Most patients with lung cancer have non-small cell lung cancer (NSCLC) subtype and have advanced disease at the time of diagnosis. Improvements in both first-line and subsequent therapies are allowing longer survival and enhanced quality of life for these patients. The cytotoxic agents pemetrexed and docetaxel and the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib are standard second-line therapies. The inhibitor of the EML4/ALK fusion protein, crizotinib, has recently become a standard second-line treatment for patients with the gene rearrangement and has promise for patients with the ROS1 rearrangement.
Keywords: Non-small cell lung cancer; Pemetrexed; Docetaxel; Gefitinib; Erlotinib, EGFR mutation, KRAS mutation, EML4/ALK rearrangement, ROS1 rearrangement; MET amplification
Abstract
Most patients with lung cancer have non-small cell lung cancer (NSCLC) subtype and have advanced disease at the time of diagnosis. Improvements in both first-line and subsequent therapies are allowing longer survival and enhanced quality of life for these patients. The median overall survival observed in many second-line trials is approximately 9 months, and many patients receive further therapy after second-line therapy. The cytotoxic agents pemetrexed and docetaxel and the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib are standard second-line therapies. For patients with EGFR mutation, a TKI is the favored second-line therapy if not already used in first-line therapy. For patients without the EGFR mutation, TKIs are an option, but many oncologists favor cytotoxic therapy. The inhibitor of the EML4/ALK fusion protein, crizotinib, has recently become a standard second-line treatment for patients with the gene rearrangement and has promise for patients with the ROS1 rearrangement.
Implications for Practice:
The landscape of first-line treatment has generated challenges for clinical decisions in second-line therapy. For the patient treated with standard chemotherapy in the first line who has a treatable molecular change, this change should be targeted. More specifically, the patient with an epidermal growth factor receptor (EGFR) mutation should be treated with an EGFR tyrosine kinase inhibitor, and the patient with EML4/ALK rearrangement should be treated with crizotinib. However, these agents are increasingly being used in the first line, and most patients do not have these molecular changes. This leaves the clinician with many challenging questions regarding second-line therapy. How should the patient without treatable mutations be treated? Which clinical trials are most promising? How should the patient treated with a targeted agent in the first line be treated in the second line? This review addresses these issues, exploring the key existing data available to help guide informed clinical decisions.
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States and in the world [1, 2]. Most patients have the non-small cell lung cancer (NSCLC) subtype and have advanced-stage disease at the time of diagnosis. The standard first-line therapy for advanced disease (defined as stage IIIB or IV) is a platinum doublet alone or with a targeted agent such as bevacizumab for four to six cycles [3]. Chemotherapy extends overall survival (OS), reduces disease-related symptoms, and improves quality of life (QoL). Historically, after completion of four to six cycles of platinum-based therapy, patients were observed, and at the time of radiographic or clinical evidence of disease progression, therapy was re-initiated. Approximately 30% of patients experience disease progression during first-line chemotherapy, and all patients with initial disease control will eventually experience disease progression and require subsequent therapy. The use of anticancer agents in these clinical situations is referred to as “second-line therapy.” Currently, there are two cytotoxic agents, pemetrexed and docetaxel, and two epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI), erlotinib and gefitinib, that are established agents in the second-line setting [4–8].
Several trials have challenged the standard of care of observation for the management of patients without progression after four cycles of platinum-based doublet first-line chemotherapy. This strategy, referred to as “maintenance therapy,” involves the administration of established second-line agents immediately after successful first-line therapy, instead of waiting for disease progression. Phase III trials of erlotinib compared with placebo and pemetrexed compared with placebo have demonstrated an improvement in progression-free survival (PFS) and OS with maintenance therapy [9, 10]. Maintenance therapy with pemetrexed is beneficial only for patients with nonsquamous histology. When a maintenance strategy is used as part of first-line therapy, the agent used is typically not considered an option for second-line or later treatment. The use of maintenance therapy has led to reconsideration of the traditional definition of second-line therapy.
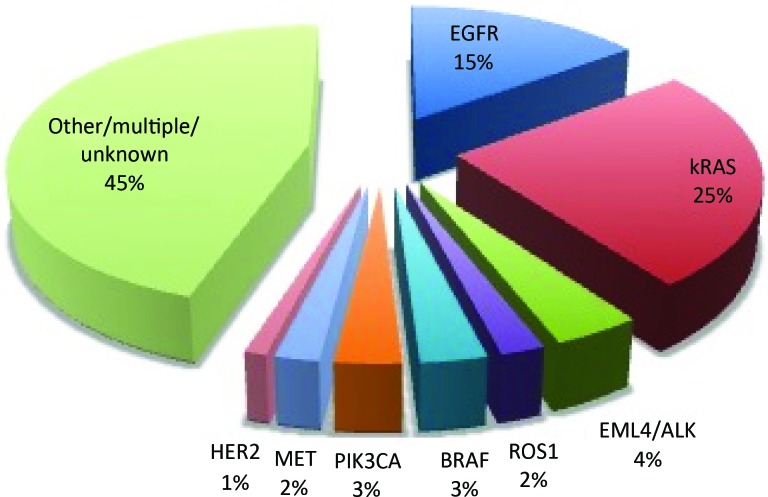
The use of a molecularly targeted agent in first-line therapy can also increase the challenge of second-line therapy selection. With complete testing, a driver mutation can now be found for more than half of adenocarcinomas of the lung [11–14] (Fig. 1). Both EGFR TKIs and crizotinib have been specifically studied in second-line therapy, but both are also approved for first-line use in patients with the relevant molecular changes (EGFR mutation and ALK rearrangement, respectively). We will explore therapeutic options for second-line therapy for patients treated with targeted therapy in the first-line setting.
Figure 1.
Approximate incidence of common mutations in adenocarcinoma. Numbers should be considered approximate to illustrate the relative frequency of these changes as they have never been all reported in the same series and estimates vary. References: EGFR [11, 12], Kirsten rat sarcoma viral oncogene homolog (kRAS) [12], echinoderm microtubule-associated protein-like 4/anaplastic lymphoma kinase (EML4/ALK) [13], ROS1 [14, 15], BRAF [12], Phosphatidylinositol-4,5-Bisphosphate 3-Kinase (PIK3CA) [12], hepatocyte growth factor receptor (MET) [12] (note: this rate refers to MET mutation; overexpression is more common), Human Epidermal Growth Factor Receptor 2 (HER2) [12], Other/unknown [12].
Docetaxel
The first agent approved for use in the second-line setting was docetaxel, and its approval was based on the results of two phase III trials (Table 1) [4, 6]. In the trial by Shepherd et al., patients were required to have a performance status of 0–2 and to have received one or more platinum-based chemotherapy regimens [4]. Patients were randomly assigned to docetaxel 100 mg/m2 every 3 weeks or best supportive care (BSC). The trial was amended because of excessive toxicity in the docetaxel arm, and the dose of docetaxel was reduced to 75 mg/m2 every three weeks. The time to disease progression and OS were statistically significantly longer in the docetaxel arm compared with the BSC arm in the intent-to-treat (ITT) patient population. These differences were more significant in the cohort of patients treated with 75 mg/m2 every 3 weeks. The QoL assessments were significantly better in the docetaxel arm as well, with significant differences in the pain and fatigue scales [15].
Table 1.
Select second-line phase III trials of cytotoxic agents
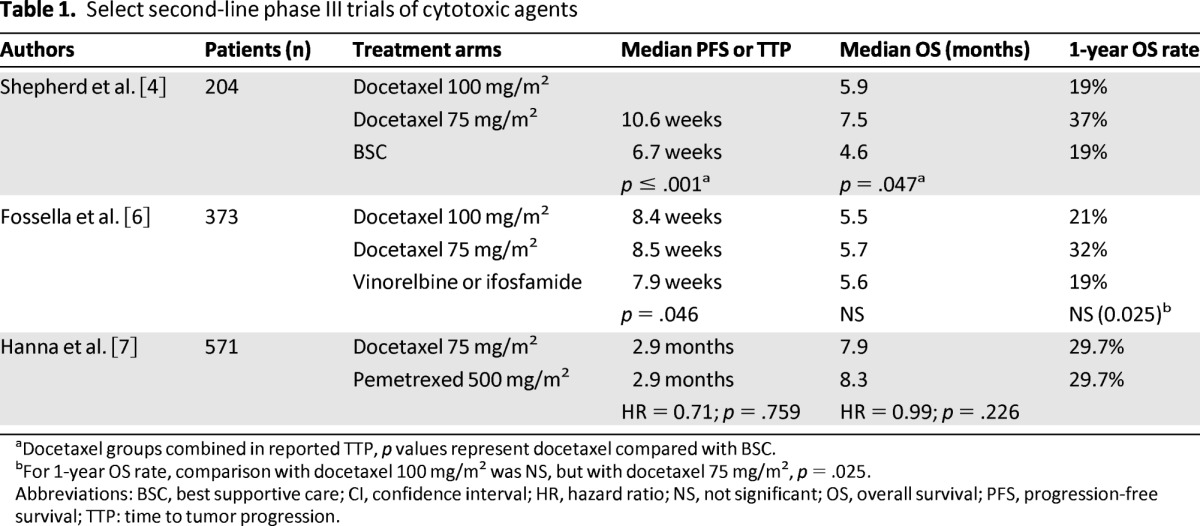
aDocetaxel groups combined in reported TTP, p values represent docetaxel compared with BSC.
bFor 1-year OS rate, comparison with docetaxel 100 mg/m2 was NS, but with docetaxel 75 mg/m2, p = .025.
Abbreviations: BSC, best supportive care; CI, confidence interval; HR, hazard ratio; NS, not significant; OS, overall survival; PFS, progression-free survival; TTP: time to tumor progression.
A second trial compared docetaxel 100 mg/m2 or 75 mg/m2 every three weeks versus vinorelbine or ifosfamide (selection of the agent was at the investigator's discretion) [6]. Patients assigned to the docetaxel arm experienced a longer PFS at 26 weeks (p = .005), but OS did not differ among the three arms. The one-year OS rate was significantly higher in the docetaxel 75 mg/m2 arm compared with the control arm. These two trials established docetaxel 75 mg/m2 as the standard second-line therapy. The clinically significant toxicities were neutropenia and febrile neutropenia. To reduce the rate of these toxicities, several trials investigated a weekly compared with the standard schedule of docetaxel every three weeks. A meta-analysis using individual patient data compared weekly with every three weeks docetaxel and revealed similar OS with both schedules [16]. Patients treated with docetaxel weekly compared with docetaxel every three weeks experienced a lower rate of neutropenia (5% vs. 18%; p < .00001) and febrile neutropenia (<1% vs. 6%; p < .00001); no significant differences were observed for anemia, thrombocytopenia, and nonhematologic toxicities. In clinical practice, both schedules are frequently used.
Clinicians are increasing their use of pemetrexed in combination with a platinum agent in the first-line setting or as single-agent maintenance therapy in patients with nonsquamous histology. The restriction to nonsquamous histology and the use of pemetrexed earlier in the disease course have reduced the availability of pemetrexed in the second-line setting.
Pemetrexed
A subsequent phase III trial compared pemetrexed 500 mg/m2 every three weeks with docetaxel 75 mg/m2 every three weeks (Table 1) [7]. The trial was designed to demonstrate the non-inferiority of pemetrexed compared with docetaxel in OS. A statistically significant difference in PFS and OS was not observed. In the docetaxel arm compared with the pemetrexed arm, a higher rate of grade 3 or 4 neutropenia (40.2% vs. 5.3%; p < .001) and febrile neutropenia (13.4% vs. 1.9%; p < .001) was observed. The rate of nonhematologic toxicities was similar. This trial established pemetrexed as an alternative to docetaxel in the second-line setting.
After completion of this trial, an interaction between pemetrexed efficacy and histology (squamous vs. nonsquamous) was detected. In a retrospective subset analysis, it was found that patients with nonsquamous histology (n = 399) experienced a superior OS with pemetrexed compared with docetaxel [17]. In contrast, patients with squamous histology (n = 172) experienced a statistically significantly inferior OS with pemetrexed. This trial and other phase III trials demonstrated that the efficacy of pemetrexed is limited to patients with nonsquamous histology tumors [10, 17]. Clinicians are increasing their use of pemetrexed in combination with a platinum agent in the first-line setting or as single-agent maintenance therapy in patients with nonsquamous histology. The restriction to nonsquamous histology and the use of pemetrexed earlier in the disease course have reduced the availability of pemetrexed in the second-line setting.
EGFR TKIs
The National Cancer Institute of Canada Clinical Trials Group performed a phase III trial (BR.21) that compared erlotinib with placebo in patients who had disease progression during one or two lines of chemotherapy and who were not eligible for further chemotherapy (Table 2). Patients assigned to the erlotinib arm experienced a statistically significantly longer PFS and OS than those in the BSC group [5]. Patients assigned to the erlotinib arm also experienced a statistically significantly longer median time to deterioration in cough, dyspnea, and pain, and improvement in physical function and global QoL [18].
Table 2.
Select second-line phase III trials of EGFR TKIs
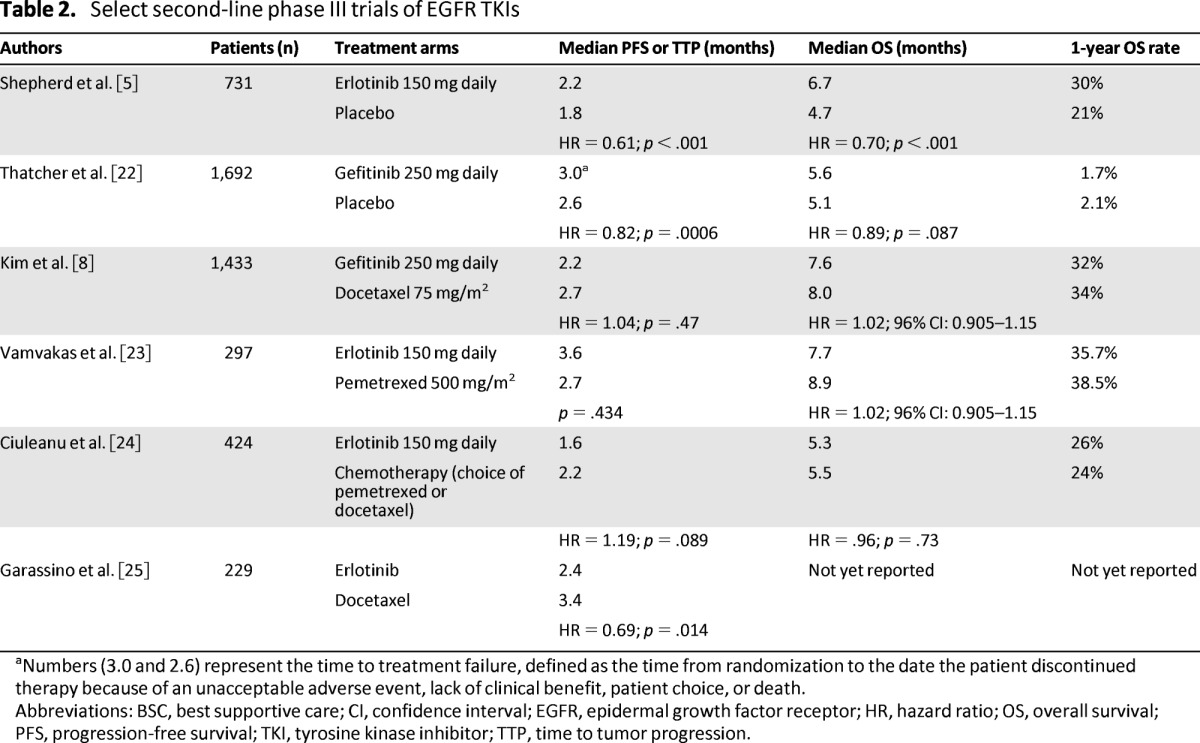
aNumbers (3.0 and 2.6) represent the time to treatment failure, defined as the time from randomization to the date the patient discontinued therapy because of an unacceptable adverse event, lack of clinical benefit, patient choice, or death.
Abbreviations: BSC, best supportive care; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase inhibitor; TTP, time to tumor progression.
Gefitinib was granted accelerated approval in the United States based on an improvement in response rate in two phase II trials [19, 20]. A phase III trial compared gefitinib with placebo in patients who were refractory or intolerant to their previous chemotherapy (Table 2). However, a statistically significant difference in the primary endpoint of OS was ultimately not observed [21]. Based on these data, the U.S. Food and Drug Administration withdrew approval for gefitinib, and it is not commercially available in the United States. Following these results, a phase III trial comparing gefitinib 250 mg daily with docetaxel continued to enroll patients who had already received at least one platinum-based regimen. The co-primary endpoint of the study, non-inferiority of gefitinib compared with docetaxel in OS, was observed [8]. Key trials comparing TKIs to placebo [5, 22] and to chemotheraphy [8, 23–25] are summarized in Table 2.
Second-Line Therapy for Patients With EGFR Wild-Type Disease
The use of EGFR mutation testing and first-line therapy has resulted in two subpopulations of patients who are eligible for second-line therapy: patients with EGFR-mutated NSCLC after EGFR TKI therapy, and patients with EGFR wild-type tumors. In contrast to the impressive results of EGFR TKI treatment for patients with EGFR-mutated NSCLC, the results in EGFR wild-type tumors are far more modest. Patients with EGFR wild-type NSCLC have a response rate to EGFR TKI therapy of 1%–7%, a median PFS of approximately 2 months, and a median OS of 6–8 months in retrospective subset analyses of second-line trials [24–26]. Two recent prospective trials have demonstrated that in the second-line setting, PFS is inferior for patients with EGFR wild-type tumors treated with erlotinib compared with those treated with cytotoxic chemotherapy [25, 27]. In one study, OS data are not yet available, and in the other trial, the results were similar between erlotinib and cytotoxic chemotherapy. These results left a clinical need to identify which patients with EGFR wild-type tumors will benefit from EGFR TKI therapy. A recent trial, PROSE, compared chemotherapy with erlotinib for the second-line treatment of unselected patients with NSCLC [28]. Overall, survival was similar in the ITT patient population. More importantly, patients were stratified based on VeriStrat (Biodesix, Boulder, CO, http://www.biodesix.com/products/veristrat/) status, a serum proteomics assay, and the trial prospectively assessed the predictive capacity of VeriStrat. Among patients with a VeriStrat “good” signature, survival was similar between chemotherapy and erlotinib. Among patients with a VeriStrat “poor” signature, survival was better with chemotherapy than with erlotinib. Findings were similar when the analysis was restricted to those with EGFR wild-type or with unknown-type disease, suggesting that VeriStrat may be able to identify patients with EGFR wild-type tumors who should preferentially be treated with cytotoxic therapy or who are unlikely to benefit from EGFR TKI therapy in the second line.
Second-Line Therapy for Patients With an EGFR Mutation After EGFR TKI
For those patients with an EGFR mutation who have disease progression on an EGFR TKI, chemotherapy remains the most often used second-line option, but a host of alternative treatments seek to challenge this standard. The simplest alternative to chemotherapy alone is the addition of an EGFR TKI to chemotherapy. This approach was initiated because it was observed that a portion of patients with EGFR mutation had “flare” of their disease when EGFR TKI therapy was withdrawn at the time of disease progression [29]. Researchers hypothesized that whereas some cells were resistant to the EGFR TKI, leading to progression, other cells remained sensitive, and that their growth was disinhibited by withdrawal of TKI. Although the combination of chemotherapy and an EGFR TKI has not improved treatment results in unselected populations, [30–34] prevention of flare and ongoing suppression of resistant clones might improve disease control over chemotherapy alone in a population selected for EGFR mutation. The treatment strategy of re-treating with a TKI after an interval with no TKI treatment has been evaluated in two retrospective series, both of which reported clinical activity of repeat EGFR TKI [35, 36]. In clinical practice, patients with EGFR mutation who have disease progression while taking a TKI often have progression in one or several locations, so called “oligoprogression.” Small retrospective case series have demonstrated the efficacy of local radiation therapy to the site(s) of progression followed by re-initiation of EGFR TKI therapy [37, 38]. The combination of afatinib with cetuximab, a monoclonal antibody against EGFR, seems promising in preliminary data. In the first 90 evaluable patients treated in this ongoing trial (NCT01090011), the disease control rate was 94%, the probability of being progression-free at six months was 42%, and the confirmed response rate was 40% (95% CI: 17–59) [39]. Final results from this study are eagerly awaited.
In clinical practice, patients with EGFR mutation who have disease progression while taking a TKI often have progression in one or several locations, so called “oligoprogression.” Small retrospective case series have demonstrated the efficacy of local radiation therapy to the site(s) of progression followed by re-initiation of EGFR TKI therapy.
EML4/ALK Rearrangements
In approximately 4% of adenocarcinomas, a gene fusion between the echinoderm microtubule-associated protein-like 4 (EML4) and the anaplastic lymphoma kinase (ALK) gene leads to a fusion gene (EML4/ALK), which contributes to carcinogenesis [40]. Crizotinib has been compared with chemotherapy for second-line treatment of patients with the EML4/ALK rearrangement. In 347 such patients randomly assigned to crizotinib or chemotherapy (either docetaxel or pemetrexed), overall response rate was superior with crizotinib (65% vs. 20%) as was PFS (7.7 months vs. 3 months; hazard ratio = 0.49; p < .0001) [41]. Crizotinib was approved by the U.S Food and Drug Administration in August 2011 and may be considered a standard second-line treatment for patients with EML4/ALK who are treated with chemotherapy in the first line.
Although targeted therapy with crizotinib for patients with EML4/ALK rearrangements certainly represents a clinical advance, the maximum PFS thus far reported is approximately 10 months, generating a need to define optimal subsequent therapy [42]. For patients whose first anticancer treatment is crizotinib, the only standard subsequent therapy is chemotherapy, which is typically given in the first-line setting, namely a platinum doublet, with consideration of the addition of bevacizumab in eligible patients. Two retrospective series suggest superior treatment outcomes with pemetrexed for patients with the EML4/ALK rearrangement [43, 44]. Heat shock protein (HSP) inhibitors and more potent and selective EML4/ALK inhibitors have also shown promise as additional molecularly directed treatment options for patients with the EML4/ALK rearrangement [45–48]. Finally, as in patients with the EGFR mutation who have oligoprogression on erlotinib, retrospective data suggest the efficacy of local ablation followed by re-treatment. In the case of EML4/ALK, this approach may be particularly promising for those with intracranial-only progression [37].
Investigational Agents
There are substantial flaws with the existing standard second-line agents, thus increasing the interest in experimental agents. Docetaxel has significant toxicities and is difficult to tolerate even as a weekly dose, pemetrexed is active only in patients with nonsquamous histology and is frequently used as first-line therapy, crizotinib is approved only for patients with EML4/ALK rearrangements, and the EGFR TKIs have limited activity in patients whose tumors do not have the mutation. Fewer than 20% of patients will have the actionable changes EGFR and EML4/ALK. In contrast, kRAS is the most common known driver gene mutation, present in approximately 25% of adenocarcinomas [49]. Recently, two treatments have emerged as promising for the patient with kRAS. MAP kinase/ERK kinase (MEK) is downstream to signaling from RAS, and selumetinib is an MEK inhibitor. A multicenter international phase II trial compared treatment with docetaxel and placebo with treatment using docetaxel plus selumetinib for patients with KRAS mutation [50]. Patients randomly assigned to selumetinib and docetaxel experienced a numerically longer OS, a statistically significantly longer PFS, and higher overall response rate than those treated with docetaxel and placebo. The rate of febrile neutropenia in the docetaxel plus selumetinib arm was 18.2% and in the docetaxel plus placebo arms was 0%. Results with HSP-90 inhibitors also look promising for patients with kRAS. A study of single-agent ganetespib, an inhibitor of HSP-90 protein, included a molecularly defined cohort of patients in whom NSCLC included a KRAS mutation [48]. These results were followed by a randomized phase IIB/III study of docetaxel and ganetespib compared with docetaxel alone, known as the Ganetespib Assessment in Lung cAncer with docetaxel (GALAXY) study (NCT: 01348126) [51]. The co-primary endpoints were PFS in patients with elevated lactate dehydrogenase levels and mutant kRAS NSCLC. An interim safety analysis revealed no evidence of benefit and possible safety concerns in patients with non-adenocarcinoma histology, and consequently, eligibility was restricted to patients with adenocarcinoma histology. Preliminary results revealed benefit from the combination therapy in patients with adenocarcinoma histology, kRAS mutation, and an elevated lactate dehydrogenase level. These results are only preliminary, and a phase III trial is required.
MET is a receptor tyrosine kinase that is activated by hepatocyte growth factor. MET expression is associated with adverse prognosis and has been implicated in resistance to EGFR TKIs [52]. A randomized phase II study compared second-line or third-line erlotinib with erlotinib plus onartuzumab, a monoclonal antibody against the MET receptor [53]. Patients were required to provide tissue, and a companion diagnostic test was included in the trial design that determined whether MET expression was present in tumors. The co-primary endpoints were PFS in the ITT patient population and in MET-positive patients. Patients who were MET diagnostic positive and assigned to erlotinib and onartuzumab therapy experienced a significant improvement in PFS and OS with the addition of onartuzumab compared with those treated with erlotinib and placebo. A phase III study comparing erlotinib with erlotinib plus onartuzumab in patients who are MET diagnostic positive is underway (NCT01456325) [54].
Programmed death 1 (PD-1) protein is a T-cell co-inhibitory receptor, and PD-L1 is one of its ligands; disruption of this pathway allows tumor cells to evade the host immune system. A phase I trial of the anti-PD-L1 antibody (BMS-936559) enrolled 207 patients with advanced cancer; 75 patients had advanced NSCLC [55]. Of patients with advanced NSCLC, 49 were assessable for response, and of these, 5 had response. Another phase I trial of an anti-PD-1 antibody (BMS-936558) included 76 assessable patients with NSCLC of whom 14 experienced an objective response. In an exploratory analysis of 25 patients from this study with NSCLC and PD-L1-positive tumors, 9 responded to the drug [56]. Phase III trials with BMS-936558 in the second-line setting are ongoing.
Conclusion
This article has summarized the current key clinical data needed to inform clinical decision-making in second-line treatment of NSCLC. In practice, clinical decisions are personalized to the individual patient—their priorities, strengths, comorbidities, and prior therapies. The goals of care in the second-line setting are similar to those in the first-line setting: to maximize duration of life, minimize toxicity, and improve QoL.
When a targeted molecular option is available, the authors strongly prefer that it be used. Therefore, for the patient with EGFR mutation who has not yet been treated with erlotinib or gefitinib, or the patient with EML4/ALK who has not yet been treated with crizotinib, the clinical decision is easy. When an experimental target such as kRAS or ROS1 is present, the authors recommend participation in a clinical trial with an agent that targets the relevant molecular pathway. Even when a specific trial drug–target match is not available, clinical trials for unselected patients should be strongly considered when available. Trials incorporating agents targeting PD-1 and PD-L1 are particularly promising.
Although targeted therapy is preferred when a target is present, most patients are still treated with cytotoxic chemotherapy in the first line and lack a drug-treatable target for second-line therapy. For the patient with nonsquamous histology who is treated with a non-pemetrexed doublet in the first line, pemetrexed is recommended for second-line therapy because of its favorable side effect profile and data indicating superior efficacy. For the patient with squamous histology or the patient treated with pemetrexed in the first line (whether as part of an initial cytotoxic doublet, as maintenance, or both), docetaxel and erlotinib are the standard options. In the absence of known EGFR mutation, existing data are not sufficient to define one of these medicines as superior to the other. In our practice, we favor docetaxel for healthy, fit, motivated patients based on superior PFS. However, we consider legitimate the choice of erlotinib or gefitinib (where available) for patients who are less fit, less healthy, or less tolerant of side effects.
Cytotoxic therapy is the most often used standard second-line therapy for patients with EGFR mutation who are treated with erlotinib as first-line therapy or for patients with EML4/ALK rearrangement treated with crizotinib as first-line therapy. Therapy consists of whatever drugs would have been otherwise used in the first line (typically carboplatin and paclitaxel with consideration of bevacizumab or carboplatin and pemetrexed with consideration of bevacizumab). The authors favor enrollment in a promising second-line clinical trial that targets the mutation present prior to resorting to cytotoxic therapy, if such a trial is available. In patients with oligoprogression on erlotinib or crizotinib, the authors consider ablation of progressive sites followed by re-initiation of the relevant targeted agent when possible.
Author Contributions
Conception/Design: Jared M. Weiss, Thomas E. Stinchcombe
Collection and/or assembly of data: Jared M. Weiss, Thomas E. Stinchcombe
Manuscript writing: Jared M. Weiss, Thomas E. Stinchcombe
Final approval of manuscript: Jared M. Weiss, Thomas E. Stinchcombe
Disclosures
Jared M. Weiss: Pfizer (C/A); Astellas, Celgene, GSK (RF). Thomas Stinchcombe. Eli Lilly, Genentech (C/A); Eli Lilly, Genentech, GSK, Pfizer, Synta (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Azzoli CG, Baker S, Jr, Temin S, et al. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 6.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The tax 320 non-small cell lung cancer study group J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 7.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 8.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 9.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 10.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 11.D'Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and l858r in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29:2066–2070. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kris MG, Kwiatkowski DJ, Iafrate AJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2011;29(suppl):CRA7506. [Google Scholar]
- 13.Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-pcr screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–6624. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 14.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 15.Dancey J, Shepherd FA, Gralla RJ, et al. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: Results of a prospective, randomized phase III trial. Lung Cancer. 2004;43:183–194. doi: 10.1016/j.lungcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Di Maio M, Perrone F, Chiodini P, et al. Individual patient data meta-analysis of docetaxel administered once every 3 weeks compared with once every week second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:1377–1382. doi: 10.1200/JCO.2006.09.8251. [DOI] [PubMed] [Google Scholar]
- 17.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 18.Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group study br. 21. J Clin Oncol. 2006;24:3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 19.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: Data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 20.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Stinchcombe TE, Socinski MA. Gefitinib in advanced non-small cell lung cancer: Does it deserve a second chance? The Oncologist. 2008;13:933–944. doi: 10.1634/theoncologist.2008-0019. [DOI] [PubMed] [Google Scholar]
- 22.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (IRESSA survival evaluation in lung cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 23.Vamvakas L, Kentepozidis NK, Karampeazis A, et al. Pemetrexed (mta) compared with erlotinib (erl) in pretreated patients with advanced non-small cell lung cancer (NSCLC): Results of a randomized phase III Hellenic Oncology Research Group trial. J Clin Oncol. 2010;28(suppl):7519a. [Google Scholar]
- 24.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): A randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 25.Garassino MC, M O, Bettini A, Floriani I, et al. Tailor: A phase III trial comparing erlotinib with docetaxel as the second-line treatment of NSCLC patients with wild-type (WT) EGFR. J Clin Oncol. 2012;30(suppl):LBA7501. [Google Scholar]
- 26.Zhu CQ, da Cunha Santos G, Ding K, et al. Role of kras and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group study br. 21. J Clin Oncol. 2008;26:4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 27.Okano Y, Asami K, Fukuda M, et al. Randomized phase III trial of erlotinib (e) versus docetaxel (d) as second- or third-line therapy in patients with advanced non-small cell lung cancer (NSCLC) who have wild-type or mutant epidermal growth factor receptor (EGFR): Docetaxel and erlotinib lung cancer trial (DELTA) J Clin Oncol. 2013;31(suppl):8006a. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 28.Lazzari C, Barni S, Aieta M, et al. Randomized proteomic stratified phase III study of second-line erlotinib (e) versus chemotherapy (ct) in patients with inoperable non-small cell lung cancer (PROSE) J Clin Oncol. 2013;31(suppl):LBA8005. [Google Scholar]
- 29.Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin Cancer Res. 2011;17:6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 31.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial–INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial–INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 33.Herbst RS, Prager D, Hermann R, et al. Tribute: A phase III trial of erlotinib hydrochloride (osi-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 34.Stinchcombe TE, Peterman AH, Lee CB, et al. A randomized phase II trial of first-line treatment with gemcitabine, erlotinib, or gemcitabine and erlotinib in elderly patients (age >/=70 years) with stage IIIb/IV non-small cell lung cancer. J Thorac Oncol. 2011;6:1569–1577. doi: 10.1097/JTO.0b013e3182210430. [DOI] [PubMed] [Google Scholar]
- 35.Heon S, Nishino M, Goldberg SB, et al. Response to EGFR tyrosine kinase inhibitor (TKI) retreatment after a drug-free interval in EGFR-mutant advanced non-small cell lung cancer (NSCLC) with acquired resistance. J Clin Oncol. 2012;30(suppl):7525a. [Google Scholar]
- 36.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weickhardt AJ, Burke JM, Gan G, et al. Continuation of EGFR/alk inhibition after local therapy of oligoprogressive disease in EGFR mutant (MT) and ALK+ non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30(suppl):7526a. [Google Scholar]
- 38.Yu HA, Drilon AEDC, Varghese AM, et al. Local therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Clin Oncol. 2012;30(suppl):7527a. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janjigian YY, Horn L, Groen HJM, et al. Activity of afatinib/cetuximab in patients (pts) with EGFR mutant non-small cell lung cancer (NSCLC) and acquired resistance (AR) to EGFR inhibitors. Paper presented at: European Society of Medical Oncology; September 30, 2012; Viena, Austria. [Google Scholar]
- 40.Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-pcr screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–6624. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 41.Shaw AT, Nakagawa K, Seto T, et al. Phase III study of crizotinib versus pemetrexed or docetaxel chemotherapy in patients with advanced ALK-positive non-small cell lung cancer (NSCLC) (PROFILE 1007). Paper presented at: European Society of Medical Oncology; September 30, 2012; Viena, Austria. [Google Scholar]
- 42.Camidge DR, Bang YJ, Kwak EL, et al. Progression-free survival (PFS) from a phase I study of crizotinib (pf-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29:2501a. [Google Scholar]
- 43.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6:774–780. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JO, Kim TM, Lee SH, et al. Anaplastic lymphoma kinase translocation: A predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011;6:1474–1480. doi: 10.1097/JTO.0b013e3182208fc2. [DOI] [PubMed] [Google Scholar]
- 45.Felip E, Barlesi F, Gandhi L, et al. Phase II activity of the HSP90 inhibitor AUY922 in patients with ALK-rearranged (ALK+) or EGFR-mutated advanced non-small cell lung cancer (NSCLC). Paper presented at: European Society of Medical Oncology; September 29, 2012; Viena, Austria. [Google Scholar]
- 46.Mehra R, Camidge DR, Sharma S, et al. First-in-human phase I study of the ALK inhibitor LDK378 in advanced solid tumors. J Clin Oncol. 2012;30(suppl):3007a. [Google Scholar]
- 47.Sequist LV, Gettinger S, Senzer NN, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28:4953–4960. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong K, Goldman JW, Paschold EH, et al. An open-label phase II study of the HSP90 inhibitor ganetespib (sta-9090) as monotherapy in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29(suppl):7500a. [Google Scholar]
- 49.Kris MG, Kwiatkowski DJ, Iafrate AJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2011;29(suppl):CRA7506. [Google Scholar]
- 50.Janne PA, Shaw AT, Pereira JR, et al. Phase II double-blind, randomized study of selumetinib (sel) plus docetaxel (doc) versus doc plus placebo as second-line treatment for advanced kras mutant non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30(suppl):7503a. [Google Scholar]
- 51.Ramalingam SS, Goss GD, Manegold C, Sr, et al. The GALAXY Trial ( NCT 01348126): A randomized IIB/III study of ganetespib (STA-9090) in combination with docetaxel versus docetaxel alone as second line therapy in patients with stage IIIB or IV NSCLC. Paper presented at: European Society of Medical Oncology; September 29, 2012; Viena, Austria. [Google Scholar]
- 52.Cappuzzo F, Marchetti A, Skokan M, et al. Increased met gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27:1667–1674. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spigel DR, Ramlau R, Daniel DB, et al. Final efficacy results from OAM4558g, a randomized phase II study evaluating metmab or placebo in combination with erlotinib in advanced NSCLC. J Clin Oncol. 2011;29(suppl):7505a. [Google Scholar]
- 54.Spigel David R, E MJ, Tony Mok, Kenneth John O'Byrne, Luis Paz-Ares, Wei Yu, Karen Rittweger, Holger C, Thurm The metlung study: A randomized, double-blind, phase iii study of onartuzumab (metmab) plus erlotinib versus placebo plus erlotinib in patients with advanced, met-positive non-small cell lung cancer (nsclc) J Clin Oncol. 2012;30(suppl) doi: 10.1016/j.cllc.2012.05.009. abstr TPS7616. [DOI] [PubMed] [Google Scholar]
- 55.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-pd-l1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]