Abstract
Gastrointestinal disease has been recognized as a major manifestation of human immunodeficiency virus infection since the earliest recognition of acquired immunodeficiency syndrome (AIDS). Originally, these disease manifestations were considered to be sequelae of the immune destruction that characterizes AIDS rather than being central to the pathogenesis of AIDS. Over time, it has become clear that the mucosal immune system in general and the intestinal immune system in particular are central to the pathogenesis of AIDS, with most of the critical events (eg, transmission, viral amplification, CD4+ T-cell destruction) occurring in the gastrointestinal tract. Compared with peripheral blood, these tissues are not easily accessible for analysis and have only begun to be examined in detail recently. In addition, although the resulting disease can progress over years, many critical events happen within the first few weeks of infection, when most patients are unaware that they are infected. Moreover, breakdown of the mucosal barrier and resulting microbial translocation are believed to be major drivers of AIDS progression. In this review, we focus on the interaction between primate lentiviruses and the gastrointestinal tract and discuss how this interaction promotes the pathogenesis of AIDS and drives immune dysfunction and progression to AIDS. This article draws extensively on work done in the nonhuman primate model of AIDS to fill gaps in our understanding of AIDS in humans.
Our understanding of the pathogenesis of acquired immunodeficiency syndrome (AIDS) has evolved dramatically since its initial discovery. Although originally believed to involve a period of viral latency, it is now clear that human immunodeficiency virus (HIV) replication occurs at a high level throughout infection. Progress in defining both molecular and cellular viral targets has also led to important discoveries that allow us to better understand the various stages of infection as well as the events leading to immunodeficiency. The cellular receptors for HIV and simian immunodeficiency virus (SIV) are the CD4 molecule on T cells and monocyte/macrophage lineage cells along with a chemokine receptor.1,2 Direct infection of CD4+ T cells leads to their destruction and to global immune deficiency, because these cells are required for induction and control of most immune responses. Infection of monocyte/macrophage lineage cells appears to be particularly important in chronic infection; these are likely major reservoirs for viral replication and persistence and might also contribute to immune deficiency.
In response to HIV or SIV infection, the host generally mounts a robust but clearly ineffectual adaptive immune response that includes both antibody and cellular responses.3,4 In most untreated patients, viral replication continues at high levels throughout infection, invariably leading to immune suppression and AIDS. However, a small percentage of infected individuals are able to control the infection and have undetectable levels of viremia, and they are therefore called long-term nonprogressors. It would be expected that such individuals would provide major clues as to how HIV infection can be controlled. However, no single answer has emerged. Furthermore, efforts to identify “correlates of immunity” in humans as well as nonhuman primate models of AIDS have yet to yield consistent, generally accepted results. Part of this difficulty may be due to the fact that the vast majority of work to understand the pathogenesis of AIDS has relied on blood samples.
Blood is easily accessible and provides a window into events happening throughout the body. However, it is increasingly clear that this window is at best hazy and does not adequately reflect events occurring in tissues, particularly in early phases of infection.5 Furthermore, the highly dynamic interplay involving the host immune response, attempts by the host to replenish cells that are destroyed, the virus, and viral evolution appear to differ in various tissue compartments.6,7
Biology of HIV/SIV
HIV and SIV are closely related lentiviruses that cause AIDS in their respective human and macaque hosts. In fact, it is now generally accepted that HIV evolved from multiple cross-species transmission events from African nonhuman primates (chimpanzees and sooty mangabeys) to humans.8-10 Similarly, SIV strains most commonly used to experimentally infect Asian macaques evolved from cross-species transmission from African nonhuman primates (sooty mangabeys).11 Like other lentiviral infections, clinical disease progression in both HIV-infected humans and SIV-infected macaques is slow despite generally robust viral replication. To infect CD4+ T cells and macrophages, HIV and SIV use 2 cellular receptors in combination: the CD4 molecule and a chemokine receptor (the 2 most common are CCR5 and CXCR4).2 The terms R5 and X4 are now used to distinguish virus strains that use CCR5 and CXCR4 as coreceptors, respectively, replacing older and somewhat misleading terminology of “macrophage tropic” and “T-cell tropic” viruses. The use of chemokine receptors in conjunction with CD4 is clearly involved in the profound immunosuppression induced by these viruses in susceptible hosts. Chemokine receptor usage is also a key factor in explaining the importance of the gastrointestinal immune system as a major target and potential reservoir of HIV/SIV.
In addition to chemokine receptor expression, the activation state of infected CD4+ lymphocytes has a significant impact on the ability of the virus to replicate successfully. As newly produced lymphocytes emerge from the thymus, they are generally considered naive in that they have never encountered their cognate antigen and are thus in a “resting” state. Naive resting cells are abundant in the blood and organized lymphoid tissues (lymph nodes, intestinal Peyer’s patches, and so on). Once the cell encounters its cognate antigen, it becomes activated and begins to produce cytokines to recruit or stimulate other cells and/or divides to produce daughter “memory” cells that also respond to this particular antigen. Cells that have previously encountered their antigen are considered memory cells, which can be distinguished by expression of specific cell surface antigens. In addition, memory cells can also be subdivided into short-lived effector memory cells, which actively secrete cytokines, and long-lived central memory cells, which may be resting or rapidly activated to mount immune responses against further exposures to the antigen. Central memory cells can be further subdivided into resting central memory (CD28+CCR7+) and transitional phenotypes (CD28+CCR7− and CD28−CCR7+),12 the latter of which may be more “activated” and supportive of viral replication.
Activated CD4+ T cells, identified in part by expression of CD25, CD69, HLA-DR, and so on, support HIV and SIV infection, whereas resting naive CD4+ T cells do not.13-15 This could partially be because resting naive CD4+ T cells generally do not express CCR5 and therefore are resistant to SIV/HIV infection. However, resting central memory cells, which express low levels of CCR5, have been shown to be significant targets for SIV in vivo.16 In addition, activated cells transcribe DNA, which logically would promote more viral replication. Moreover, intracellular factors have been identified that might be involved in the differential susceptibility of resting and activated cells to infection.17,18
Recent reports suggest that the preference of HIV/SIV for the mucosal immune system may be due, in part, to the use of mucosal homing molecules that target infection to mucosal lymphocytes or at least in transport of HIV to intestinal tissues. Studies by Arthos et al indicate that HIV is able to selectively bind the α4β7 integrin molecule on lymphocytes, which directs migration and homing of these cells to the intestine.19 Furthermore, although this binding does not appear to directly result in viral fusion and entry, it does apparently result in activation of the cell, which could lead to increased susceptibility of these and neighboring cells to infection. Importantly, α4β7 binding leads to increased activation and expression of lymphocyte function antigen 1 (another integrin consisting of a heterodimer of CD11a and CD18), which has an important role in formation of junctions between lymphocytes and thus could contribute to cell-to-cell transmission of HIV.19,20 Clearly, additional studies are needed to elucidate the role that these receptors and others have in the mucosal pathogenesis of HIV infection and AIDS.
Gastrointestinal Mucosa and AIDS
It is evident from studies of SIV-infected macaques and HIV-infected humans that mucosal tissues are not only primary sites of viral transmission but also the major sites for viral replication and CD4+ T-cell destruction, regardless of route of transmission. Understanding the basis of this central role of mucosal tissues in the pathogenesis of AIDS is critical for efforts to develop strategies to prevent or treat AIDS.
Normal gastrointestinal function requires balanced interactions among different organ systems (digestive, immune, nervous), multiple different cell types from each organ system, and the intestinal microbiome.5,21-23 Disruptions of sufficient magnitude in any of these components can have consequences that reach beyond the clinical manifestation of diarrhea. In the case of HIV and SIV infection, the initial target is the intestinal immune system. It is becoming clear, however, that this has a negative impact on intestinal structure and function that begins early in infection and might favor disease progression via microbial translocation and generalized immune activation.24-29 Selected major components of this tightly ordered sequence of events that are thrown off balance by the targeted destruction of the CD4+ T cells are discussed in more detail in the following text.
The Mucosal Immune System
The intestinal immune system is considered the largest single immunologic organ in the body, containing upward of 40% of all lymphocytes.30,31 When the rest of the mucosal immune system is included (from lungs, reproductive tract, urinary tract, mammary glands, and so on), the mucosal immune system dwarfs the systemic immune system. Furthermore, in contrast to the systemic immune system, most of the CD4+ T cells in the mucosal immune system are CCR5+, activated memory CD4+ T cells. This is of enormous importance with respect to the pathogenesis of AIDS because these are the preferred cellular targets for HIV/SIV infection.32-34 This is also reflected in the fact that productive infection of peripheral CD4+ T cells is rare (0.01% to 1%),35 whereas infection of mucosal CD4+ T cells is quite common; it is estimated that 60% of mucosal memory CD4+ T cells become infected within days of infection at the time of peak viremia.36
The unique challenges faced by the mucosal tissues and the immune system have resulted in a structurally and functionally distinct mucosal immune system. In the case of the intestine, this system includes inductive organized lymphoid tissues and diffuse effector lymphoid tissues (Figure 1). The inductive sites in the intestine and most other mucosal sites include widely scattered but well-organized lymphoid follicles, best exemplified by solitary and aggregated (Peyer’s patches) lymphoid follicles. In general, these are found in the tonsils and the terminal portion of the small intestine (particularly the ileum) as well as the terminal portion of the large intestine (rectum), cecum, and appendix.
Figure 1.
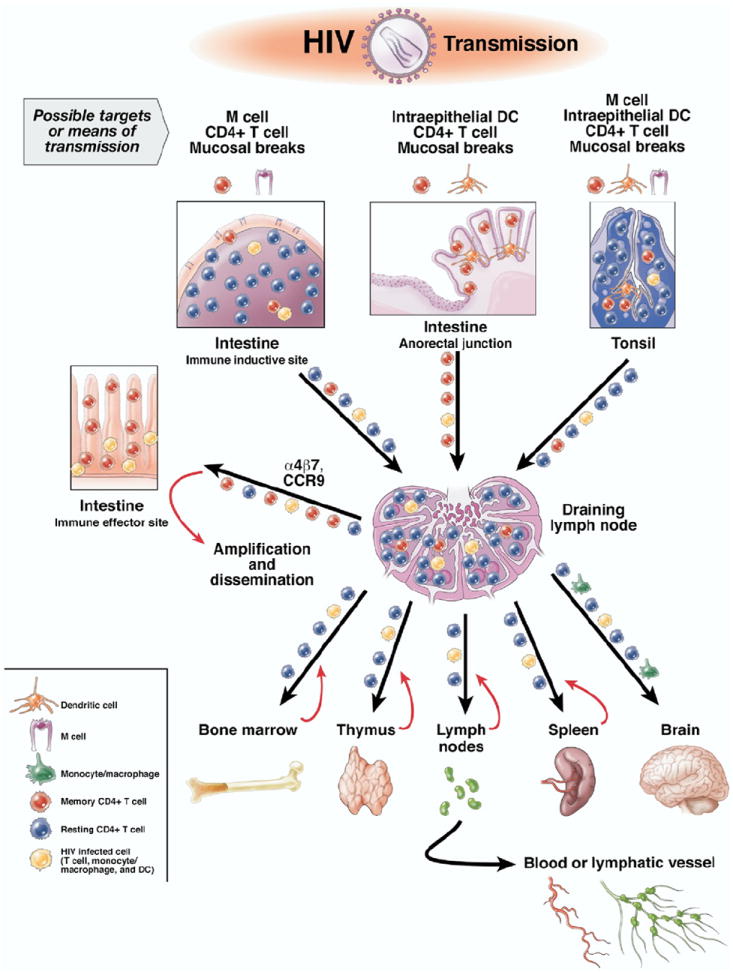
Most infections with HIV occur via mucosal surfaces. In the gastrointestinal tract, this involves the anorectal mucosa and possibly tonsil. In addition to mucosal breaks that facilitate transmission, there are several other cellular targets for mucosal transmission. This includes M cells in intestine and tonsil associated with immune inductive sites and intraepithelial DCs, particularly in the anorectal mucosa. Once the virus crosses the mucosa, it can encounter numerous CD4+ cells, but most T cells in immune inductive sites are resting and are not likely to support infection. In contrast, a high percentage of memory CD4+ T cells that express CCR5 are present in mucosal immune effector sites, which serve as a major site for amplification of the initial infection. Infected cells and virus reach these sites by taking advantage of normal trafficking patterns facilitated by molecules such as α4β7 and CCR9. Regional draining lymph nodes serve as a nexus where infected mucosal homing lymphocytes encounter systemic lymphocytes and disseminate the infection throughout the body.
In addition to the organized lymphoid tissues of the inductive arm of the mucosal immune system, there is an even larger pool of immune cells diffusely scattered throughout mucosal tissues that serves as the “effector” arm of the mucosal immune system.37 The effector arm consists of large numbers of various subsets of lymphocytes, macrophages, dendritic cells (DCs), and other immune cells that are scattered diffusely throughout the lamina propria and epithelium of mucosal tissues.37 These cells are responsible for performing the major effector functions of the intestinal immune response that are initiated in inductive sites.
Immunophenotypic Composition of the Mucosal Immune System
Mucosal tissues contain the majority of all the lymphocytes and macrophages in the body. From an anatomic perspective, the lymphocyte populations can be divided into those present in epithelium (intraepithelial lymphocytes) and those in the underlying lamina propria (lamina propria lymphocytes). The lamina propria lymphocytes can be further subdivided into those from inductive sites (organized lymphoid nodules) and effector sites (diffuse lamina propria). More than 90% of the intraepithelial lymphocytes are CD3+ T cells, approximately 80% of which express CD8.37,38 In addition, approximately 10% of intraepithelial lymphocytes express the γδ T-cell receptor.39 In contrast, the lamina propria contains almost all of the various lymphoid phenotypes,37 including a vast reservoir of CD4+ T cells. In normal humans and nonhuman primates, the ratio of CD4+ to CD8+ T cells in the lamina propria is similar to that of the peripheral blood and lymph nodes.37,38,40 However, in contrast to peripheral lymphoid tissues, a much larger percentage of mucosal CD4+ T cells express CCR5, have a memory phenotype, and express markers of cell activation, particularly if lamina propria lymphocytes from the diffuse lamina propria are examined separately from organized lymphoid nodules.5,31,37,38,41,42 Furthermore, a large percentage of CD4+ T cells also produce cytokines in situ, indicating that they are activated, terminally differentiated effector cells.37
Combined, these data indicate that the largest pool of activated, terminally differentiated, memory CCR5+CD4+ T cells resides in mucosal tissues (particularly the diffuse lamina propria) and not in peripheral blood or lymph nodes. HIV and SIV preferentially infect these memory CCR5+CD4+ T cells in immune effector sites (diffuse lamina propria), causing rapid depletion of these cells by 21 days after infection. Subsequently, most infected cells in the intestine are present in immune inductive sites, represented by organized lymphoid nodules in the lamina propria.5,43,44 This dramatic and rapid loss of CD4+ T cells in mucosal effector sites in SIV-infected macaques is associated with subclinical opportunistic infections as well as significant alterations in intestinal structure and function.26,27,45
Of additional importance is a subset of CD4+ T cells known as Th17 cells because they produce interleukin (IL)-17 and IL-22 but not interferon gamma or IL-4.46 Of particular relevance for this discussion is the role these cells are likely to have in enterocyte homeostasis and production of antimicrobial defensins, both of which are critical for maintenance of the mucosal barrier.47,48 Recent evidence indicates that Th17 cells are even more profoundly depleted than CD4+CCR5+ T cells in the intestinal mucosa of HIV- and SIV-infected individuals.28 The loss of Th17 cells provides a possible direct link between CD4+ T-cell destruction and dysfunction of the intestinal mucosa.
Interactions Between the Mucosal Immune System and Intestinal Structure and Function
Alterations in intestinal structure and function associated with HIV/SIV infection have long been recognized.27,49-52 Histologically, villus atrophy and increased epithelial apoptosis in the villus tips were often linked to increased proliferation of crypt cells, leading to crypt hyperplasia. This lesion of “crypt hyperplastic villous atrophy” had been associated with mucosal T-cell activation in vitro.53,54 However, in the case of AIDS, the dominant feature was one of immune suppression rather than activation. Thus, it was not clear how the two were related. Over time, however, it has become clear that immune activation is a major feature of SIV and HIV infection and there is increasing evidence that intestinal immune dysfunction can result in structural changes to the intestinal mucosa and cause breakdown of the intestinal epithelial barrier.47,48,55-57 The molecular basis for damage to the intestinal epithelial barrier is now coming into focus, aided by functional genomic approaches and their frequent application to studies of the pathogenesis of AIDS.
Normal function of the mucosal barrier requires not only an intact epithelium joined by tight junctions, but also coordinated function of multiple cell types that occupy distinct anatomic positions and maintain reciprocal interrelationships.58 The sudden and massive destruction of activated effector memory CD4+, CCR5+ cells and Th17 cells would be expected to disrupt this communication network linking epithelial cells and the intestinal immune system, which is believed to operate primarily via diffusible molecules such as cytokines, growth factors, locally derived hormones, and products of arachidonic acid metabolism.59 A consequence of this disruption is likely deprivation of epitheliotrophic factors required for epithelial cell growth, maintenance, and renewal, leading to increased epithelial cell apoptosis and death (Figure 2). In support of this concept, significant down-modulation of genes regulating intestinal epithelial cell growth and renewal, along with increased expression of inflammation and immune activation genes and activated caspase-3 protein expression in epithelial cells, has been observed in primary HIV infection.60-62 Additionally, increases in proinflammatory cytokine production in the colon as early as 6–10 days following SIV infection63 and in the intestine of HIV-infected patients64-66 could further facilitate mucosal damage by activating myosin light chain kinase (MLCK)67 (Figure 3). MLCK has been implicated in initiation of damage to the intestinal epithelial barrier in other gastrointestinal diseases such as inflammatory bowel disease.68 MLCK selectively phosphorylates myosin II regulatory light chains, which is followed by perijunctional actin-myosin contraction and redistribution of tight junction proteins zonula occludens 1 (ZO-1) and occludin, leading to alterations in integrity of the mucosal barrier69 (Figure 3). Although the role of MLCK has not been directly examined in AIDS, a study of HIV-infected patients showed cytoskeletal changes in intestinal epithelial cells from the colon and jejunum, which would be expected as a result of MLCK activation.70
Figure 2.
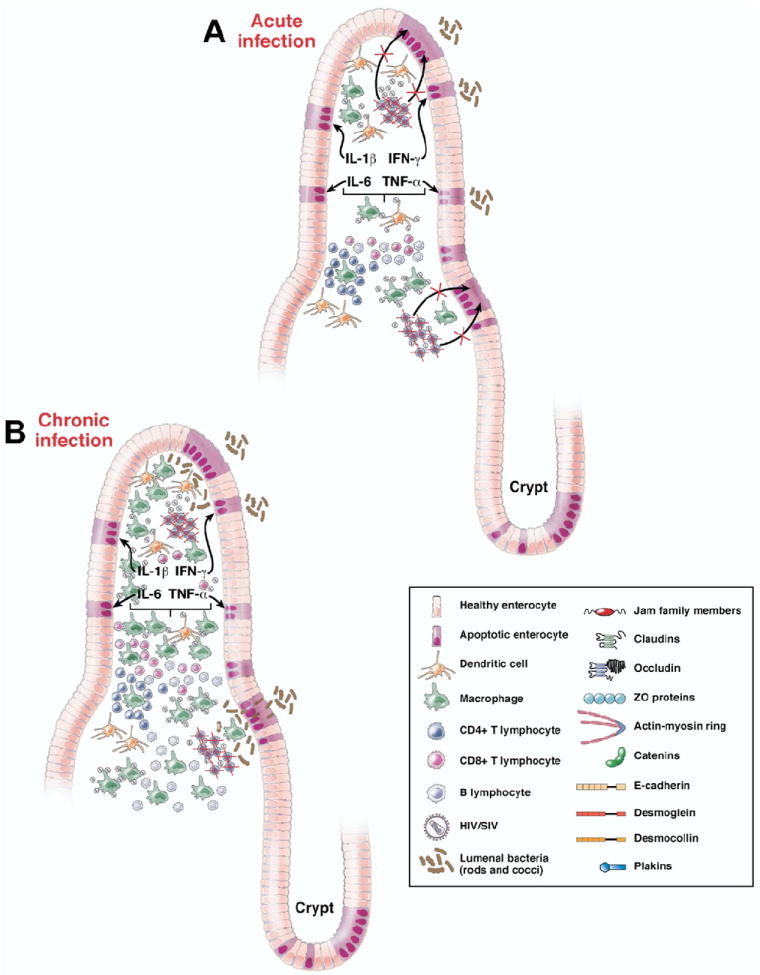
Schematic representation of pathological changes underlying intestinal epithelial barrier disruption during HIV/SIV infection. (A) Acute HIV/SIV infection depletes the mucosa of CD4+CCR5 effector memory T cells and Th17 cells, leading to disruption of the bidirectional communication that links the intestinal epithelium with the mucosal immune system necessary for the growth, maintenance, and renewal of epithelial cells and leading to increased epithelial cell apoptosis. (B) During chronic HIV/SIV infection, in addition to the continual destruction of CD4+CCR5 effector memory T cells and Th17 cells there is increased infiltration of inflammatory cells into the lamina propria leading to more proinflammatory cytokine production and widespread distribution of the intestinal epithelial barrier facilitating translocation of lumenal bacteria.
Figure 3.
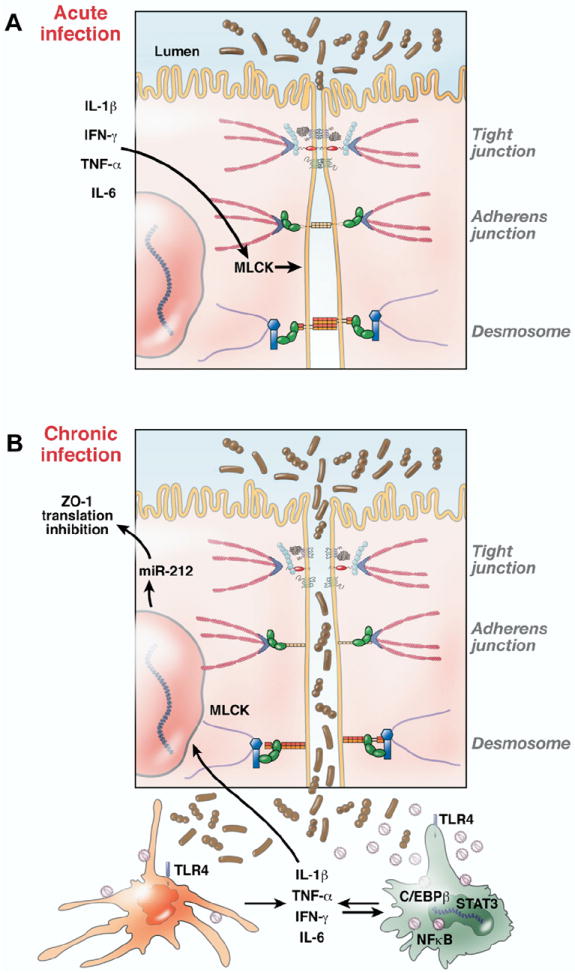
Schematic representation of possible molecular mechanisms underlying intestinal epithelial barrier disruption during HIV/SIV infection. (A) Normal intestinal mucosal barrier integrity is maintained by junctional complexes between intestinal epithelial cells (tight junctions, desmosomes, etc). Proinflammatory cytokines (eg, tumor necrosis factor α, IL-1β, interferon gamma) produced by DCs and macrophages in response to local viral replication can also exacerbate epithelial injury by activating factors such as MLCK, which can alter the structure and integrity of tight junctions. (B) As infection progresses, loss of epithelial integrity leads to increased translocation of luminal bacteria and bacterial products that in turn locally activate innate immune cells (eg, DCs and macrophages) to produce more proinflammatory cytokines, thus creating a localized inflammatory environment. Proinflammatory cytokines also activate a variety of immune cells, including CD4+ T cells, which increase the ability of these cells to support viral replication. The underlying molecular mechanism of macrophage activation is partly evident from the constitutive expression of STAT3 and C/EBPβ, proinflammatory transcription factors activated by IL-6 and interferon gamma. Whereas STAT3 is antiapoptotic, C/EBPβ can transactivate the HIV/SIV LTR, resulting in persistent inflammation, immune activation, and increased virus production. Select micro-RNAs such as miR-212, which is up-regulated in the intestine during inflammation, can also contribute to epithelial barrier breakdown by down-regulating the expression of the tight junction protein ZO-1.
Whatever the mechanism(s), altered intestinal epithelial permeability permits entry of bacteria and their products (eg, lipopolysaccharides), some of which bind to TLR4 receptors expressed on immune cells such as DCs and macrophages. This results in their activation, leading to localized cytokine production and activation of other immune cells such as CD4+ T cells that can also serve as substrates for HIV/SIV. In support of this concept, recent work examining IL-6 signal transduction in the gastrointestinal tract of rhesus macaques chronically infected with SIV revealed constitutive activation of the signal transducer and activator of transcription 3 (STAT3) pathway, which was particularly evident in macrophages71 (Figure 3). STAT3 has been shown to activate antiapoptotic genes such as Bcl-Xl72 in intestinal immune cells; in doing so, it might allow macrophages to survive longer and at the same time maintain an inflammatory milieu by producing more proinflammatory cytokines, which in turn can constantly activate MLCK to cause barrier disruption (Figures 2 and 3). It is also interesting to note emerging links between the STAT3 pathway and Th17 cells which, as noted previously, have a role in enterocyte homeostasis.73 Apart from STAT3, intestinal macrophages also express high levels of CCAAT/enhancer binding protein β (C/EBPβ), a proviral and proinflammatory transcription factor activated by IL-6 and interferon gamma that can, in alliance with nuclear factor κB, transactivate the HIV/SIV long terminal repeat (LTR) and thus maintain high-level viral replication. This provides a mechanistic link between inflammation and activation of viral replication in vivo74 (Figures 2 and 3).
MicroRNAs, which also regulate gene expression, have potential roles in the pathogenesis of AIDS-associated intestinal epithelial barrier disruption.75 It was recently shown that miR-212 expression is increased ~32-fold in the intestinal mucosa of patients with alcoholic liver disease and that this microRNA can down-regulate ZO-1 protein expression76; ZO-1 is a major component of epithelial tight junctions, and its loss adversely affects the integrity of the mucosal barrier (Figure 3).
Transmission of HIV/SIV
Although transmission of HIV occurs via contaminated blood products and intravenous drug abuse during reuse of contaminated needles, the dominant means of HIV transmission worldwide is sexual transmission via mucosal surfaces. While this clearly includes vaginal transmission associated with heterosexual intercourse and mother-to-child transmission during birth, the focus of this review is the mucosa of the gastrointestinal tract. The primary site of transmission in the gastrointestinal tract is the anorectal mucosa and possibly the oral mucosa.
Transmission of HIV/SIV at mucosal surfaces can occur through several means (Figure 1). It is clear that breaks in the mucosa can facilitate HIV/SIV transmission by allowing the virus direct access to target cells and/or the vascular and lymphatic systems. However, nonhuman primate studies have also shown that viral transmission can occur across an intact mucosa and that physical factors, such as the thickness of the epithelium, can have a dramatic impact on the efficiency of transmission.77
The primary target cell for viral transmission via mucosal sites varies, depending on the tissue. With regard to the anorectal mucosa, the anus is lined by several layers of stratified squamous epithelium-containing intraepithelial DCs that can facilitate transmission of the virus across the epithelium.78,79 Although the mechanisms by which DCs facilitate transmission are currently debated, they appear to be independent of CD4, CCR5, or DC-SIGN, because intraepithelial DCs do not express these receptors. Interestingly, antibodies or compounds that block only CD4 or CCR5 are sufficient to completely block transmission of SIV to macaques via the vaginal mucosa, which has a similar anatomy to the anorectal mucosa.80,81 The rectal mucosa is also considered a major site of HIV transmission. In contrast to the anus, the rectum and colon are lined by a single layer of columnar epithelial cells. Intraepithelial DCs are not present in the rectal mucosa. However, subepithelial (lamina propria) DCs are present just beneath the rectal epithelium, and many of these and other cells in the lamina propria do express DC-SIGN, CD4, and CCR5.82-84
Whereas the rectum and most of the rest of the intestinal tract lack intraepithelial DCs, they have specialized epithelial cells known as microfold cells, or simply M cells, over organized lymphoid nodules that sample intestinal contents and present antigen to underlying lymphocytes and macrophages. A variety of pathogens take advantage of M cells to gain entry into the body.85 Although there are no definitive data from primates to indicate that HIV/SIV uses this pathway, there are data from other animal models that suggest that HIV might utilize the M cell to cross the mucosa.86
The oral mucosa does not appear to be a major route of sexual transmission; however, it could be a major route of mother-to-child transmission via breast milk. The palatine tonsil is fairly unique among mucosal tissues in that it has M cells, intraepithelial DCs, crypts, and natural breaks in the mucosa designed to expose underlying lymphocytes and macrophages directly to the oral microenvironment. Thus, the tonsil provides multiple possible routes for the virus to encounter T cells and macrophages.
Whereas HIV/SIV can clearly cross intact mucosal surfaces, the intact mucosa does represent a selective barrier in contrast to intravenous transmission.87 In studies examining mucosal transmission, it is clear that only a very small subset of the quasi-species within a host are transmitted.88,89 In fact, recent studies indicate that in early HIV infection, usually only 1 or 2 viruses are successfully transmitted across the mucosa,90 despite the fact that viral “swarms” are usually present in infected semen or body fluids. Some of this is probably related to the type of viruses that are shed and thus available for mucosal transmission, and some is related to the cellular receptors on the target cells. For example, despite the presence of both R5 and X4 HIV strains in approximately 50% of chronically infected patients, R5 viruses are clearly dominant in primary mucosal transmission. Although the underlying mechanism(s) for the selective transmission of R5 viruses across mucosal surfaces is unknown, it is likely related to the fact that mucosal tissues contain large numbers of optimal viral target cells (activated memory CD4+CCR5+ T cells), which could serve as initial targets for transmission and/or for viral amplification and dissemination of virus to systemic lymphoid tissues.
Early Targets of Infection, Amplification, and Viral Dissemination
HIV/SIV can undergo limited replication within DCs in mucosal surfaces that contain them78,91 (anus and tonsil [lined by stratified squamous epithelium]); however, the primary substrates for HIV/SIV replication are memory CD4+ T cells that express CCR5 (hereafter referred to as “primary target cells”). How the virus reaches these cells, which are abundant in the lamina propria of all mucosal tissues (Figure 1), varies depending on the route and site of transmission.
In the case of the rectal mucosa, once the virus crosses the epithelium either via small mucosal breaks or M cells, the virus will encounter a high density of primary target cells to support significant levels of viral replication (amplification). It is worth noting that, in the case of M cells, they form an intraepithelial pocket containing CD4+ memory T cells and DCs in close proximity, which would greatly facilitate HIV/SIV replication.85,92 After local replication and amplification, it is likely that virus and virus-infected cells migrate to draining lymph nodes and then on to the rest of the body.
Few studies have focused on transmission of HIV/SIV from the anal mucosa, but data obtained from studies of vaginal transmission are likely instructive because of the similar structure of the tissues. In this setting, intraepithelial DCs appear to have a major role. Although there are significant numbers of primary target cells in the lamina propria,40 there are also data that indicate that DCs can rapidly carry virus to regional lymph nodes.78 In this case, it appears that spread to regional lymph nodes occurs before there is significant local replication of virus in the lamina propria. This is likely because the virus subverts normal trafficking patterns of intraepithelial DCs that bring antigen to immune inductive sties (regional lymph nodes).
In contrast to mucosal transmission, which provides a selective barrier, based on the ability of the virus to contact target cells either directly or by utilizing existing biological processes, intravenous transmission poses no such barrier. Thus, the virus quickly disseminates to all tissues, including those that support high levels of viral replication (mucosal tissues). Whereas virus is readily found in tissues by 14 days after infection, it is difficult to find infected cells in tissues by in situ hybridization or immunohistochemistry before that time, except in effector sites in mucosal lymphoid tissues such as the lamina propria of the intestinal tract, where significant numbers of productively infected cells have been detected within 3 to 4 days of intravenous inoculation.93 Combined, these data suggest that replication in mucosal tissues is not only important for transmission but also critical for initial viral replication and amplification, regardless of the route of transmission.
Nonprogressive SIV Infection in African Nonhuman Primates
In addition to pathogenic infections in humans and Asian macaques, primate lentiviral infections can also lead to a lack of (or at least extremely slow) disease progression despite persistent viral replication, as occurs in the natural African nonhuman primate hosts of SIV, or transient SIV replication and clearance upon cross-species transmission in partially permissive hosts (reviewed by Pandrea et al94). The effects of SIV infection on the intestinal mucosa have been examined in 2 natural hosts of SIV: African green moneys (Chlorocebus sabaeus) and the sooty mangabey (Cercocebus atys).95-97 These studies have shown a profound and rapid loss of intestinal CD4+CCR5+ T cells virtually indistinguishable from what has been described in HIV-infected humans and SIV-infected macaques. Yet, despite this rapid and profound loss of the primary target cells and persistent viral replication, this cell population was restored and progression to disease was averted.94-97 A similar outcome was observed in SIVagm-infected rhesus macaques, which were able to completely control the infection.95 One conclusion of these studies was that while acute loss of mucosal CD4+CCR5+ T cells might be necessary for disease, it is not sufficient for disease progression and other factors are important. The most likely additional factor responsible for driving disease progression identified so far is immune activation. In SIV-infected African nonhuman primates, the level of immune activation remains near baseline whereas the immune system is persistently activated in HIV-infected humans and SIV-infected macaques. As noted previously, a major driver of this persistent immune activation is believed to be microbial translocation, associated with a leaky gut.24 Increased levels of microbial products such as bacterial lipopolysaccharide were notably absent from SIV-infected African nonhuman primates. Furthermore, experimental immune activation in SIV-infected African nonhuman primates by intravenous administration of lipopolysaccharide has been shown to cause significant but transient increases in viral replication and CD4+ T-cell depletion in these animals.98 These data suggest that the key determinant of whether a primate lentivirus infection is progressive (leading to AIDS) or nonprogressive is the induction of persistent immune activation. Moreover, the main driver of this immune activation appears to be a leaky intestinal mucosal barrier, although a causal relationship remains to be established. Thus, understanding the mechanisms whereby the mucosal barrier is compromised during HIV and SIV infection may be critical for modifying the outcome of infection.
Antiretroviral Therapy and the Intestinal Tract
The potential for antiretroviral therapy (ART) to restore mucosal CD4+ T cells has only begun to be examined and has been particularly difficult to assess in acute infection. Small studies in humans and SIV-infected macaques have suggested near-complete restoration of mucosal CD4+ T cells when treatment is initiated early.99,100 In contrast, other studies have not shown a significant restoration of mucosal CD4+ T cells either early or late in infection.101-103 In summary, although CD4+ T cells in the peripheral blood have been reported to fully reconstitute in patients on ART, there is considerable controversy regarding the capacity for patients to restore intestinal CD4+ T cells, particularly when they are treated in the early stages of infection. This controversy is in part a result of how “early” is defined. Clearly, in macaque studies, if ART is started early enough (within 42 days), restoration of mucosal CD4+ T cells can occur. However, in humans, acutely infected patients are seldom started on ART because treatment guidelines generally recommend waiting to initiate therapy until blood CD4+ T-cell counts have decreased to reduce the risk of drug side effects as well as the potential for selecting for drug-resistant strains of HIV. Furthermore, even in situations in which ART has resulted in undetectable levels of viral replication in peripheral blood, virus replication continues in the intestinal mucosa.101-103 These data suggest that ongoing viral replication and CD4+ T-cell destruction occur in the intestine of patients on ART, despite what appears to be complete suppression of viral replication in the blood. Thus, the major challenge for antiretroviral control of HIV infection appears to be in mucosal tissues, particularly the intestine.
Early Immune Response
As outlined previously, HIV and SIV subvert the immune system by attacking and eliminating the very cells responsible for initiating immune responses. Activated memory CD4+ T cells provide essential “help” for cell-mediated (cytotoxic T lymphocytes [CTLs]) and humoral (B-cell) immune responses, particularly upon primary antigen exposure. It is now clear that both HIV and SIV selectively infect and destroy memory CD4+ T cells (both central and effector cells), resulting in subsequent impairment of immune responses to not only the infecting virus but other antigens as well. This tropism for memory CD4+ T cells eventually leads to profound immunodeficiency and likely underlies the fact that effective immunity and clearance of the infection have yet to be documented in an HIV-infected patient. This rapid and profound elimination of memory CD4+ T cells in the host undoubtedly affects the immune system from the onset of infection; understanding these consequences is confounded at least in part by the compartmentalization, dynamics, and resilience of the immune system, especially in mucosal tissues.
Acute SIV infection elicits early and relatively robust immune responses in SIV-infected macaques. Within 1– 4 weeks of SIV infection, marked increases in CD8+ (5- to 10-fold) and NK cell (2- to 3-fold) proliferation are observed in the blood.104 Interestingly, most of this proliferation appears to be nonspecific, because few of the responding CD8+ T cells are specific for SIV antigens during peak viremia.105 Similarly, few of the CD8+ T cells and even fewer CD4+ T cells are virus specific in HIV-infected patients.106
Mucosal tissues are also major sites for generation of virus-specific immune responses. Using tetramer technology in genetically defined macaques, strong virus-specific CTL responses have been detected in mucosal sites within 14–21 days of infection.105,107 In both intravenously and rectally inoculated macaques, virus-specific CTLs appear to emerge simultaneously in blood and intestines, although the percentages of mucosal CTLs often exceed those in the blood in both early and chronic infection.105,108,109 Interestingly, few virus-specific CTLs were detected in the gut of vaginally inoculated animals using similar (tetramer) techniques,107 which could reflect differences in CTL development or homing, depending on the route of transmission, but this observation remains to be fully explored. In addition to cell-mediated immune responses, infection with SIV or HIV also generates diverse antibody responses, although some strains of SIV are poor at eliciting neutralizing antibody production. Regardless, neither robust cellular nor humoral immune responses are sufficient to clear the infection, and correlates of immunity to SIV and HIV remain to be determined.
Although there is consensus that early infection with SIV and HIV results in robust early immune responses, it is also apparent that the magnitude as well as quality of the immune response diminishes with time. CTL-mediated killing is more rapid in early versus chronic HIV infection.110 Moreover, CD4+ immune responses to tetanus toxin and hepatitis C virus (in coinfected patients) also decline as the disease progresses.111 In addition, SIV studies using tetramer technology have shown that the levels of SIV-specific CTLs diminish with time.105 Thus, the development of AIDS seems to be a gradual process despite the fact that most of the memory CD4+ T cells are eliminated within days of infection and never fully restored, at least in animals that progress to AIDS. However, in the majority of animals, sustained increases in CD4+ T-cell turnover throughout SIV infection usually result in maintenance of “threshold levels” of mucosal CD4+ T cells (5%–10% of normal values), which seem sufficient to maintain immune function, although subclinical opportunistic infections are frequently found in macaques within weeks of infection.45 Therefore, the ongoing destruction of memory CD4+ T cells is likely balanced by continuous proliferation of these cells in attempts to maintain this threshold. Evidence for this comes from studies showing that macaques that fail to maintain proliferation of memory CD4+ T cells rapidly progress to AIDS.112
Conclusions
The gastrointestinal tract has long been recognized as an important player in HIV transmission; it is becoming clear that it also is a key component of the pathogenesis of AIDS rather than just a passive victim of a failing immune system. In fact, it is probably useful to think of AIDS as a mucosal disease. In this context, several critical questions about the transmission of HIV, the pathogenesis of AIDS, and development of a vaccine arise and are summarized in the following text.
Although the anorectal and possibly oral mucosa are sites of HIV transmission, the determinants of transmission are not well defined. A “short list” of targets has been described (Figure 1), but it is not clear how often these are used at each of the potential sites of mucosal transmission. Further, the viral determinants that could favor transmission and the implications of the establishment of infection by a limited number of founder viruses are not well understood.
HIV and SIV preferentially infect activated memory CD4+ T cells that express CCR5; most of the T cells of this phenotype reside in the intestine and other mucosal sites. The recognition that progressive HIV and SIV infection is linked to immune activation, which in turn is linked to a leaky gut, has only recently focused intense interest on the effects of HIV and SIV infection on the intestinal epithelial barrier. The details of how infection and loss of intestinal CD4+ T cells lead to this leaky gut are unclear, but multiple avenues of investigation have begun to be explored, including the role of Th17 cells and regulation of the integrity of epithelial tight junctions (Figure 3). As the steps linking HIV infection to breakdown of the intestinal barrier become clear, therapeutic targets will hopefully be found. If it were possible to prevent or decrease the breakdown of the mucosal barrier through therapeutic means, this could greatly slow AIDS disease progression; natural nonhuman primate hosts of SIV that are persistently infected experience acute loss of intestinal CD4+ T cells but apparently do not have a leaky gut or chronic immune activation and rarely progress to AIDS.
As we recognize that the primary targets of HIV and SIV infection are in mucosal sites (such as the intestine), we realize that we do not have a clear understanding of how to induce mucosal immune responses in these sites. Is local immunization required? How can we generate mucosal effector memory T-cell responses? What role do mucosal antibodies have in preventing HIV transmission? These and similar, seemingly basic, questions about mucosal immunology need to be answered if we are to develop an effective vaccine for AIDS that protects the key target cells in the mucosa. Until an effective vaccine can be developed, continued prevention efforts through education, condom use, and microbicide development remain the most promising approach to slowing the spread of HIV infection.
Acknowledgments
Funding
Supported in part by Public Health Service grant RR000164.
Abbreviations used in this paper
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- C/EBPβ
CCAAT/enhancer binding protein β
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- HIV
human immunodeficiency virus
- IL
interleukin
- MLCK
myosin light chain kinase
- SIV
simian immunodeficiency virus
- STAT3
signal transducer and activator of transcription 3
- ZO-1
zonula occludens 1
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Moore JP, Kitchen SG, Pugach P, et al. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- 3.Koff WC, Johnson PR, Watkins DI, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 4.Picker LJ, Watkins DI. HIV pathogenesis: the first cut is the deepest. Nat Immunol. 2005;6:430–432. doi: 10.1038/ni0505-430. [DOI] [PubMed] [Google Scholar]
- 5.Veazey RS, DeMaria M, Chalifoux LV, et al. The gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 6.Ryzhova EV, Crino P, Shawyer L, et al. Simian immunodeficiency virus encephalitis: analysis of envelope sequences from individual brain multinucleated giant cells and tissue samples. Virology. 2002;297:57–67. doi: 10.1006/viro.2002.1395. [DOI] [PubMed] [Google Scholar]
- 7.Horton H, Vogel T, O’Connor D, et al. Analysis of the immune response and viral evolution during the acute phase of SIV infection. Vaccine. 2002;20:1927–1932. doi: 10.1016/s0264-410x(02)00069-5. [DOI] [PubMed] [Google Scholar]
- 8.Marx PA, Li Y, Lerche NW, et al. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a West African pet sooty mangabey. J Virol. 1991;65:4480–4485. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao F, Bailes E, Robertson DL, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 10.Gao F, Yue L, White AT, et al. Human infection by genetically diverse SIVSM-related HIV-2 in west Africa. Nature. 1992;358:495–499. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- 11.Apetrei C, Kaur A, Lerche NW, et al. Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J Virol. 2005;79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picker LJ, Reed-Inderbitzin EF, Hagen SI, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zack JA, Arrigo SJ, Weitsman SR, et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson M, Stanwick TL, Dempsey MP, et al. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–60. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou CS, Ramilo O, Vitetta ES. Highly purified CD25- resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci U S A. 1997;94:1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 17.Chiu YL, Soros VB, Kreisberg JF, et al. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 18.Robichaud GA, Barbeau B, Fortin JF, et al. Nuclear factor of activated T cells is a driving force for preferential productive HIV-1 infection of CD45RO-expressing CD4+ T cells. J Biol Chem. 2002;277:23733–23741. doi: 10.1074/jbc.M201563200. [DOI] [PubMed] [Google Scholar]
- 19.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RP. How HIV guts the immune system. N Engl J Med. 2008;358:2287–2289. doi: 10.1056/NEJMcibr0802134. [DOI] [PubMed] [Google Scholar]
- 21.McKenna P, Hoffmann C, Minkah N, et al. The macaque gut microbiome in health, lentiviral infection and chronic enterocolitis. PLoS Pathog. 2008;4:e20. doi: 10.1371/journal.ppat.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orandle MS, Veazey RS, Lackner AA. Enteric ganglionitis in rhesus macaques infected with simian immunodeficiency virus. J Virol. 2007;81:6265–6275. doi: 10.1128/JVI.02671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batman PA, Miller AR, Sedgwick PM, et al. Autonomic denervation in jejunal mucosa of homosexual men infected with HIV. AIDS. 1991;5:1247–1252. doi: 10.1097/00002030-199110000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 25.Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:114–117. [PubMed] [Google Scholar]
- 26.Stone JD, Heise CC, Miller CJ, et al. Development of malabsorption and nutritional complications in simian immunodeficiency virus-infected rhesus macaques. AIDS. 1994;8:1245–1256. doi: 10.1097/00002030-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Heise C, Miller CJ, Lackner A, et al. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 28.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpstone D, Neild P, Crane R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45:70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald TT, Spencer J. Lymphoid cells and tissues of the gastrointestinal tract. In: Heatley RH, editor. Gastrointestinal and hepatic immunology. Cambridge: Cambridge University Press; 1994. pp. 1–23. [Google Scholar]
- 31.Schieferdecker HL, Ullrich R, Hirseland H, et al. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J Immunol. 1992;149:2816–2822. [PubMed] [Google Scholar]
- 32.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veazey RS, Marx PA, Lackner AA. Importance of the state of activation and/or differentiation of CD4(+) T cells in AIDS pathogenesis. Trends Immunol. 2002;23:129. doi: 10.1016/s1471-4906(01)02171-8. [DOI] [PubMed] [Google Scholar]
- 35.Brenchley JM, Hill BJ, Ambrozak DR, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattapallil JJ, Douek DC, Hill B, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 37.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 38.Veazey RS, Rosenzweig M, Shvetz DE, et al. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 39.Viney J, MacDonald TT, Spencer J. Gamma/delta T cells in the gut epithelium. Gut. 1990;31:841–844. doi: 10.1136/gut.31.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. 2003;187:769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 41.James SP, Graeff AS, Zeitz M. Predominance of helper-inducer T cells in mesenteric lymph nodes and intestinal lamina propria of normal nonhuman primates. Cell Immunol. 1987;107:372–383. doi: 10.1016/0008-8749(87)90245-0. [DOI] [PubMed] [Google Scholar]
- 42.Zeitz M, Greene WC, Peffer NJ, et al. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988;94:647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]
- 43.Veazey RS, Marx PA, Lackner AA. The mucosal immune system: primary target for HIV infection and fundamental component of AIDS pathogenesis. Trends Immunol. 2001;22:626–633. doi: 10.1016/s1471-4906(01)02039-7. [DOI] [PubMed] [Google Scholar]
- 44.Veazey RS, Tham IC, Mansfield KG, et al. Identifying the target cell in primary SIV infection; highly activated “memory” CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lackner AA, Vogel P, Ramos RA, et al. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994;145:428–439. [PMC free article] [PubMed] [Google Scholar]
- 46.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 47.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Heise C, Vogel P, Miller CJ, et al. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques: functional, pathological and morphological changes. Am J Pathol. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 50.Batman PA, Miller AR, Forster SM, et al. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. J Clin Pathol. 1989;42:275–281. doi: 10.1136/jcp.42.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cummins AG, LaBrooy JT, Stanley DP, et al. Quantitative histological study of enteropathy associated with HIV infection. Gut. 1990;31:317–321. doi: 10.1136/gut.31.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ullrich R, Zeitz M, Heise W, et al. Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med. 1989;111:15–21. doi: 10.7326/0003-4819-111-1-15. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira RC, Forsyth LE, Richman PI, et al. Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T-cell-mediated response in human small intestine. Gastroenterology. 1990;98:1255–1263. doi: 10.1016/0016-5085(90)90342-x. [DOI] [PubMed] [Google Scholar]
- 54.MacDonald TT, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988;167:1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDonald TT, Spencer J. Cell-mediated immune injury in the intestine. Gastroenterol Clin North Am. 1992;21:367–386. [PubMed] [Google Scholar]
- 56.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 58.Traber PG. Epithelial cell growth and differentiation. V. Transcriptional regulation, development, and neoplasia of the intestinal epithelium. Am J Physiol. 1997;273:G979–G981. doi: 10.1152/ajpgi.1997.273.5.G979. [DOI] [PubMed] [Google Scholar]
- 59.Shanahan F. Intestinal lymphoepithelial communication. Adv Exp Med Biol. 1999;473:1–9. doi: 10.1007/978-1-4615-4143-1_1. [DOI] [PubMed] [Google Scholar]
- 60.George MD, Wehkamp J, Kays RJ, et al. In vivo gene expression profiling of human intestinal epithelial cells: analysis by laser microdissection of formalin fixed tissues. BMC Genomics. 2008;9:209. doi: 10.1186/1471-2164-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sankaran S, George MD, Reay E, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sankaran S, Guadalupe M, Reay E, et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A. 2005;102:9860–9865. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abel K, Rocke DM, Chohan B, et al. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reka S, Garro ML, Kotler DP. Variation in the expression of human immunodeficiency virus RNA and cytokine mRNA in rectal mucosa during the progression of infection. Lymphokine Cytokine Res. 1994;13:391–398. [PubMed] [Google Scholar]
- 65.Olsson J, Poles M, Spetz AL, et al. Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4, and beta-chemokines. J Infect Dis. 2000;182:1625–1635. doi: 10.1086/317625. [DOI] [PubMed] [Google Scholar]
- 66.McGowan I, Elliott J, Fuerst M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 67.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blair SA, Kane SV, Clayburgh DR, et al. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 69.Shen L, Black ED, Witkowski ED, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 70.Clayton F, Kapetanovic S, Kotler DP. Enteric microtubule depolymerization in HIV infection: a possible cause of HIV-associated enteropathy. AIDS. 2001;15:123–124. doi: 10.1097/00002030-200101050-00019. [DOI] [PubMed] [Google Scholar]
- 71.Mohan M, Aye PP, Borda JT, et al. Gastrointestinal disease in simian immunodeficiency virus-infected rhesus macaques is characterized by proinflammatory dysregulation of the interleukin-6-Janus kinase/signal transducer and activator of transcription3 pathway. Am J Pathol. 2007;171:1952–1965. doi: 10.2353/ajpath.2007.070017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atreya R, Neurath MF. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr Drug Targets. 2008;9:369–374. doi: 10.2174/138945008784221116. [DOI] [PubMed] [Google Scholar]
- 73.de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohan M, Aye PP, Borda JT, et al. CCAAT/enhancer binding protein beta is a major mediator of inflammation and viral replication in the gastrointestinal tract of SIV-infected rhesus macaques. Am J Pathol. 2008;173:106–118. doi: 10.2353/ajpath.2008.080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 76.Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 77.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 78.Hu JJ, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 80.Veazey RS, Shattock RJ, Pope M, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 81.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz AJ, Alvarez X, Lackner AA. Distribution and immunophenotype of DC-SIGN expressing cells in SIV-infected and uninfected macaques. AIDS Res Hum Retro. 2002;18:1021–1029. doi: 10.1089/08892220260235380. [DOI] [PubMed] [Google Scholar]
- 83.Jameson B, Baribaud F, Pohlmann S, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poonia B, Wang X, Veazey RS. Distribution of simian immunodeficiency virus target cells in vaginal tissues of normal rhesus macaques: implications for virus transmission. J Reprod Immunol. 2006;72:74–84. doi: 10.1016/j.jri.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Neutra MR, Frey A, Kraehenbuhl J-P. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 86.Amerongen HM, Weltzin R, Farnet CM, et al. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4:760–765. [PubMed] [Google Scholar]
- 87.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rychert J, Lacour N, Amedee AM. Genetic analysis of simian immunodeficiency virus expressed in milk and selectively transmitted through breastfeeding. J Virol. 2006;80:3721–3731. doi: 10.1128/JVI.80.8.3721-3731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neildez O, Le Grand R, Caufour P, et al. Selective quasispecies transmission after systemic or mucosal exposure of macaques to simian immunodeficiency virus. Virology. 1998;243:12–20. doi: 10.1006/viro.1997.9026. [DOI] [PubMed] [Google Scholar]
- 90.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spira AI, Marx PA, Patterson BK, et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pope M, Betjes MGH, Romani N, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 93.Sasseville VG, Du Z, Chalifoux LV, et al. Induction of lymphocyte proliferation and severe gastrointestinal disease in macaques by a nef gene variant of SIVmac239. Am J Pathol. 1996;149:163–176. [PMC free article] [PubMed] [Google Scholar]
- 94.Pandrea I, Sodora DL, Silvestri G, et al. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pandrea I, Gautam R, Ribeiro RM, et al. Acute loss of intestinal CD4+ T cells in not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gordon SN, Klatt NR, Bosinger SE, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silvestri G, Paiardini M, Pandrea I, et al. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pandrea I, Gaufin T, Brenchley JM, et al. Experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J Immunol. 2008;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.George MD, Reay E, Sankaran S, et al. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehandru S, Poles MA, Tenner-Racz K, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anton PA, Mitsuyasu RT, Deeks SG, et al. Multiple measures of HIV burden in blood and tissue are correlated with each other but not with clinical parameters in aviremic subjects. AIDS. 2003;17:53–63. doi: 10.1097/00002030-200301030-00008. [DOI] [PubMed] [Google Scholar]
- 103.Poles MA, Boscardin WJ, Elliott J, et al. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr. 2006;43:65–68. doi: 10.1097/01.qai.0000230524.71717.14. [DOI] [PubMed] [Google Scholar]
- 104.Kaur A, Hale CL, Ramanujan S, et al. Differential dynamics of CD4(+) and CD8(+) T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J Virol. 2000;74:8413–8424. doi: 10.1128/jvi.74.18.8413-8424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Veazey RS, Lifson JD, Schmitz JE, et al. Dynamics of simian immunodeficiency virus-specific cytotoxic T-cell responses in tissues. J Med Primatol. 2003;32:194–200. doi: 10.1034/j.1600-0684.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 106.Betts MR, Ambrozak DR, Douek DC, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reynolds MR, Rakasz E, Skinner PJ, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veazey RS, Gaudin M-C, Mansfield KG, et al. Emergence of simian immunodeficiency virus-specific CD8+ T cells in the intestine of macaques during primary infection. J Virol. 2001;75:10515–10519. doi: 10.1128/JVI.75.21.10515-10519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stevceva L, Kelsall B, Nacsa J, et al. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J Virol. 2002;76:9–18. doi: 10.1128/JVI.76.1.9-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Asquith B, Edwards CT, Lipsitch M, et al. Inefficient cytotoxic T lymphocyte-mediated killing of HIV-1-infected cells in vivo. PLoS Biol. 2006;4:e90. doi: 10.1371/journal.pbio.0040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harcourt G, Gomperts E, Donfield S, et al. Diminished frequency of hepatitis C virus specific interferon gamma secreting CD4+ T cells in human immunodeficiency virus/hepatitis C coinfected patients. Gut. 2006;55:1484–1487. doi: 10.1136/gut.2005.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Picker LJ, Hagen SI, Lum R, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]


