This article reviews the benefits and risks of low-dose computed tomography (LDCT) screening in light of the results of the National Lung Screening Trial and other randomized trials critically appraises current economic evaluations of LDCT screening, and a subsequent discussion highlights guideline recommendations for implementation strategies. The article concludes by endorsing the use of LDCT screening in institutions capable of responsible implementation of screening in both medical and economical terms.
Keywords: Lung cancer, Screening, Low-Dose CT, Implementation
Abstract
The National Lung Screening Trial (NLST) has sparked new interest in the adoption of lung cancer screening using low-dose computed tomography (LDCT). If adopted at a national level, LDCT screening may prevent approximately 18,000 lung cancer deaths per year, potentially constituting a high-value public health intervention. Before incorporating LDCT screening into practice, health care institutions need to consider the risks associated with LDCT screening and the impact of LDCT screening on health care costs, as well as other remaining areas of uncertainty, including the unknown cost-effectiveness of LDCT screening. This article will review the benefits and risks of LDCT screening in light of the results of the NLST and other randomized trials, it will discuss the additional health care costs associated with LDCT screening from the perspective of health care payers, and it will examine the published cost-effectiveness analyses of LDCT screening. A subsequent discussion highlights guideline recommendations for implementation strategies, the goals of which are to ensure that those eligible for LDCT screening derive the benefits while minimizing the risks of screening and avoiding an unnecessary escalation in screening-related costs. The article concludes by endorsing the use of LDCT screening in institutions capable of responsible implementation of screening in both medical and economic terms. The key elements of responsible implementation include the development of standardized screening practices, careful selection of screening candidates, and the creation of prospective registries that will mitigate current areas of uncertainty regarding LDCT screening.
Implications for Practice:
The National Lung Screening Trial (NLST) has fueled new interest in lung cancer screening by demonstrating a 20% reduction in lung cancer mortality with the use of low-dose computed tomography (LDCT). If adopted a national level, LDCT screening could avoid over 18,000 premature deaths per year, but several areas of uncertainty exist regarding the benefits and harms of screening at the community at large. This article seeks to inform clinicians about the current evidence supporting LDCT screening and provides a framework for efficient implementation of lung cancer screening programs. The authors review the benefits and harms of LDCT screening based on the results of the NLST and other studies, highlight current areas of uncertainty, and critically appraise studies of health care costs and cost-effectiveness of screening. A set of recommendations ensues to endorse screening practices that avoid unnecessary harms and costs while assuring that eligible patients derive the benefits of screening.
Introduction
The rationale for lung cancer screening is straightforward. The disease represents a common and serious public health problem, with more than 226,000 estimated new cases and 160,000 deaths in the United States in 2012 [1]. When detected at earlier stages, lung cancer is often curable by surgery, and 5-year overall survival can be as high as 70% [2]. In most cases, lung cancer presents at an advanced stage when detected by symptoms, carrying a poor prognosis that is reflected by a 5-year overall survival of <5% [1]. Screening can potentially decrease the high mortality rate associated with lung cancer by detecting the disease at its earlier stages in asymptomatic high-risk individuals, thereby enabling those individuals to undergo potentially curative surgery [3, 4]. Despite this compelling rationale, the enthusiasm for lung cancer screening has been tempered by several older observational studies and randomized trials that failed to demonstrate a clear benefit of screening [5–10]. Multiple sources of bias in observational studies prevent an accurate interpretation of results, including lead time, length of time, overdiagnosis, and healthy volunteer biases [3, 11, 12]. In addition, screening modalities evaluated in earlier randomized studies consisted of chest x-rays with or without sputum cytology, and this screening methods proved to have no effect on lung cancer mortality [13–16]. These disappointing results generated much skepticism about the role of lung cancer screening, and any potential benefits of this practice remained unrealized until the release of the National Lung Screening Trial (NLST) results [17]. This article briefly reviews the benefits and harms of lung cancer screening with low-dose computed tomography (LDCT) in light of the NLST findings, discusses the economic implications of adopting LDCT screening programs at a national level, and considers potential strategies for efficient implementation of lung cancer screening programs.
The NLST: Study Design and Efficacy Results
The NLST enrolled high-risk asymptomatic individuals, defined as those of age 55 to 74 years who had a smoking history of 30 pack-years or more and who were either current smokers or former smokers who had quit within 15 years prior to enrollment. From August 2002 through April 2004, 53,454 individuals were randomly assigned to annual LDCT screening scans versus chest x-rays for three consecutive years, after which they underwent follow-up. After a median follow-up of 6.5 years, the study showed a statistically significant relative reduction of 20% and 6.7% in lung cancer and overall mortality, respectively. For each lung cancer death avoided, 320 individuals underwent LDCT screening. In the LDCT arm, 57% of screening-detected lung cancer cases had TNM stages I or II compared with 39% in the chest x-ray arm, supporting the hypothesis that LDCT screening reduces mortality by detecting lung cancer at earlier stages. Lung cancer accounted for 24% of deaths in the trial. After excluding these deaths, LDCT screening resulted in a nonsignificant 3.2% reduction in overall mortality, suggesting that screening findings unrelated to lung cancer (e.g., interstitial lung disease) did not contribute to the overall mortality benefit of LDCT screening.
LDCT Screening: Efficacy Results From Other Randomized Trials
At least eight randomized trials have compared the impact of annual LDCT screening versus usual care (no screening, five trials) or chest x-rays (three trials, including NLST) on lung cancer and overall mortality [18]. Other than the NLST, only the Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays (DANTE) and the Danish Lung Cancer Screening Trial (DLCST) studies have reported efficacy results, and these trials found no statistically significant difference in cancer-specific or overall mortality between LDCT screening and usual care, respectively [19, 20]. The remaining five trials are either actively accruing patients or waiting completion of follow-up. Both the DANTE and DLCST trials had a much smaller patient sample size (n = 2,472 and n = 4,104, respectively) than the NLST (n = 53,454) and included patients with less exposure to tobacco than the those included in the NLST study (minimum smoking history of 20 pack-years for both DANTE and DLCST vs. 30 pack-years for NLST). These differences raise questions about whether the DANTE and DLCST studies were underpowered to detect differences in cancer-specific and overall mortality of the magnitude seen in the NLST study and whether the inclusion of patients at lower risk for lung cancer in the DANTE and DLCST trials diluted any potential benefits of LDCT screening for higher-risk patients. It is hoped that the results of the other ongoing trials will consolidate the evidence about the effectiveness of LDCT screening. Until those results become available, the only solid evidence of benefit exists for patients who meet the inclusion criteria of the NLST.
Risks of LDCT Screening
False Positive Results
Based on a systematic review of 8 randomized trials and 13 cohort studies, it appears that approximately 20% of the LDCT scans performed during each screening round detect suspicious noncalcified lung nodules or other findings that require further workup for a possible diagnosis of lung cancer [18]. More than 90% of these screening findings are benign and constitute false positive screening results. The risks associated with false positive results include the inconvenience and morbidity of confirmatory imaging tests and invasive procedures (i.e., lung biopsies or surgeries) in addition to the emotional stress related to a possible diagnosis of lung cancer. In the NLST, the burden of follow-up tests was considerable for patients who had a positive result after each LDCT screening round: on average, 50% of these patients underwent a follow-up CT scan, 8% had a positron emission tomography (PET) or PET-CT scan, and approximately 10% underwent an invasive procedure, including percutaneous biopsy, bronchoscopy, or surgery [17]. Few studies have evaluated the impact of LDCT screening results on health-related quality of life domains, including emotional stress. The Dutch-Belgian Randomized Lung Cancer Screening (NELSON) trial reported an increase in lung cancer-specific distress in patients who had an indeterminate result (i.e., a finding that required a follow-up CT) at two months after the initial LDCT screening compared with patients who had no screening or who had a negative screening result. This difference disappeared at two years, suggesting that a positive screening result can lead to short-term emotional distress that is reversible over time [21]. Additional studies and longer follow-up times are necessary to determine the impact of LDCT screening on mental and physical quality of life domains. Also of concern is how primary care providers will respond to the additional workup required by the detection of suspicious findings that will ultimately prove to be benign in most cases.
Overdiagnosis
LDCT screening can potentially detect tumors that otherwise would not cause symptoms or result in death over the patient's lifetime, a phenomenon known as overdiagnosis. The actual rate of overdiagnosis remains unclear for LDCT screening. In the LDCT arm of the NLST, the incidence of lung cancer was 13% higher than in the chest x-ray arm, suggesting an overdiagnosis rate of 10%–15%. The lead time associated with LDCT screening could have accounted, at least in part, for the difference in incidence seen in the NLST, resulting in an overestimated rate of overdiagnosis. Because all patients diagnosed with lung cancer receive treatment, no prospective studies of LDCT screening are available to determine the natural history of screening-detected, untreated lung cancers, which ultimately would inform the rate of overdiagnosis. An approximate estimate of overdiagnosis rate, based on lead time or tumor doubling times, is possible through complex mathematical modeling studies and is an area of active investigation [22, 23]. Despite its unknown frequency, overdiagnosis remains a concern for institutions offering LDCT screening programs, given the risks of medical complications and costs associated with unnecessary surgeries in overdiagnosed cases.
Medical Complications
The NLST is the only study that has reported on the incidence of medical complications associated with LDCT screening. The total incidence of complications was 1.4% in the LDCT arm and 1.6% in the chest x-ray arm. All complications resulted from the subsequent workup of suspicious screening findings and/or surgery for lung cancer. Major complications, as defined by the NLST (e.g., hemothorax, lung collapse), occurred infrequently after LDCT screening. Major complications occurred in 0.06% of LDCT screening results that were suspicious but ultimately benign (false positive). Of the LDCT screening scans that detected lung cancer, 11.2% were associated with major complications, the majority of which occurred after lung cancer surgery [17, 18].
Deaths resulting from complications of screening were very rare in the NLST. In the LDCT screening arm, 16 patients died within 60 days after an invasive diagnostic procedure. Of these 16, 10 had lung cancer and may have died of their cancer or from surgical complications. Because procedure-related deaths are unlikely to have occurred beyond the 60-day window, these results suggest a very low likelihood of dying as a result of medical complications from screening.
Radiation Exposure
A recent systematic review estimates that LDCT screening will cause one cancer death from radiation exposure per 2,500 persons screened, based on risk models from atomic bombings and medical imaging studies [18, 24, 25]. In the NLST study, LDCT screening resulted in one lung cancer death avoided per 320 persons screened, showing that the benefits of screening outweigh the risks from radiation exposure by eightfold. Despite this apparently favorable risk-benefit ratio, additional long-term follow-up data are necessary to inform the actual risk of radiation-induced cancers in patients who underwent the three LDCT screening rounds in the NLST.
The Post-NLST Era: Economic Implications of LDCT Screening Programs
The NLST has left many unanswered questions that deserve consideration before widespread implementation of LDCT screening occurs. Some of these are listed in Table 1 [26–29]. An overarching issue, and one that will be fundamentally influenced by these areas of uncertainty, is the economic impact of widespread LDCT screening. Estimating the economic burden and cost-effectiveness of screening will be critical so that eligible individuals derive the benefit of screening and health care systems can allocate resources for this intervention efficiently and judiciously.
Table 1.
Examples of areas of uncertainty regarding the use of LDCT screening
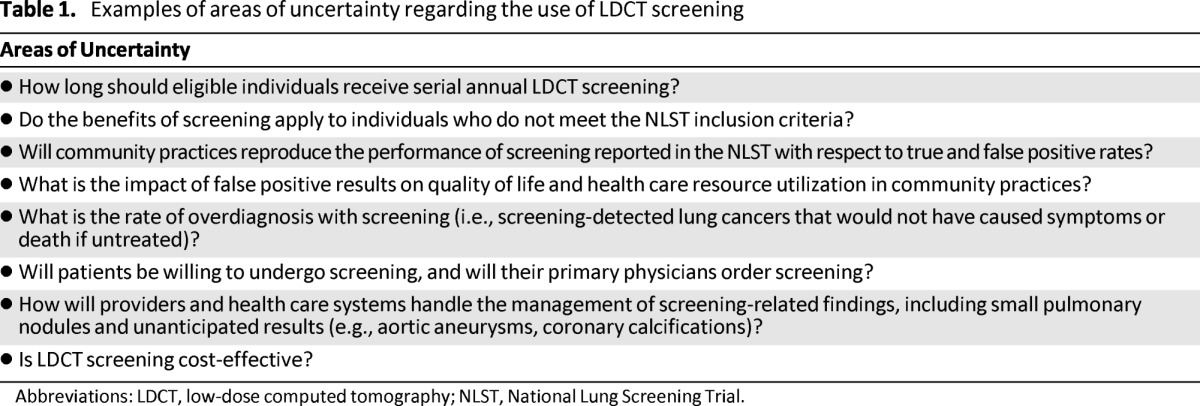
Abbreviations: LDCT, low-dose computed tomography; NLST, National Lung Screening Trial.
Health Care Costs of LDCT Screening
One economic evaluation estimated that $4.4 billion in U.S. health care costs would be added by an LDCT screening program for a population of 18 million individuals ages 50 to 64 years with a minimum smoking history of 30 pack-years [24]. The study did not report the amount of the reimbursement fee (or price) for an LDCT scan used to estimate the costs of screening, modeled costs for individuals who were on average younger than the NLST population, and it assumed a larger number of candidates for screening than the estimated national number of individuals who meet the NLST selection criteria. Given these limitations, this cost study may have overestimated the national health care costs associated with LDCT screening.
Our group developed a budget impact model to project the annual costs of adopting LDCT screening at the national level from the perspective of public and commercial health care payers. The model was informed by the resource utilization data reported in the NLST and estimated the number of eligible screening candidates in the United States based on NLST selection criteria, data from the National Health Interview Survey, and data from a recently published mathematical model study [30, 31]. For an estimated 8 million screening candidates, LDCT screening will add $2.2 to $3.3 billion in annual national health care costs to attain adherence rates of 50% to 75%, respectively, at a unit price of $300 per LDCT scan [25, 32]. These adherence rates allow a more realistic estimation of the costs of LDCT screening as they reflect the current adherence for colorectal and breast cancer screening, respectively.
The results of both economic models suggest that, if adopted at a national level, LDCT screening may add substantially to national health care costs. According to our model, the national costs of LDCT screening programs will largely depend on the number of individuals screened and the adherence rate. If, for example, all individuals in the United States in the age range included in the NLST (55–74 years) underwent screening, irrespective of their smoking history, the annual national costs of screening would increase sharply to $19.1 billion, but many of these individuals would be at a much lower risk for lung cancer and would not benefit from screening. These economic projections indicate that candidates for screening will need to be selected carefully to avoid an unnecessary escalation in health care costs.
Despite being potentially costly, LDCT screening may offer high societal value as a public health intervention. Assuming that 320 individuals need to undergo screening to avoid one lung cancer death, a national LDCT screening program would save more than 18,000 lives annually, at an average cost of $170,500 per life saved (2012 U.S. dollars).
Cost-Effectiveness of LDCT Screening
Six published studies estimated the cost-effectiveness of LDCT screening versus no screening for high-risk individuals in the United States [24, 33–37]. All analyses reported standard incremental cost-effectiveness ratios as their primary metric of cost-effectiveness.
Multiple methodological differences and limitations apply to these studies, making the interpretation of their results difficult. All of these cost-effectiveness analyses used data from observational studies to estimate the effectiveness of LDCT screening, and therefore are subject to several types of biases, including selection, lead time, length time, and overdiagnosis. In addition, the populations included in the cost-effectiveness analyses were heterogeneous with regard to key criteria used to define screening eligibility, including age and smoking histories. This heterogeneity also prevents an accurate comparison of the cost-effectiveness ratios reported across the studies. Finally, most of the studies did not account for the costs of false positive results, including follow-up PET-CT scans and lung biopsies, nor did they account for the impact of overdiagnosis on cost, which could have resulted in overly optimistic cost-effectiveness ratios.
Aside from the methodological limitations of these studies, the cost-effectiveness ratios of LDCT screening reported by these analyses varied substantially, from approximately $4,000 per life-year gained to more than $250,000 per quality-adjusted life-year gained (all costs are adjusted for U.S. 2012 dollars) [36, 37]. This wide range of reported cost-effectiveness ratios precludes any conclusion about whether LDCT screening is cost-effective.
McMahon et al. explored the plausible hypothesis that LDCT screening could be cost-effective if screening is used as a “teachable moment” to encourage smoking cessation [37]. In sensitivity analysis, the authors assumed that screening would double smoking cessation rates from 3% to 6%. In this scenario, LDCT screening was cost-effective, with incremental cost-effectiveness ratios of approximately $50,000 and $90,000 per quality-adjusted life-year for women and men, respectively. Although preliminary, this analysis suggests that a smoking cessation effect would substantially improve the cost-effectiveness of LDCT screening, warranting further studies on the role of screening as a “teachable moment” for smoking cessation and the integration of smoking cessation interventions into LDCT screening programs.
A cost-effectiveness analysis based on NLST data is currently underway. It is hoped that the results of this analysis will provide a conclusive answer about whether LDCT screening offers good value for the money spent.
Initial Implementation of LDCT Screening Programs
Because LDCT screening can add substantially to health care costs and because the cost-effectiveness of screening remains unknown, health care systems need to develop responsible strategies for screening implementation to ensure that eligible candidates derive the benefits of screening while avoiding any potential harm and unnecessary cost of screening. Published guidelines recommend several elements of an implementation plan that encourage rational use of health care resources related to screening, as discussed below [18, 26, 38–41].
The main element of a responsible implementation plan is the use of integrated and standardized screening practices at the hospital level with the goal of promoting efficient use of health care resources related to screening. Medical centers offering LDCT screening will need to assemble a multidisciplinary team comprised of specialists who are familiar with the potential benefits and harms of screening, including radiologists, pulmonologists, thoracic surgeons, and medical and radiation oncologists [38–40]. This multidisciplinary team will be in charge of developing standardized protocols for imaging acquisition, LDCT scan reports, and diagnostic algorithms for subsequent tests and procedures following each LDCT screening result. Multiple guidelines are available to assist screening centers in designing their own algorithms for evaluation of newly detected lung nodules, including the Fleischner Society and the National Comprehensive Cancer Network guidelines for lung cancer screening (Fig. 1) [39, 41]. Adherence to standard practices and diagnostic algorithms will minimize the use of unnecessary tests and procedures while ensuring timely institution of therapies for screening detected cancers, thereby improving patient outcomes and avoiding escalation in costs.
Figure 1.
Proposed model for initial implementation of lung cancer screening with low-dose computed tomography.
Another important aspect of program implementation will be the appropriate selection of high-risk individuals for screening. Until new studies show whether the benefits of screening apply to broader populations, we recommend that programs limit the use of LDCT screening to those individuals who meet the NLST eligibility criteria. This will minimize the burden of screening on patients, including the anxiety related to test results and the use of confirmatory imaging tests and biopsies in those with false-positive results, and will avoid unnecessary escalation of health care costs. Many primary care physicians may not be fully aware of the selection criteria for screening and may refer patients for screening [28, 42]. Screening centers should bear the responsibility of carefully determining high-risk individuals who will most likely benefit from screening. Those who would not benefit from screening should be referred back to their primary providers, who should be educated about eligibility criteria and how to refer appropriate candidates. Screening centers can facilitate this referral process by creating telephone hotlines that inform patients and providers about screening eligibility or by developing electronic referral orders that help determine whether an individual is eligible.
The third important element of the implementation model relates to data collection and continuous assessment (Fig. 1). We suggest that screening centers create prospective longitudinal registries that capture patient-level information as they undergo serial screening scans over years. Longitudinal registries represent an excellent opportunity to address current gaps in knowledge about lung cancer screening. For example, registries could track true and false positive rates of screening to confirm the validity of LDCT screening in routine practice outside clinical trials. In most cases, a false positive result will consist of a stable pulmonary nodule followed over at least 2 years, highlighting the importance of data collection at multiple time points [17, 43]. Other relevant information that registries should obtain includes data about health care resource utilization and information about patient quality of life after a positive screening result, using standard health-related quality of life instruments [44]. These data would help address the uncertainty about the health care costs of screening; would serve as a platform for future cost-effectiveness analysis of screening-related interventions, including referral to smoking-cessation programs; and would help us understand the impact of screening on multiple domains of quality of life, including anxiety.
The expertise and resources necessary for effective and efficient implementation of screening programs are most often available in university or large multispecialty referral hospitals, including the availability of multidisciplinary care and research infrastructure. In fact, these types of institutions represented most of the participants in the NLST [18]. We therefore recommend that screening programs should initially be implemented in academic or referral medical centers, and we discourage the use of LDCT screening in practices that cannot offer an integrated multidisciplinary approach for patients.
Conclusion
Since the release of the NLST results in November 2010, an increasing number of guidelines and medical societies have endorsed the use of LDCT screening for high-risk individuals, including the National Comprehensive Cancer Network guidelines, the American College of Chest Physicians, and the American Cancer Society [18, 39, 45]. Following these new guidelines, hospitals and clinics across the country will likely implement LDCT screening as part of routine care in the near future [26].
LDCT screening is a potentially valuable public health intervention because it can save thousands of lives every year. In adopting LDCT screening, health care systems need to acknowledge the potential risks, additional costs, and the several areas of uncertainty related to the screening process, including cost-effectiveness. We endorse the use of LDCT screening in practices that are capable of implementing LDCT screening responsibly, both in medical and economical terms. The key elements for responsible implementation of LDCT screening include the development of standardized practices, careful selection of screening candidates, and the creation of prospective screening registries that will help mitigate the remaining areas of uncertainty regarding lung cancer screening with LDCT.
Acknowledgments
This research was supported by the Moyer Foundation and the other contributors to the Gregory Fund for Early Cancer Detection Research.
Footnotes
Editor's Note: For another perspective on LDCT screening, see the commentary, “Screening for Lung Cancer With Low-Dose Computed Tomography,” by Anthony Miller, on pages 897–899 of this issue.
Author Contributions
Conception/Design: Bernardo H.L. Goulart
Collection and/or assembly of data: Bernardo H.L. Goulart
Data analysis and interpretation: Scott D. Ramsey, Bernardo H.L. Goulart
Manuscript writing: Scott D. Ramsey, Bernardo H.L. Goulart
Final approval of manuscript: Scott D. Ramsey, Bernardo H.L. Goulart
Disclosures
The authors indicated no financial relationships.
Reference
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Robinson BM, Kennedy C, McLean J, et al. Node-negative non-small cell lung cancer: Pathological staging and survival in 1765 consecutive cases. J Thorac Oncol. 2011;6:1691–1696. doi: 10.1097/JTO.0b013e31822647fd. [DOI] [PubMed] [Google Scholar]
- 3.Patz EF, Jr., Goodman PC, Bepler G. Screening for lung cancer. N Engl J Med. 2000;343:1627–1633. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- 4.Patz EF, Jr., Rossi S, Harpole DH, Jr, et al. Correlation of tumor size and survival in patients with pathologic stage lA nonsmall cell lung cancer. Chest. 2000;117:1568–1571. doi: 10.1378/chest.117.6.1568. [DOI] [PubMed] [Google Scholar]
- 5.Brett GZ. The value of lung cancer detection by six-monthly chest radiographs. Thorax. 1968;23:414–420. doi: 10.1136/thx.23.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss W, Boucot KR. The Philadelphia Pulmonary Neoplasm Research Project. Early roentgenographic appearance of bronchogenic carcinoma. Arch Intern Med. 1974;134:306–311. [PubMed] [Google Scholar]
- 7.Berlin NI, Buncher CR, Fontana RS, et al. The National Cancer Institute Cooperative Early Lung Cancer Detection Program. Results of the initial screen (prevalence) Early lung cancer detection: Introduction Am Rev Respir Dis. 1984;130:545–549. doi: 10.1164/arrd.1984.130.4.545. [DOI] [PubMed] [Google Scholar]
- 8.Flehinger BJ, Melamed MR, Zaman MB, et al. Early lung cancer detection: Results of the initial (prevalence) radiologic and cytologic screening in the Memorial Sloan-Kettering study. Am Rev Respir Dis. 1984;130:555–560. doi: 10.1164/arrd.1984.130.4.555. [DOI] [PubMed] [Google Scholar]
- 9.Fontana RS, Sanderson DR, Taylor WF, et al. Early lung cancer detection: Results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984;130:561–565. doi: 10.1164/arrd.1984.130.4.561. [DOI] [PubMed] [Google Scholar]
- 10.Frost JK, Ball WC, Jr., Levin ML, et al. Early lung cancer detection: Results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984;130:549–554. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- 11.Morrison AS. The effects of early treatment, lead time and length bias on the mortality experienced by cases detected by screening. Int J Epidemiol. 1982;11:261–267. doi: 10.1093/ije/11.3.261. [DOI] [PubMed] [Google Scholar]
- 12.Patz EF., Jr Lung cancer screening, overdiagnosis bias, and reevaluation of the Mayo Lung Project. J Natl Cancer Inst. 2006;98:724–725. doi: 10.1093/jnci/djj226. [DOI] [PubMed] [Google Scholar]
- 13.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 14.Marcus PM, Bergstralh EJ, Fagerstrom RM, et al. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst. 2000;92:1308–1316. doi: 10.1093/jnci/92.16.1308. [DOI] [PubMed] [Google Scholar]
- 15.Kubik AK, Parkin DM, Zatloukal P. Czech Study on Lung Cancer Screening: Post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer. 2000;89:2363–2368. doi: 10.1002/1097-0142(20001201)89:11+<2363::aid-cncr9>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Hocking WG, Hu P, Oken MM, et al. Lung cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. J Natl Cancer Inst. 2010;102:722–731. doi: 10.1093/jnci/djq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: A systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: Status after five annual screening rounds with low-dose CT Thorax. 2012;67:296–301. doi: 10.1136/thoraxjnl-2011-200736. [DOI] [PubMed] [Google Scholar]
- 20.Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: Three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445–453. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]
- 21.van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: The NELSON trial. Eur Respir J. 2011;38:154–161. doi: 10.1183/09031936.00123410. [DOI] [PubMed] [Google Scholar]
- 22.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: Importance of methods and context. J Natl Cancer Inst. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reich JM. A critical appraisal of overdiagnosis: Estimates of its magnitude and implications for lung cancer screening. Thorax. 2008;63:377–783. doi: 10.1136/thx.2007.079673. [DOI] [PubMed] [Google Scholar]
- 24.Pyenson BS, Sander MS, Jiang Y, et al. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff (Millwood) 2012;31:770–779. doi: 10.1377/hlthaff.2011.0814. [DOI] [PubMed] [Google Scholar]
- 25.Goodman A. Screening with low-dose helical CT scans cuts lung cancer deaths in heavy smokers. Oncology Business Review. [Accessed June 24, 2013]. Available at http://www.oncbiz.com/blog/2010/11/screening-with-low-dose-helical-ct-scans-cuts-lung-cancer-deaths-in-heavy-smokers/
- 26.Arenberg D, Kazerooni EA. Setting up a lung cancer screening program. J Natl Compr Canc Netw. 2012;10:277–285. doi: 10.6004/jnccn.2012.0024. [DOI] [PubMed] [Google Scholar]
- 27.Aberle DR, Henschke CI, McLoud TC, et al. Expert opinion: Barriers to CT screening for lung cancer. J Thorac Imaging. 2012;27:208. doi: 10.1097/RTI.0b013e318253d74d. [DOI] [PubMed] [Google Scholar]
- 28.Ravenel JG. Lung cancer screening: Confession of a skeptic. J Thorac Imaging. 2012;27:207. doi: 10.1097/RTI.0b013e318257f435. [DOI] [PubMed] [Google Scholar]
- 29.Leventhal W. Primary care perspective on lung cancer screening. J Thorac Imaging. 2012;27:209–210. doi: 10.1097/RTI.0b013e318256f2d7. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control. Current smoking. [Accessed June 24, 2013]. Available at http://www.cdc.gov/nchs/data/nhis/earlyrelease/201106_08.pdf.
- 31.Ma J, Ward EM, Smith R, et al. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119:1381–1385. doi: 10.1002/cncr.27813. [DOI] [PubMed] [Google Scholar]
- 32.Goulart BH, Bensink ME, Mummy DG, et al. Lung cancer screening with low-dose computed tomography: Costs, national expenditures, and cost-effectiveness. J Natl Compr Canc Netw. 2012;10:267–275. doi: 10.6004/jnccn.2012.0023. [DOI] [PubMed] [Google Scholar]
- 33.Chirikos TN, Hazelton T, Tockman M, et al. Screening for lung cancer with CT: A preliminary cost-effectiveness analysis. Chest. 2002;121:1507–1514. doi: 10.1378/chest.121.5.1507. [DOI] [PubMed] [Google Scholar]
- 34.Mahadevia PJ, Fleisher LA, Fric KD, et al. Lung cancer screening with helical computed tomography in older adult smokers: A decision and cost-effectiveness analysis. JAMA. 2003;289:313–322. doi: 10.1001/jama.289.3.313. [DOI] [PubMed] [Google Scholar]
- 35.Marshall D, Simpson KN, Earle CC, et al. Potential cost-effectiveness of one-time screening for lung cancer (LC) in a high risk cohort. Lung Cancer. 2001;32:227–236. doi: 10.1016/s0169-5002(00)00239-7. [DOI] [PubMed] [Google Scholar]
- 36.Wisnivesky JP, Mushlin AI, Sicherman N, et al. The cost-effectiveness of low-dose CT screening for lung cancer: Preliminary results of baseline screening. Chest. 2003;124:614–621. doi: 10.1378/chest.124.2.614. [DOI] [PubMed] [Google Scholar]
- 37.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood DE. Maximizing the benefit and minimizing the risks of lung cancer screening. J Thorac Imaging. 2012;27:211–212. doi: 10.1097/RTI.0b013e318256c22f. [DOI] [PubMed] [Google Scholar]
- 39.Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;10:240–265. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen MP, Corey J, Siewert B. Establishing a computed tomography screening clinic. J Thorac Imaging. 2012;27:220–223. doi: 10.1097/RTI.0b013e3182587cf8. [DOI] [PubMed] [Google Scholar]
- 41.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: A statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 42.Klabunde CN, Marcus PM, Han PK, et al. Lung cancer screening practices of primary care physicians: Results from a national survey. Ann Fam Med. 2012;10:102–110. doi: 10.1370/afm.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croswell JM, Baker SG, Marcus PM, et al. Cumulative incidence of false-positive test results in lung cancer screening: A randomized trial. Ann Intern Med. 2010;152:505–512. W176–W180. doi: 10.7326/0003-4819-152-8-201004200-00007. [DOI] [PubMed] [Google Scholar]
- 44.van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON) Br J Cancer. 2010;102:27–34. doi: 10.1038/sj.bjc.6605459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]



