinoma (UC; ClinicalTrials.gov identifier NCT0126178) are ongoing.
Unlike Fairey et al. and the MSKCC experience with GC, Weight et al. [29] performed a retrospective study that demonstrated a lack of pathological downstaging with neoadjuvant chemotherapy for MIBC. This group evaluated 117 patients who underwent open RC at the Cleveland Clinic for MIBC (T2-T4a, N0–2, M0) from January 2006 to November 2007. They found that of the 29 patients (25%) who received neoadjuvant chemotherapy (20 patients received GC, 4 received M-VAC, and 5 received other regimens), only 2 patients (7%) achieved pT0. Eighteen patients (62%) had non-organ-confined residual cancer consistent with disease progression and overall median progression-free survival (PFS) of 10.5 months. The median time interval from date of diagnosis of MIBC to RC was 208 days in those patients receiving neoadjuvant chemotherapy versus 48 days for the immediate RC cohorts. Possible reasons for these poor outcomes in the study by Weight et al. include selection bias, with more aggressive disease selected for the neoadjuvant cohort combined with use of non-M-VAC regimens, and/or substantial delay in performing RC (in the study by Grossman et al. [22], date of diagnosis to RC was 115 days). Taken together, although GC appears to have similar efficacy and less toxicity compared with M-VAC, making GC an attractive alternative in the neoadjuvant setting, a randomized prospective trial comparing neoadjuvant GC and M-VAC is needed but is unlikely to be performed. A comparative trial of GC and M-VAC is unlikely to occur due to the difficult accrual seen in modern perioperative chemotherapy trials in patients with MIBC.
Despite level 1 evidence supporting a survival benefit for cisplatin-based combination therapy, the utilization of neoadjuvant chemotherapy is dismal. The reasons for underutilization are many and include patient preference and physician bias as well as difficulty in the administration of cisplatin-based chemotherapy to an older patient population with coexisting medical problems.
Underutilization of Neoadjuvant Therapy in MIBC
Despite level 1 evidence supporting a survival benefit for cisplatin-based combination therapy, the utilization of neoadjuvant chemotherapy is dismal [30–34]. The reasons for underutilization are many and include patient preference and physician bias as well as difficulty in the administration of cisplatin-based chemotherapy to an older patient population with coexisting medical problems [30, 35]. A retrospective review of 238 patients (145 patients with preoperative clinical stage T2 or higher) who underwent RC for MIBC between 2003 and 2008 found that only 17% (25 of 145) of patients who underwent RC for bladder cancer received cisplatin-based neoadjuvant chemotherapy. Renal function was adequate (creatinine clearance [CrCl] >60 ml per minute) for neoadjuvant cisplatin-based chemotherapy in 67% of the MIBC patients. Patients who received neoadjuvant chemotherapy had higher pT0 rates (29% vs. 8%) compared with those who did not receive neoadjuvant chemotherapy [36]. The authors suggest that in addition to an older patient population with coexisting medical problems, concerns related to toxicity and the modest nature of the benefit from neoadjuvant chemotherapy might explain the underutilization.
Ineligibility for Neoadjuvant Cisplatin-Based Therapy in MIBC
Although the underutilization of neoadjuvant chemotherapy is a major problem, many older patients are unfit for cisplatin-based combination treatment for MIBC. In an effort to enhance uniformity in clinical trials, Galsky et al. published a consensus definition of patients unfit for cisplatin based on at least one of the following criteria: ECOG performance status of 2, CrCl <60 mL per minute, grade ≥2 hearing loss, grade ≥2 neuropathy, and/or New York Heart Association class 3 heart failure [37]. Many centers will treat patients with a glomerular filtration rate (GFR) ≥50 or 55 mL per minute. Furthermore, the clinician may also consider alternative individual patient factors on a case-by-case basis for cisplatin treatment determination, as there are not absolute guidelines regarding cisplatin eligibility.
To ascertain the prevalence of hearing loss in an increasingly aging U.S. population, Agrawal et al. performed a national cross-sectional survey with audiometric testing in 5,742 U.S. adults aged 20–69 years from 1999 to 2004 [38]. They found that in 2003–2004, 16.1% of U.S. adults (29 million) had speech-frequency hearing loss. In addition, hearing loss occurred earlier in participants with smoking and cardiovascular disease. Individuals diagnosed with bladder cancer are likely to have increased baseline hearing loss not only due to their advanced age at diagnosis but also based on smoking history as well as possible cardiovascular disease. Inzitari et al. sought to examine predictors of motor-performance decline by performing a longitudinal review in 1,052 persons ranging from 65 to 84 years old who performed normally at baseline [39]. They found 166 patients had motor-performance decline at 3-year follow-up. Distal symmetrical neuropathy was significant among several factors associated with motor-performance decline. Baseline distal symmetrical neuropathy is an important consideration with the use of cisplatin-based chemotherapy to prevent motor-performance decline, which is associated with disability, institutionalization, and death. Baseline distal symmetrical neuropathy and/or hearing loss further reduce an older person's eligibility for cisplatin-based chemotherapy.
The vast majority of MIBC patients are unfit for neoadjuvant cisplatin-based therapy secondary to renal insufficiency. Dash et al. completed a retrospective review in the MSKCC Department of Urology RC database in patients who underwent RC for bladder cancer with category pT3 or higher or any node-positive disease between January 1990 and March 2005 [40]. Serum creatinine (SCr) was measured before and after cystectomy using GFR with formulas by Cockroft-Gault (CG), Jelliffe, and the Modification of Diet in Renal Disease. Using the CG formula, the overall proportion of patients ineligible for adjuvant cisplatin-based chemotherapy was 28%, and >40% of patients who were >70 years old were ineligible.
In a separate study of 194 patients who underwent RC for MIBC, SCr immediately before and nadir SCr 1–3 months after surgery were used to calculate CrCl and GFR [41]. A cutoff of CrCl ≥60 mL per minute or GFR ≥60 mL per minute per 1.73 m2 was used to determine eligibility for chemotherapy. Nearly 40% of patients were ineligible for cisplatin-based neoadjuvant chemotherapy due to poor renal function. Furthermore, RC did not affect eligibility for chemotherapy based on renal function.
To meet the challenges presented by elderly MIBC patients with renal insufficiency, Hussain et al. completed a small study in 23 patients who received GC therapy with the cisplatin component split dosed (35 mg/m2) on days 1 and 8 every 21 days for a maximum of four cycles [42]. Although limited to a small number of patients, they found that split-dosed GC therapy was well tolerated and effective in MIBC patients with a GFR >40 mL per minute using the CG formula. These studies highlight the difficulty with cisplatin-based therapy in patients with MIBC and the need for the development of novel therapies with improved tolerability.
The Case for Bladder Cancer: An Ideal Model for the Neoadjuvant Paradigm To Accelerate Drug Development
Potential Ethical and Clinical Implications
Neoadjuvant trials in MIBC patients with a high risk of recurrence and associated mortality that exploit actionable molecular targets are both ethically and clinically warranted. The I-SPY2 breast cancer trial [11], a study endorsed by the FDA and institutional review boards across >20 different sites along with individual sponsors and researchers, was determined to be ethical in its design for the treatment of locally advanced breast cancer in the neoadjuvant setting. In this trial, all patients who were enrolled were given the current standard of care and assigned to receive an investigational drug based on the breast cancer patient's individual tumor biology and/or biomarkers. Unlike the I-SPY2 model, the bladder cancer neoadjuvant model includes potential candidates with multiple comorbidities often deemed “unfit” for cisplatin-based chemotherapy. Consequently, novel investigational agents may not always be able to be tested with a backbone of cisplatin-based therapy. There is a pressing need for alternative treatment options in this setting for patients with bladder cancer. Clinical implications such as risk of disease progression due to treatment delay, ineffectiveness, or side effects must be weighed against the potential gains in tumor downstaging and other outcome measures including survival. Short-window trials of investigational agents with pre- and post-treatment tumor tissue analysis may set the stage for trials with longer treatment times prior to definitive management.
Using pT0 as a Marker for Overall Outcome
Given the NCI-mandate to support trial designs that improve efficiency and shorten development time coupled with the demonstrated efficacy of neoadjuvant therapy in bladder cancer and association with pT0, the neoadjuvant paradigm for accelerated drug development in bladder cancer must be pursued. Studies have found that pT0 is associated with improved outcome in MIBC patients treated with neoadjuvant cisplatin-based chemotherapy [17, 22, 23] and suggest that it may serve as an optimal endpoint. The 5-year survival rate for patients achieving pT0 with neoadjuvant M-VAC is 85% [23]. Sonpavde et al. completed a retrospective study of patients who received RC alone versus three cycles of neoadjuvant M-VAC chemotherapy before RC for bladder cancer from the SWOG-S8710/INT-0080 trial [17]. The study evaluated the pathologic response following RC with OS in a subset of patients who received neoadjuvant M-VAC and RC with negative margins. Sixty-eight (44.2%) of the 154 patients who received neoadjuvant M-VAC had a pathological response lower than P2 (P0, Pa, P1, or carcinoma in situ) at RC; 46 patients (29.9%) were P0, and the remainder had P2 disease or higher or did not undergo RC. Neoadjuvant chemotherapy plus RC with negative margins with pT0 and lymph node-negative disease correlated with improved OS. Despite these data, which strongly support pT0 as a marker for overall outcome in the neoadjuvant setting with standard chemotherapy, clinical trials will be needed to determine whether this is the case for new molecularly targeted therapies. Furthermore, pT0 is not currently a true surrogate for survival; therefore, PFS and OS are important endpoints to include.
The Potential for Less Genomic Instability at an Earlier Disease State
There may be a greater likelihood of successful therapy at an earlier disease state that is characterized by less genomic instability compared with the advanced or metastatic setting. Blaveri et al. studied 98 bladder tumors of diverse stages and grades by array-based comparative genomic hybridization (CGH) [43]. Array CGH analysis showed significant increases in copy number alterations and genomic instability with increasing stage and worse outcome (pTa vs. pT1, p = .0003; pTa vs. pT2-T4, p = .02; pT1 vs. pT2-T4, p = .03). Furthermore, a worse outcome in muscle-invasive tumors was associated with the fraction of genomes altered independent of other clinicopathological parameters.
Pre- and Post-Treatment Tumor Tissue Collection Is the Standard of Care
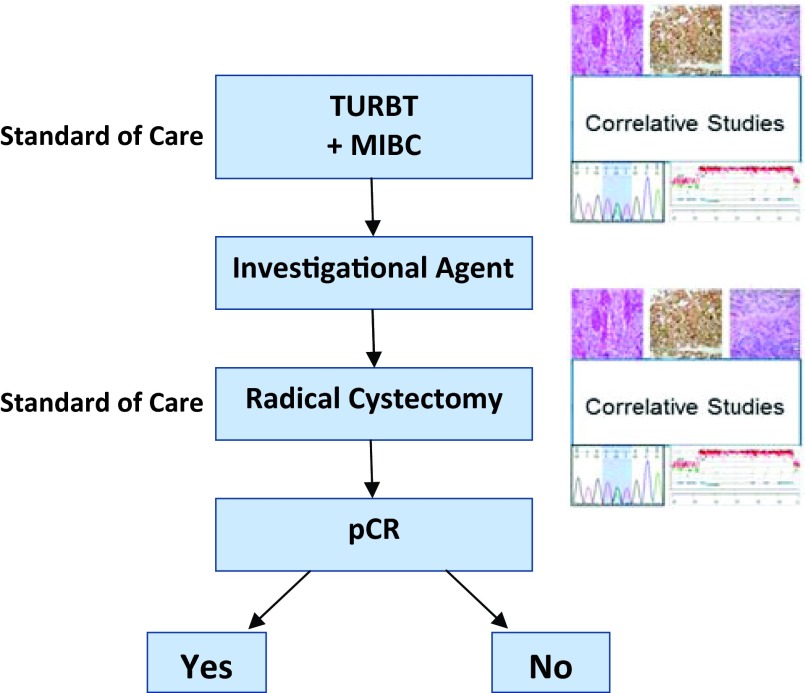
Unlike neoadjuvant clinical trials for early breast cancer, pre- and post-treatment tumor tissue collection in patients with MIBC is completed as the standard of care (Fig. 1). In addition to neoadjuvant cisplatin-based combination chemotherapy, an aggressive surgical approach with RC with bilateral pelvic lymph node dissection remains the gold standard for surgical care of MIBC. The RC specimen provides pathological staging while allowing for important science studies that may be correlated with tumor-response assessment. These post-treatment specimens can be compared with pretreatment specimens obtained during the standard of care transurethral resection of the bladder tumor. In the best-case scenario, the post-treatment specimen will result in pT0; however, this limits the ability to perform post-treatment molecular analyses. Short-window studies to evaluate pre- and post-treatment samples in MIBC patients who are not candidates for cisplatin-based therapies are warranted to understand the effect on tumor biology.
Figure 1.
Neoadjuvant paradigm in bladder cancer.
Abbreviations: MIBC, muscle-invasive bladder cancer; pCR, pathological complete response; TURBT, transurethral resection of the bladder tumor.
The Evolving, Strong, Biological Rationale for Targeted Therapeutics in Bladder Cancer
An improved understanding of the biology of bladder cancer will allow for the design and implementation of trials evaluating novel agents directed against molecular targets that exist in patients with muscle-invasive disease. The distinct natural histories of non-MIBC and MIBC are clearly driven by their disparate molecular profiles. In contrast to non-MIBC, which is predominantly characterized by alterations in FGFR3 and HRAS, MIBC is characterized by loss-of-function mutations in major tumor suppressor genes including TP53 (also known as p53), RB1 (also known as RB), and PTEN, leading to an aggressive, lethal phenotype. The nonoverlapping distribution of genetic alterations within the PIK3CA (also known as PI3K)-AKT1 (also known as AKT)-mammalian target of rapamycin (mTOR) pathway, the receptor tyrosine kinase (RTK)-Rat Sarcoma (RAS)-Rapidly Accelerated Fibrosarcoma (RAF) pathway, and the EGFR (also known as ERBB) family suggest that these alterations may represent driver events and thus should be evaluated as potential drug targets.
The distinct natural histories of non-MIBC and MIBC are clearly driven by their disparate molecular profiles. In contrast to non-MIBC, which is predominantly characterized by alterations in FGFR3 and HRAS, MIBC is characterized by loss-of-function mutations in major tumor suppressor genes including TP53 (also known as p53), RB1 (also known as RB), and PTEN, leading to an aggressive, lethal phenotype.
PI3K-AKT-mTOR Pathway
The PI3K-AKT signal transduction pathway is known to be aberrantly activated in a number of solid tumors, and a wide variety of mutations within multiple members of this pathway have been observed in UC. In select series, mutations of PIK3CA, the gene encoding for the catalytic subunit of PI3K, have been detected in up to 17% of UC samples [44]. Deletions and mutations of PTEN, a phosphatase protein that negatively regulates the activation of PI3K, on chromosome 10 are documented as occurring in 9% of UC cases and result in high-grade, invasive UC when combined with inactivation of p53 in genetically engineered mouse models [44–46]. Activating mutations in AKT in bladder cancer leads to upregulation of multiple downstream proteins involved in cell-cycle progression and inhibition of apoptosis [47]. Activation of the PI3K-AKT-mTOR pathway correlates with tumor progression and reduced survival in patients with UC of the bladder [48]. Iyer et al. investigated the genetic basis of a durable remission in a metastatic bladder cancer patient treated with everolimus, an mTOR inhibitor [49]. Whole-genome analysis revealed a TSC1 mutation in this patient who achieved a durable response while on everolimus. Subsequent targeted sequencing revealed TSC1 mutations in approximately 8% of 109 additional bladder cancers evaluated. TSC1 mutations appeared to correlate with everolimus sensitivity. This study highlights the importance of tumor tissue genomic analysis as a potential tool for therapeutic decision making.
RAS-Mitogen-Activated Protein Kinase (MEK)-ERK Pathway
The RAS-MEK-extracellular-signal-regulated kinase (ERK) signal transduction pathway is also known to have profound effects on proliferative, apoptotic, and differentiation pathways. Deregulated signaling can lead to unrestrained cellular growth and proliferation, ultimately resulting in tumor formation [50]. A number of molecular events in invasive UC activate MEK/ERK signaling, including HRAS mutations (∼10%), mutations of FGFR3 (∼10%–15%), and mutations or amplification of ERBB2 [51].
ERBB Family
Targeting EGFR in non-small cell lung cancer patients harboring EGFR mutations with drugs like erlotinib and gefitinib have yielded response rates as high as 60% [52, 53]. Cetuximab, a monoclonal antibody inhibitor of EGFR, was evaluated with or without paclitaxel in a phase II trial in patients with advanced urothelial tract carcinoma [54]. Wong et al. randomly assigned 39 patients to the single-agent cetuximab arm versus the combination arm. Either arm would close if 7 of the initial 15 patients progressed at the first disease evaluation at 8 weeks. The single-agent arm closed after 9 of the 11 patients progressed; however, the combination arm had an overall response rate of 25%, with a median PFS of 16.4 weeks and a median OS of 42 weeks. In a phase II trial, Pruthi et al. evaluated erlotinib, an EGFR tyrosine kinase inhibitor in 20 patients with MIBC in the neoadjuvant setting. The primary endpoint was pT0 in the RC specimen. Five patients (25%) were pT0, 7 patients (35%) were clinically downstaged (pT1 or lower), and 15 (75%) had organ-confined disease [55].
Hussain et al. investigated the safety and efficacy of trastuzumab, carboplatin, gemcitabine, and paclitaxel (TCGP) in patients with advanced UC prospectively evaluated for Her-2/neu overexpression [56]. Fifty-seven of 109 registered patients were positive for Her-2/neu (48.6% positive by immunohistochemistry). Ongoing neoadjuvant clinical trials incorporating novel targeted agents in MIBC include studies of cisplatin, gemcitabine, and sorafenib tosylate (ClinicalTrials.gov identifier NCT01222676), dasatinib (ClinicalTrials.gov identifier NCT00706641), lapatinib (ClinicalTrials.gov identifier NCT0124566), and sunitinib (ClinicalTrials.gov identifier NCT00526656) (Table 2). The gemcitabine, cisplatin, and sunitinib trial was closed early due to toxicity concerns [57].
Table 2.
Ongoing phase II neoadjuvant targeted therapy trials for muscle-invasive or locally advanced urothelial cancer

aClinicalTrials.gov identifier is shown for each trial. Accessed November 30, 2012.
Abbreviations: GC, gemcitabine, cisplatin; MDACC, M.D. Anderson Cancer Center; M-VAC, methotrexate, vinblastine, doxorubicin, cisplatin; pCR, partial complete response.
The Next Challenge: Matching Patients and Molecular Targets
Based on a report from the clinical trial design task force of the NCI Investigational Drug Steering Committee [58], phase I trial designs can be optimized to minimize patient risks by incorporating the following elements: (a) development of accelerated titration designs that maximize a patient's chance of being treated at an active dose; (b) movement beyond safety and dose selection in phase I trials to identification of target patient population and preliminary evidence of target inhibition, especially in the setting of molecularly targeted drugs; and (c) use of phase 0 trials to assess a drug effect on a molecular target in a small number of patients. An example of a phase I trial that included a specific target patient population involved malignant melanoma patients with the BRAF V600E mutation that responded to the BRAF inhibitor PLX4032 [59]. Development of predictive biomarkers may assist in matching the individual patient with an appropriate molecular target. SWOG, for example, has sponsored a phase II neoadjuvant trial using the “CO-eXpression ExtrapolatioN,” or COXEN, algorithm [60, 61], a method of using expression microarray for identification of drug sensitivity in vitro followed by comparison to individual patient tumor gene expression. Based on this comparison, an optimal therapy would be obtained and would minimize the patient's exposure to inert investigational therapies.
Conclusion
A mere 5% of investigational agents tested in phase III cancer clinical trials ultimately make it to the bedside. It may take a decade or more for current bladder cancer clinical trials to lead to paradigm-shifting therapies. To accelerate significant discoveries at the bench into targeted weapons against bladder cancer at the bedside, novel trial designs will need to be developed. To meet the challenges of future drug development in bladder cancer, national experts from the bladder cancer community convened at a workshop at an NCI conference entitled “Novel Neoadjuvant Therapy for Bladder Cancer” to discuss how best to move the field forward. Exploration of drug targets and therapeutics using the neoadjuvant setting was one of the key recommendations offered [62]. New therapies are desperately needed for patients with advanced bladder cancer, and use of the neoadjuvant paradigm is a promising strategy to accelerate drug development.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: David D. Chism, Michael E. Woods, Matthew I. Milowsky
Collection and/or assembly of data: David D. Chism, Michael E. Woods, Matthew I. Milowsky
Manuscript writing: David D. Chism, Michael E. Woods, Matthew I. Milowsky
Final approval of manuscript: David D. Chism, Michael E. Woods, Matthew I. Milowsky
Disclosures
Michael E. Woods: BeaconLBS (C/A); Matthew I. Milowsky: J&J, Astellas, Exelixis, Dendreon (RF). The other author indicated no financial relationships.
Section editor: Derek Raghavan: Sanofi, Gerson Lehrman (C/A)
Reviewer “A”: None
Reviewer “B”: Millennium, Genentech, Dendreon (RF)
Reviewer “C”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Seymour L, Ivy SP, Sargent D, et al. The design of phase II clinical trials testing cancer therapeutics: Consensus recommendations from the clinical trial design task force of the National Cancer Institute Investigational Drug Steering Committee. Clin Cancer Res. 2010;16:1764–1769. doi: 10.1158/1078-0432.CCR-09-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee AE, Farrell AT, Pazdur R, et al. The role of the U.S. Food and Drug Administration review process: Clinical trial endpoints in oncology. The Oncologist. 2010;15(suppl 1):13–18. doi: 10.1634/theoncologist.2010-S1-13. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JR, Ning YM, Farrell A, et al. Accelerated approval of oncology products: The Food and Drug Administration experience. J Natl Cancer Inst. 2011;103:636–644. doi: 10.1093/jnci/djr062. [DOI] [PubMed] [Google Scholar]
- 4.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 5.Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: The pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152–1160. doi: 10.1016/S1470-2045(12)70348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: An updated meta-analysis of 13 randomized control trials. J Thorac Oncol. 2010;5:510–516. doi: 10.1097/JTO.0b013e3181cd3345. [DOI] [PubMed] [Google Scholar]
- 7.Robova H, Rob L, Halaska MJ, et al. High-dose density neoadjuvant chemotherapy in bulky IB cervical cancer. Gynecol Oncol. 2013;128:49–53. doi: 10.1016/j.ygyno.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Tajima H, Ohta T, Kitagawa H, et al. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp Ther Med. 2012;3:787–792. doi: 10.3892/etm.2012.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 10.Rock EP, Kennedy DL, Furness MH, et al. Patient-reported outcomes supporting anticancer product approvals. J Clin Oncol. 2007;25:5094–5099. doi: 10.1200/JCO.2007.11.3803. [DOI] [PubMed] [Google Scholar]
- 11.Barker AD, Sigman CC, Kelloff GJ, et al. I-SPY 2: An adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 12.Lips EH, Mukhtar RA, Yau C, et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res Treat. 2012;136:35–43. doi: 10.1007/s10549-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: Results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 15.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 16.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 17.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109. doi: 10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 19.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Adjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis of individual patient data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:189–199. doi: 10.1016/j.eururo.2005.04.005. discussion 199–201. [DOI] [PubMed] [Google Scholar]
- 20.Paz-Ares LG, Solsona E, Esteban E, et al. Randomized phase III trial comparing adjuvant paclitaxel/gemcitabine/cisplatin (PGC) to observation in patients with resected invasive bladder cancer: Results of the Spanish Oncology Genitourinary Group (SOGUG) 99/01 study. J Clin Oncol. 2010;28(suppl 18s):LBA4518a. [Google Scholar]
- 21.Cognetti F, Ruggeri EM, Felici A, et al. Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: An Italian, multicenter, randomized phase III trial. Ann Oncol. 2012;23:695–700. doi: 10.1093/annonc/mdr354. [DOI] [PubMed] [Google Scholar]
- 22.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 23.International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: A randomised controlled trial. International Collaboration of Trialists. Lancet. 1999;354:533–540. [PubMed] [Google Scholar]
- 25.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:202–205. doi: 10.1016/j.eururo.2005.04.006. discussion 205–206. [DOI] [PubMed] [Google Scholar]
- 26.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 27.Dash A, Pettus JA, IV, Herr HW, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer. 2008;113:2471–2477. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairey AS, Daneshmand S, Quinn D, et al. Neoadjuvant chemotherapy with gemcitabine/cisplatin vs. methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive urothelial carcinoma of the bladder: A retrospective analysis from the University of Southern California. Urol Oncol. 2012 doi: 10.1016/j.urolonc.2012.07.005. '[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Weight CJ, Garcia JA, Hansel DE, et al. Lack of pathologic down-staging with neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder: A contemporary series. Cancer. 2009;115:792–799. doi: 10.1002/cncr.24106. [DOI] [PubMed] [Google Scholar]
- 30.David KA, Milowsky MI, Ritchey J, et al. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: A report from the National Cancer Data Base. J Urol. 2007;178:451–454. doi: 10.1016/j.juro.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 31.Miles BJ, Fairey AS, Eliasziw M, et al. Referral and treatment rates of neoadjuvant chemotherapy in muscle-invasive bladder cancer before and after publication of a clinical practice guideline. Can Urol Assoc J. 2010;4:263–267. doi: 10.5489/cuaj09134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feifer AH, Taylor JM, Tarin TV, et al. Maximizing cure for muscle-invasive bladder cancer: Integration of surgery and chemotherapy. Eur Urol. 2011;59:978–984. doi: 10.1016/j.eururo.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donat SM. Integrating perioperative chemotherapy into the treatment of muscle-invasive bladder cancer: Strategy versus reality. J Natl Compr Canc Netw. 2009;7:40–47. doi: 10.6004/jnccn.2009.0003. [DOI] [PubMed] [Google Scholar]
- 34.Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: Evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185:72–78. doi: 10.1016/j.juro.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Schrag D, Mitra N, Xu F, et al. Cystectomy for muscle-invasive bladder cancer: Patterns and outcomes of care in the Medicare population. Urology. 2005;65:1118–1125. doi: 10.1016/j.urology.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117:276–282. doi: 10.1002/cncr.25429. [DOI] [PubMed] [Google Scholar]
- 37.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–2438. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: Data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- 39.Inzitari M, Carlo A, Baldereschi M, et al. Risk and predictors of motor-performance decline in a normally functioning population-based sample of elderly subjects: The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2006;54:318–324. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 40.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 41.Canter D, Viterbo R, Kutikov A, et al. Baseline renal function status limits patient eligibility to receive perioperative chemotherapy for invasive bladder cancer and is minimally affected by radical cystectomy. Urology. 2011;77:160–165. doi: 10.1016/j.urology.2010.03.091. [DOI] [PubMed] [Google Scholar]
- 42.Hussain SA, Palmer DH, Lloyd B, et al. A study of split-dose cisplatin-based neo-adjuvant chemotherapy in muscle-invasive bladder cancer. Oncol Lett. 2012;3:855–859. doi: 10.3892/ol.2012.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaveri E, Brewer JL, Roydasgupta R, et al. Bladder cancer stage and outcome by array-based comparative genomic hybridization. Clin Cancer Res. 2005;11:7012–7022. doi: 10.1158/1078-0432.CCR-05-0177. [DOI] [PubMed] [Google Scholar]
- 44.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aveyard JS, Skilleter A, Habuchi T, et al. Somatic mutation of PTEN in bladder carcinoma. Br J Cancer. 1999;80:904–908. doi: 10.1038/sj.bjc.6690439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Askham JM, Platt F, Chambers PA, et al. AKT1 mutations in bladder cancer: Identification of a novel oncogenic mutation that can co-operate with E17K. Oncogene. 2010;29:150–155. doi: 10.1038/onc.2009.315. [DOI] [PubMed] [Google Scholar]
- 48.Sun CH, Chang YH, Pan CC. Activation of the PI3K/Akt/mTOR pathway correlates with tumour progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology. 2011;58:1054–1063. doi: 10.1111/j.1365-2559.2011.03856.x. [DOI] [PubMed] [Google Scholar]
- 49.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chappell WH, Steelman LS, Long JM, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: Rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujita J, Yoshida O, Yuasa Y, et al. Ha-ras oncogenes are activated by somatic alterations in human urinary tract tumours. Nature. 1984;309:464–466. doi: 10.1038/309464a0. [DOI] [PubMed] [Google Scholar]
- 52.Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: Results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 54.Wong YN, Litwin S, Vaughn D, et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol. 2012;30:3545–3551. doi: 10.1200/JCO.2012.41.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pruthi RS, Nielsen M, Heathcote S, et al. A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: Clinical and pathological results. BJU Int. 2010;106:349–354. doi: 10.1111/j.1464-410X.2009.09101.x. [DOI] [PubMed] [Google Scholar]
- 56.Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: Results of a multicenter phase II National Cancer Institute trial. J Clin Oncol. 2007;25:2218–2224. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]
- 57.Galsky MD, Hahn NM, Powles T, et al. Gemcitabine, cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2013;11:175–181. doi: 10.1016/j.clgc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Ivy SP, Siu LL, Garrett-Mayer E, et al. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: A report from the clinical trial design task force of the National Cancer Institute Investigational Drug Steering Committee. Clin Cancer Res. 2010;16:1726–1736. doi: 10.1158/1078-0432.CCR-09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flaherty K, Puzanov I, Sosman J, et al. Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol. 2009;27(suppl 15s):9000a. [Google Scholar]
- 60.Lee JK, Havaleshko DM, Cho H, et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci U S A. 2007;104:13086–13091. doi: 10.1073/pnas.0610292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith SC, Baras AS, Lee JK, et al. The COXEN principle: Translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in cancer. Cancer Res. 2010;70:1753–1758. doi: 10.1158/0008-5472.CAN-09-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dreicer R. The future of drug development in urothelial cancer. J Clin Oncol. 2012;30:473–475. doi: 10.1200/JCO.2011.39.5566. [DOI] [PubMed] [Google Scholar]