Abstract
Attention can profoundly shape the experience of pain. However, little is known about the neural mechanisms that support directed attention to nociceptive information. In the present study, subjects were cued to attend to either the spatial location or intensity of sequentially presented pairs of painful heat stimuli during a delayed match to sample discrimination task. We hypothesized that attention-related brain activation would be initiated following the presentation of the attentional cue and would be sustained through the discrimination task. Conjunction analysis confirmed that bilateral portions of the posterior parietal cortex (intraparietal sulcus, IPS and superior parietal lobule) exhibited this sustained activity during attention to spatial but not intensity features of pain. Analyses contrasting activation during spatial and intensity attention tasks revealed that the right IPS region of the posterior parietal cortex was consistently more activated across multiple phases of the spatial task. However, attention to either feature of the noxious stimulus was associated with activation of fronto-parietal areas (IPS and frontal eye fields) as well as priming of the primary somatosensory cortex. Taken together, these results delineate the neural substrates that support selective amplification of different features of noxious stimuli for utilization in discriminative processes.
Introduction
Top-down attentional bias established by the cognitive task affects neuronal activity even before stimulus presentation [5]. During nociceptive processing such pre-stimulus effects can be seen in expectation paradigms. Expectation of pain activates brain regions that are known to be activated by painful stimuli alone [46]. Expectations of lower pain not only decrease subjective pain experience but also decrease pain-related activations [31].
In addition to general anticipation, attention to specific dimensions of a sensory event may also dramatically shape processing by producing changes in neural activity before the stimulus has been presented. Spatial cueing in vision experiments frequently increases activity in areas of occipital cortex that retinotopically correspond to the cued location [25, 20]. Feature cues, on the other hand, increase activity in areas that are known to process the feature inside and outside spatial spotlight of attention [57, 53, 56, 47]. Finally, direction of spatial attention modulates event-related potentials produced by pain [34, 33].
Top-down attention has been shown to engage posterior parietal cortex (PPC) and superior frontal cortex [including, frontal eye fields (FEF) and dorsolateral prefrontal cortex (DLPFC)] in both spatial [11, 22, 9, 25, 6, 51] and feature [21, 54, 53, 23] attention in vision studies. Although brain mechanisms supporting top-down attention to specific stimulus dimensions have been well characterized in visual and auditory modalities, little remains known about the mechanisms that support spatial and feature attention for nociceptive information. Our group has previously shown the existence of the dorsal (consisting of posterior parietal and prefrontal cortices) and ventral (consisting of insula and prefrontal cortex) processing streams engaged by discrimination of location versus intensity of painful stimuli [42, 43]. Those studies were designed to isolate activation related to the comparison of specific features of noxious stimuli with information retrieved from memory of a previous stimulus. Attention is critically important for the acquisition of the target features of sensory stimuli and is an integral part of the discrimination process. However, it remains unclear how much of this discrimination related activation is related to the direction of attention.
To identify brain activation associated with attention to specific features of pain, subjects were cued to attend to either pain intensity or pain location before the delivery of noxious stimuli. Their attentional performance was assessed by the use of a two alternative, delayed match-to-sample task. Functional MRI was used to characterize brain activity during all four phases of the delayed match to sample task. These phases include the period following the cue (cue maintenance period), the period where subjects were acquiring noxious information (acquisition period), the memory period between stimuli, and the discrimination period. We hypothesized that attention related activation during the cue maintenance phase would be sustained across multiple phases of the discrimination task.
Methods
Subjects
Both psychophysical and MRI components of the study were completed by 18 right-handed healthy volunteers, 9 males and 9 females (age 20–33 years, mean: 27 years). Fifteen subjects were white, one Hispanic, one African American, and one Indian. One additional subject was withdrawn from the study due to extreme sensitivity to the heat stimuli during the training session. All subjects gave written, informed consent acknowledging that they would experience painful stimuli, all procedures and manipulations were clearly explained, and they were free to withdraw at any time. All procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine.
Stimulation procedures
A thermal stimulator with a 16 × 16 mm contact surface (Medoc TSA II) was used for noxious heat stimulation. The probe was placed on a special holder, after which the stimulated body region was positioned on the surface of the thermode. A baseline temperature was 35°C. The stimulus temperature was changed with rise and fall rates of 6°C/s. To minimize sensitization or adaptation, each experimental series was delivered to previously unstimulated skin areas.
Psychophysical training
Initially, all subjects were trained with thirty-two 5-s-duration stimuli (35–49°C) applied to the arm to give them experience rating pain. Then subjects practiced the discrimination task by using four out of twelve series of stimulations that were subsequently used in the scanner to ensure that they could adequately discriminate the stimuli and also to familiarize them with the task..
Experimental task
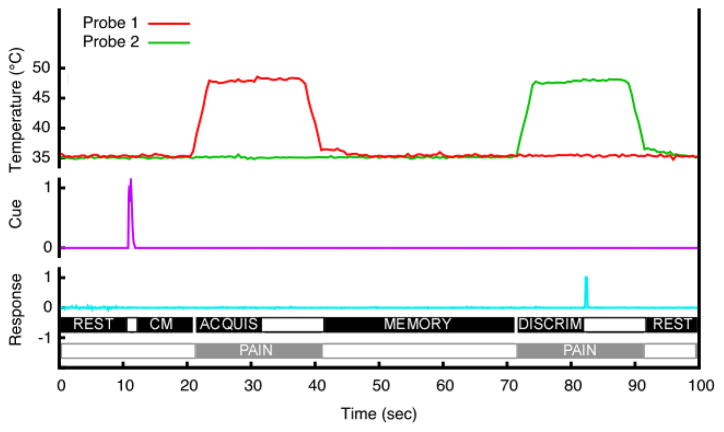
A two-alternative, forced-choice paradigm using pairs of thermal stimuli was used to identify brain regions involved in different parts of match-to-sample task (Fig. 1). These stimuli were applied to the posterior aspect of the lower left leg, and two separate probes remained positioned on a subject for the duration of the series (baseline temperature 35°C). This task (including time intervals for individual periods) was designed to parallel two previous studies on spatial and intensity discrimination of pain in our lab as closely as possible [42, 43]. For each discrimination trial, 20 seconds after task initiation, a sound cue was delivered through headphones instructing subjects to pay attention to location (two beeps, 200 ms tone with a 100 ms intertone interval) or intensity (one beep, 200 ms tone). After a 10 second cue maintenance period, the first noxious heat stimulus (48°C) was applied for 20 seconds (T1, acquisition period). A 30 s memory period followed the heat stimulation, after which a second 20 s stimulus was delivered at various temperatures or locations (T2, discrimination period). For intensity-cued trials, subjects received both stimuli at the same location, and, for location-cued trials, both stimuli were delivered at the same intensity (48°C). In location trials, T2 could be delivered at the same location (50% of trials) or delivered either 4 cm (25% of trials) or 16 cm (25% of trials) away from T1 using a separate probe. The second probe remained at 4 or 16 cm distance from the first probe for the duration of each MRI series. At the end of each series both probes were repositioned. In intensity trials, T2 could be 48 (50% of trials), 49 (25% of trials), or 50°C (25% of trials).
Figure 1.
The temporal sequence of the discrimination task. Ten seconds before T1, a sound cue instructed subjects to attend to either the location or intensity of the stimulus. Noxious stimuli (T1 and T2) were then delivered sequentially for 20 seconds each with an interstimulus (memory) interval of 30 seconds. Before the end of T2, subjects had to indicate whether T2 was different (or same) from T1 in location or intensity depending on the cue. The period between the end of the cue and beginning of T1 was considered the cue maintenance period. The discrimination period was defined as the first 75% of the time interval between the beginning of T2 and subject’s response. The corresponding time period in T1 was defined as acquisition period. Thus, regressors for the acquisition and discrimination period were unique for every discrimination pair.
Approximately 10% of all trials were catch-trials, introduced to monitor whether subjects were performing feature-specific discriminations rather than simply identifying differences between stimuli. In intensity catch trials, subjects were instructed to perform an intensity discrimination, but T2 (48°C) was delivered at a spatially distinct location from T1 (48°C). In spatial catch trials, subjects were instructed to perform a spatial discrimination task, but stimuli of different intensities (T1=48°C, T2=50°C) were delivered at the same location.
In all tasks, subjects were required to indicate whether T2 was the same or different as cued feature of T1 by pressing a button with the index or middle fingers of the right hand, respectively. Subjects were instructed that the determination was to be made as soon as the decision was reached but before the end of the second stimulus. Each 456 s series contained 4 pairs of comparisons, and the experiment contained 12 series per subject. One subject completed only 7 series due to physical discomfort from the head coil (data included). Location and intensity trials were pseudorandomized, and task order was counterbalanced across subjects.
Psychophysical assessment and analysis
For both the training and fMRI acquisition series, subjects’ responses to discrimination were recorded using a digital chart recorder (Power-Lab: ADInstruments, Sydney, Australia). These real-time data then were processed using custom-written programs within the IDL software package (Research Systems, Boulder, CO). Response latencies and error rates were examined using repeated-measures ANOVA to identify effects of the stimulus feature (intensity vs location), as well as type of trial (“same”, “different”, “catch”) on the ability to discriminate. The chart recorder data was also used to construct regressors for the fMRI analysis.
Subjective evaluation of task difficulty, pain intensity, and pain unpleasantness were acquired with a visual analog scale (VAS) at the end of each series. The scales had a 0–10 range and were 15 cm long. Subjects were instructed that the ratings should reflect the overall experience of all eight stimuli within the whole series. Ratings of individual stimuli were not obtained to minimize confounds arising from having subjects provide intensity ratings while being instructed to attend to location. Therefore direct comparison of task difficulty between the intensity and location trials was not possible. Also, these ratings do not provide the ability to assess intensity and unpleasantness on a stimulus by stimulus basis. At the end of the experiment, subjects were queried as to the strategy they used during the discrimination and memory in both intensity and location tasks.
Image acquisition and processing
Functional data were acquired on a 1.5 T General Electric echo-speed Horizon LX scanner with 1.5T HD 8 Channel High Res Brain Array Coil [Invivo Corporation, Gainesville, FL]. For functional imaging, blood oxygenation level-dependent images were acquired continuously in each contiguous plane by using echo-planar imaging [echo time (TE), 40ms; repetition time (TR), 2s; 28 × 5-mm-thick slices; 3.75 × 3.75 mm in-plane resolution; flip angle, 80°; no slice gap]. During fMRI acquisition series, subjects were requested to close their eyes.
High-resolution structural scans were acquired using a BRAVO sequence (inversion time, 600 ms; TR, 11.41ms; flip angle, 12°; TE, 4.77ms; section thickness, 1 mm with no gap between sections; number of sections, 160; in-plane resolution, 0.9375 × 0.9375 mm).
The functional image analysis package FSL [Center for Functional Magnetic Resonance Imaging of the Brain (FMRIB), University of Oxford, Oxford, UK] was used for image processing and statistical analysis. The functional data were movement corrected, spatially smoothed by 5 mm with a 3-D isotropic Gaussian kernel, and temporally filtered by a nonlinear high-pass filter with a cutoff period of 60 s. Each subject’s functional images were registered to their structural data using a six-parameter linear 3-D transformation and then were initially transformed into standard stereotaxic space (as defined by Montreal Neurologic Institute [62]) using a 12-parameter affine transformation. To further minimize spatial variation between subjects, these linearly transformed data were then nonlinearly transformed into standard space [3, 4].
Statistical analysis of regional signal changes within the brain
Statistical analysis of the regional signal changes was performed on each acquisition series (first level analyses) using a general linear modeling approach with nonparametric local autocorrelation correction [18, 65]. In all analyses, the relationship of the predictive model function to MRI signal intensity was evaluated by calculating a t-statistic on a voxel-by-voxel basis. These t values were then converted to Z scores to allow p values to be calculated on the basis of Gaussian random field theory [66, 19, 16]. The predictive model functions for the general linear modeling analysis were derived as follows:
We created 17 regressors for analysis of cue maintenance, acquisition, memory, discrimination, and pain periods. These regressors were structured to assess activity in different types of trials (Table 1). All regressors (except for pain) were orthogonalised to the pain regressor. In addition, each memory regressor was orthogonalised to memory of a different task (intensity vs. space) and to cue maintenance periods of both tasks. Similarly, cue maintenance periods were orthogonalised to cue maintenance period of a different task and memory periods of both tasks. The goal of such orthogonalisation was to be able to separate a true baseline when subjects performed no task and to exclude activations attributable to pain from discrimination and acquisition related activations.
Table 1.
Regressor structure used for first level analysis.
| Same | Space Different | Catch | Same | Intensity Different | Catch | |
|---|---|---|---|---|---|---|
| Cue Maintenance | 1 | 1 | 1 | 2 | 2 | 2 |
| Acquisition | 3 | 4 | 5 | 6 | 7 | 8 |
| Memory | 9 | 9 | 9 | 10 | 10 | 10 |
| Discrimination | 11 | 12 | 13 | 14 | 15 | 16 |
| Pain | 17 | 17 | 17 | 17 | 17 | 17 |
Numbers represent separate regressors used to identify brain activation associated with a given time period, stimulus condition, and cognitive task. Depending on the phase of experimental task (cue maintenance, memory, pain) one regressor may encompass multiple stimulus conditions.
To generate regressors, the real-time data from the chart recorder were processed using customized programs within the IDL software package. Each of the 17 regressors had a period of interest scaled as +1. For analysis of the acquisition periods we only included the stimulus pairs in which T2=T1 to avoid confounders arising from the use of different temperatures or areas. Cue maintenance period regressors were generated using the time interval between the delivery of a sound cue and the onset of the first stimulus in a pair. The first 75% of discrimination period (T2 onset-response choice) was selected for generation of discrimination regressors to ensure that that motor activity associated with response selection did not confound assessment of discrimination-related activation. The corresponding time in T1 was used to generate acquisition regressors. Thus, regressors for the acquisition and discrimination period were unique for every discrimination pair. Memory regressors included the time between the end of the first stimulus and onset of the second stimulus. Pain regressors included whole T1 and T2 periods. The auditory cues were not modeled in the regression analysis since they were so brief (each tone 200 ms) relative to the TR (2000ms).
All regressors were convolved with a gamma-variate model of the hemodynamic response (delay 6s, SD 3 s) and its temporal derivative [8, 28, 65]. They then were filtered temporally using the same parameters that were applied to the functional images.
We performed interseries fixed effects (second level) analyses within each subject separately for each period, and proceeded to intersubject group analysis (third level) using a random effects model. Clusters of voxels exceeding a Z-score>2.3 and p<0.05 (mixed effect, corrected for multiple comparisons) were considered statistically significant [66]. A random-effects analysis was performed to determine whether regions uniquely activated during either spatial or intensity trials exhibited differential magnitudes of activity in each period of the task. Finally, conjunction analyses were performed in order to identify regions commonly activated between spatial and intensity tasks in each experimental period [41].
In order to assess the influence of attention across all phases of the delayed match to sample task, we performed separate conjunction analyses for both spatial and intensity tasks. We hypothesized that areas that were important in the cue instructed direction of attention would exhibit common activation across multiple phases of the task. Since activation maps identified during the memory period differed substantially from those present in all other phases, we also performed this analysis with the memory period excluded.
Results
Psychophysics
During discrimination of pairs of noxious stimuli, VAS pain intensity was 3.4 ± 0.2, VAS pain unpleasantness was 3.2 ± 0.2 (mean ± SEM). Thus, the thermal stimuli were consistently rated as painful, indicating that the spatial and intensity discriminations were performed on noxious stimuli.
Discrimination task difficulty was 1.992 ± 0.117 (mean ± SEM, pooled for both spatial and intensity discrimination tasks). Analysis of response latencies revealed that spatial discrimination was significantly faster than intensity discrimination (F = 120.24, p < 0.0001; Fig. 2). When collapsed across both task types, response latencies varied according to the magnitude of the difference between T2 and T1, as well as for catch versus standard trials (F = 53.90, p < 0.0001). Response latencies during “same” trials were significantly longer than trials different by 1°C or 4 cm (difference 1) (F=47.08, p<0.0001), than trials different by 2°C or 16 cm (difference 2) (F=128.95, p<0.0001), and catch trials (F=129.94, p < 0.0001).
Figure 2.
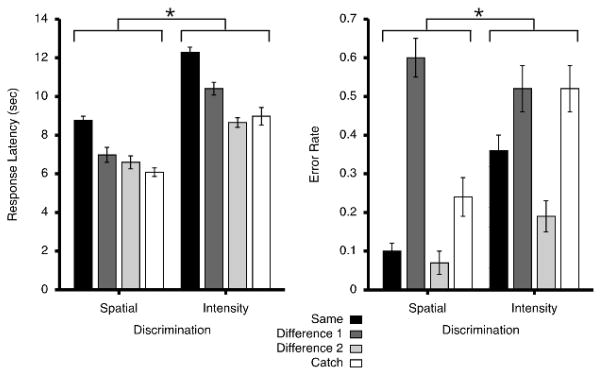
Behavioral responses during spatial and intensity discriminations (mean ± SEM). Difference 1 corresponds to the smaller difference either in location (4 cm) or intensity (1°C). Difference 2 corresponds to the larger difference in location (16 cm) or intensity (2°C). Both error rates (*, p = 0.0038) and response latencies (*, p< 0.0001) were significantly lower for spatial compared to intensity discriminations. As location or temperature differences between T1 and T2 stimuli decreased, response latencies and error rates became significantly larger, indicating that discrimination of noxious heat stimuli became more difficult. Although performance on catch trials varied between spatial and intensity tasks, the magnitude of the increase in the error rates between “catch” and “same” trials was almost identical between the two.
Subjects made more correct discriminations in spatial than intensity tasks (F = 11.45, p = 0.0038). When collapsed across both task types, error rates varied according to the magnitude of the difference between T2 and T1, as well as for catch versus standard trials (F = 28.31, p < 0.0001). “Same” trials had significantly lower error rates than trials different by 1°C or 4 cm (difference 1) (F=22.56, p=0.0002). However, there was no significant difference in error rates between “same” trials and those different by 2°C or 16 cm (difference 2) (F=3.5059, p=0.08).
Error rates for catch trials were higher than those of the “same” trials (F=13.95, p= 0.0018). Both spatial and intensity catch trials had error rates that were only 0.14 – 0.16 higher than those of the corresponding “same” trials. If subjects had been responding only to differences of two stimuli and had disregarded the cue, error rates in the spatial catch trial would have been predicted to be 81% (100 – intensity difference 2), and error rates in the intensity catch trials would have been predicted to be 93% (100 – spatial difference 2). Thus, subjects clearly performed the majority of discriminations within the cued task.
Pain-induced activations
Noxious heat stimuli produced activations and deactivations similar to ones commonly seen in other pain imaging studies (Fig. 3). In particular, activations in bilateral thalamus, putamen, caudate, insula, secondary somatosensory cortex (SII), as well as contralateral leg region of SI were detected. Deactivations were seen in a set of brain areas consistent with the default-mode network – posterior cingulate cortex (PCC), precuneus and ventromedial prefrontal cortex (VMPFC).
Figure 3.
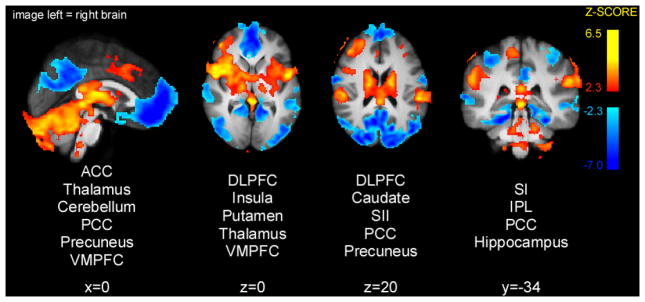
Brain activations and deactivations during painful heat stimulations (T1 and T2 periods). Noxious stimuli produced activation in thalamus, putamen, caudate, insula, SI and SII, as well as deactivations in PCC, precuneus and VMPFC. Slice locations are relative to standard Montreal Neurologic Institute (MNI) stereotaxic space.
Cue maintenance period activations
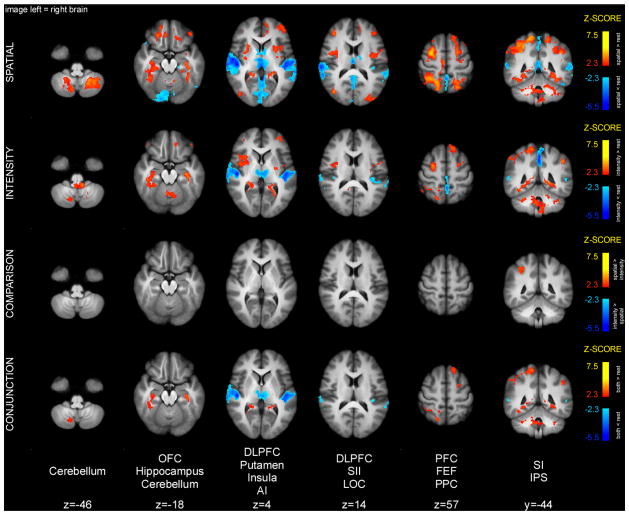
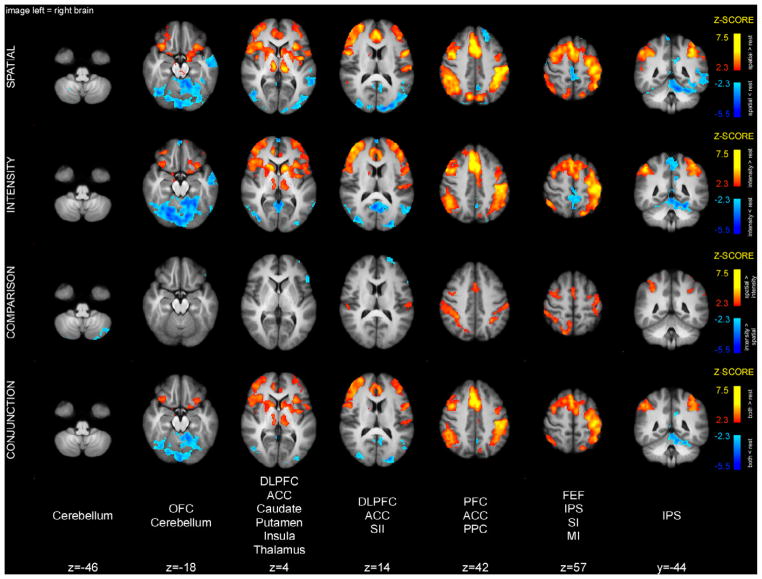
The cue maintenance period was characterized to a large extent by a common pattern of activity between the spatial and intensity tasks (Fig. 4). Specifically, conjunction analysis of spatial and intensity cue maintenance periods detected significant activation overlap in parts of the cerebellum, hippocampus, FEF, intraparietal sulcus (IPS), as well as contralateral SI. The FEF and IPS are believed to constitute the fronto-parietal attentional network that can be activated by top-down attention in multiple sensory modalities. In addition, conjunction analysis detected significant deactivation overlap in the primary auditory cortex (AI) and thalamus. Deactivation in the AI during the cue maintenance period might be related to activation of this area before/during the presentation of the cue. Similar deactivations were detected during the memory period.
Figure 4.
Brain activations and deactivations during cue maintenance period. Conjunction analysis of spatial and intensity cue maintenance periods detected common activations in the cerebellum, hippocampus, FEF, IPS and SI. These activations are consistent with retrieval of cue meaning, top-down attentional engagement and priming of early somatosensory areas. The spatial task produced greater activation in right IPS. Slice locations are relative to standard MNI stereotaxic space.
Spatial cue maintenance periods were associated with greater activation of the contralateral (right) brain regions surrounding the IPS compared to intensity cue maintenance periods (Table 2). In contrast, no region exhibited greater activation during the intensity cue maintenance period as compared to the spatial cue maintenance period.
Table 2.
Local maxima of differences between spatial and intensity task-related activity.
| Region | Cue maintenance spatial > intensity | Acquisition spatial > intensity | Acquisition intensity > spatial | Memory spatial > intensity | Discrimination spatial > intensity | Discrimination intensity > spatial | Three period conjunction spatial |
|---|---|---|---|---|---|---|---|
| IPS, R | 34; −48; 48 (3.47) | 42; −36; 42 (4.40) | 32; −44; 40 (4.21) | 44; −40; 38 (2.86) | |||
| IPS, L | −24; −58; 52 (3.00) | −30; −50; 40 (3.84) | −30; −48; 38 (3.06) | ||||
| SPL, R | 8; −78; 48 (3.76) | 32; −70; 34 (3.35) | 16; −74; 50 (3.60) | ||||
| SPL, L | −6; −78; 42 (3.55) | −16; −70; 52 (3.64) | |||||
| FEF, R | 26; 4; 54 (3.36) | 30; −4; 46 (3.99) | |||||
| FEF, L | −26; −10; 54 (3.07) | ||||||
| DLPFC, R | 46; 42; 14 (3.14) | ||||||
| Occipital, R | 48; −82; 4 (3.43) | ||||||
| Occipital, L | −36; −92; −6 (3.33) | ||||||
| Frontal pole, L | −2; 60; −8 (3.75) | −10; 60; 32 (3.37) | |||||
| Temporal pole, R | 56; 10; −4 (4.66) | ||||||
| Temporal pole, L | −52; 12; −8 (3.16) | ||||||
| Amygdala, R | 26; −2; −14 (3.89) | ||||||
| ACC | 4; 42; 24 (4.57) | 2; 0; 54 (3.64) | |||||
| Insula, R | 36; 24; 0 (3.75) | ||||||
| Insula, L | −40; 14; −12 (3.37) | −30; 26; −2 (3.21) | |||||
| SII, R | 58; −26; 24 (3.11) | ||||||
| SII, L | −58; −20; 22 (4.22) | ||||||
| Cerebellum, R | 32; −72; −40 (3.24) | ||||||
| Cerebellum, L | −44; −76; −42 (3.26) | ||||||
| IFG, L | −52; 26; −4 (3.62) |
X, Y, Z coordinates are relative to Montreal Neurologic Institute (MNI) stereotaxic space. Peak Z-scores were obtained from group analysis and are listed within the parenthesis. Abbreviations: Intraparietal sulcus: IPS, superior parietal lobule: SPL, frontal eye fields: FEF, dorsolateral prefrontal cortex: DLPFC, anterior cingulate cortex: ACC, inferior parietal lobule: IPL, secondary somatosensory cortex: SII, inferior frontal gyrus: IFG.
Acquisition period activations
In contrast with the cue maintenance period, the pattern of activity during the acquisition period was quite different between the spatial and intensity tasks (Fig. 5). In fact, conjunction analysis did not detect any clusters of overlapping activity between spatial and intensity acquisition periods. Direct comparisons revealed that the spatial task produced greater activation in regions involved in top-down attention, such as portions of the superior parietal lobule (SPL), including the precuneus, right IPS, and right FEF, compared to intensity task (Table 2). Acquisition of spatial features of pain also produced activation in left IPS, and right DLPFC.
Figure 5.
Brain activations and deactivations during the acquisition period. Conjunction analysis did not detect any clusters of overlapping activity during the spatial and intensity acquisition periods. The spatial task produced greater activation in right SPL, IPS and FEF. Activity in these areas likely represents top-down attention to the stimulus, as well as engagement of the dorsal stream of sensory information processing. Intensity task produced greater activation in LOC. Slice locations are relative to standard MNI stereotaxic space.
In contrast to the spatial task, the intensity task produced higher activity in lateral occipital cortex (LOC) bilaterally. Also, acquisition of intensity features of pain produced activation of ACC.
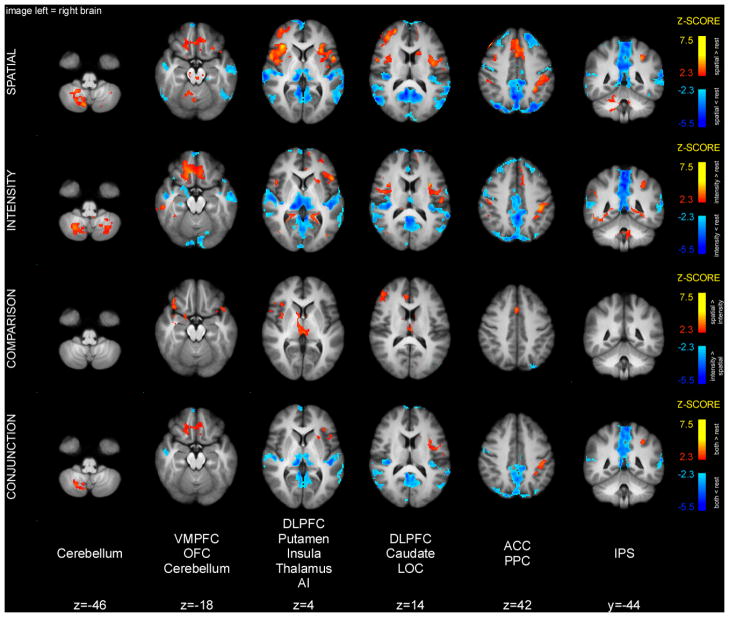
Memory period activations
Conjunction analysis of spatial and intensity memory periods detected shared activity in the cerebellum, OFC, insula, parts of frontal operculum, and left IPS (Fig. 6). In addition, there were common deactivations in areas that constitute the default-mode network (posterior cingulate cortex and precuneus), as well as bilateral AI. Direct comparison of the two conditions detected greater insular, ACC, and DLPFC activity in the spatial compared to the intensity task (Table 2). In addition, the spatial task produced smaller deactivations of the thalamus and perigenual ACC than the intensity task, thus producing apparently greater activation.
Figure 6.
Brain activations and deactivations during the memory period. Conjunction analysis of activation during the spatial and intensity memory periods detected clusters of overlapping activity in the cerebellum, OFC, insula, frontal operculum, and left IPS. The spatial task produced greater activation in the right insula, DLPFC as well as ACC. Slice locations are relative to standard MNI stereotaxic space.
Discrimination period activations
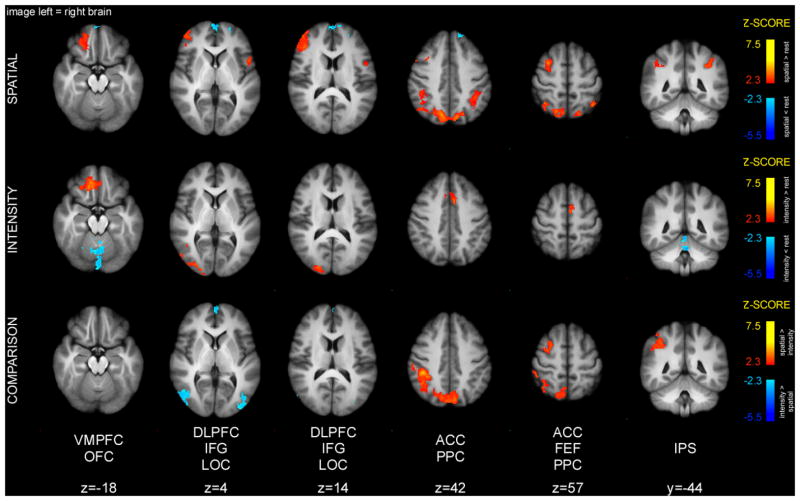
Conjunction analysis of spatial and intensity discrimination periods detected overlapping activity in bilateral thalamus, caudate, putamen, insula, SII, ACC, OFC, DLPFC, frontal operculum, IPS, as well as left primary motor cortex (MI) (Fig. 7). Many of these areas were previously implicated in discriminatory processing of nociceptive information. In addition, conjunction analysis detected significant deactivation overlap in parts of the cerebellum.
Figure 7.
Brain activations and deactivations during discrimination period. Conjunction analysis of spatial and intensity discrimination periods detected clusters of overlapping activity in bilateral thalamus, caudate, putamen, insula, ACC, OFC, DLPFC, frontal operculum, IPS, as well as left paracentral lobule. The spatial task produced greater activation in SII, ACC, FEF, and PPC. Intensity discrimination produced greater activity in cerebellum, and left DLPFC. Slice locations are relative to standard MNI stereotaxic space.
Spatial discrimination produced greater activity in SII, ACC, as well as FEF, SPL, and IPS (Table 2). Intensity discrimination produced greater activity in the cerebellum, as well as left DLPFC.
Common activations across different phases of the delayed match to sample task
No clusters of activity overlapped sufficiently between the cue maintenance, acquisition, memory and discrimination periods to be judged significant by the conjunction analysis. However, when the memory period was excluded, bilateral IPS and SPL preserved their activity throughout cue maintenance, acquisition, and discrimination periods of the spatial task (Fig. 8, Table 2). In contrast, no cluster of brain activity showed large enough overlap between these periods in the intensity task to be considered statistically significant.
Figure 8.
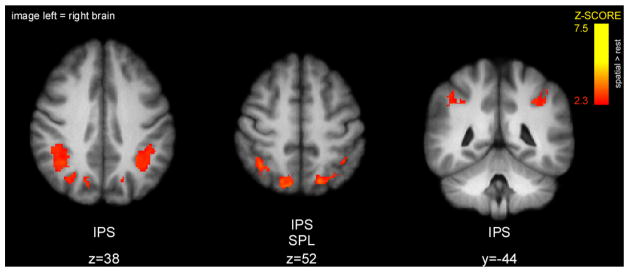
Clusters of overlapping activity between cue maintenance, acquisition, and discrimination periods in spatial task as detected by conjunction analysis. Bilateral regions of IPS and SPL were active not only during orientation of attention (cue maintenance period), but also maintained their activity during stimulus presentation (acquisition and discrimination periods). Slice locations are relative to standard MNI stereotaxic space.
Discussion
Top-down attentional direction to spatial or intensity features of painful stimuli activated the primary somatosensory cortex, FEF and IPS prior to presentation of noxious stimuli. The role of the FEF and IPS in top-down attention has been extensively studied in vision, as well as auditory and somatosensory domains [25, 10, 37, 21, 52]. However, to our knowledge this is the first study to confirm that these regions support top-down attention to features of painful stimuli. Moreover, activity in top-down attentional brain areas (most consistently, the IPS) was greater in spatial compared to intensity tasks not only during attention alone (cue maintenance period), but also during the experience of pain itself (acquisition and discrimination periods). Finally, IPS and SPL activity was preserved through the cue maintenance, acquisition, and discrimination periods of the spatial task. Therefore, these regions of PPC uniquely support the direction of attention to spatial features of noxious stimuli and likely contribute to the acquisition of information necessary to correctly perform the discrimination task.
Implications of discrimination performance
Both error rates and response latencies were significantly different between spatial versus intensity discrimination tasks. Matching these two tasks for difficulty is challenging partly because spatial information is available in the early phases of stimulation, while intensity information temporally summates and becomes more discriminable in the later phases of stimulation. The differences between these two tasks are evident even during the discrimination of “same” (i.e., same location or equal intensity) pairs. However, these differences represent a major strength in the experimental design because they provide strong evidence that subjects were attending to the instructed features of the noxious stimuli rather than simply detecting differences.
Brain activity during the cue maintenance period
Both spatial and intensity cue maintenance periods were characterized by activation in cerebellum, hippocampus, prefrontal cortex, FEF, parts of the PPC (including IPS), as well as SI contralateral to the stimulation site. Activity in these brain areas likely represents several cognitive processes related to both nonspecific processing of the cue, as well as more specific top-down attention and its down-stream effects.
Conceptually, one of the early processes that must follow sensory processing of the cue is retrieval of episodic memory to invest this cue with meaning. Retrieval of episodic memory is likely associated with the hippocampal activity during cue maintenance period of the present experiment but this interpretation is speculative given the absence of an auditory control stimulus without meaning [49, 17, 45, 27].
After the cue is invested with meaning it is transformed into an attentional set. IPS and superior frontal cortex (including FEF and DLPFC) constitute the top-down attentional network in both spatial [11, 22, 9, 25, 6, 51], and non-spatial attentional tasks [21, 54, 53, 23] in other sensory modalities. Thus activation of the prefrontal cortex, FEF and IPS during the cue maintenance period would be consistent with the formation and/or storage of the attentional set.
Fronto-parietal brain areas implicated in top-down attention are well positioned to prepare early somatosensory areas for impending pain. Monkey anatomical connectivity studies show that FEF has reciprocal connections with the IPS [26]. In addition, monkey posterior parietal areas (7b, 7ip) have reciprocal connections with primary and secondary somatosensory cortices [7]. In the present study, SI was active during both spatial and intensity cue maintenance periods in the complete absence of painful stimulation. Consistent with this observation, attentional “priming” of early sensory areas is seen in other sensory modalities [35, 29, 25, 20, 64, 56], and is directly linked to activity in top-down attentional areas [24, 39, 58, 50, 32, 59, 44].
The spatial task produced greater activations in right IPS compared to the intensity task in the cue maintenance period. It is likely that IPS plays a unique role in the spatial attentional task. For example, it has been previously shown that IPS has a spatial topographic organization and therefore may contribute to the processing of spatial information [67, 60, 61, 2].
Brain activity during the acquisition period
Although subjects experienced the same stimuli during the acquisition period, it was the only period that did not have any shared activity between spatial and intensity tasks after variability related to the presence of pain was regressed out. The absence of shared activity combined with marked differences in brain activation provides further evidence that subjects engaged different processes for each discrimination task.
PPC (SPL and IPS) and FEF exhibited greater activity in spatial compared to intensity acquisition periods. This difference likely represents a distinct role of PPC in processing of spatial features of noxious stimuli. Notably, the difference of activation of frontoparietal attentional areas is qualitatively preserved from the cue maintenance period where subjects only oriented their attention and did not experience noxious stimuli.
Brain activity during the memory period
The pattern of brain activity during memory period is qualitatively very different from other periods. It is no longer characterized by right lateralized activity in top-down attentional brain areas, such as IPS and FEF. In fact, both spatial and intensity memory periods produced activity in the left frontal operculum and PPC. This is consistent with previous findings by our group. Both areas are associated with verbal processing and their activation during the memory phase may be associated with a verbal mnemonic strategy [42]. Also, both spatial and intensity memory produced activation of the ACC and the anterior insula. Such memory related activation of both structures is a well documented feature of rating tasks that involve the recall of specific features of both noxious and innocuous stimuli [30, 1, 36].
Brain activity during the discrimination period
Both spatial and intensity discrimination periods were characterized by common patterns of activity in the thalamus, putamen, insula, ACC, PFC, FEF, PPC, as well as MI. This common activity likely represents top-down attention to the stimulus, common steps in decision-making, as well as preparation for the motor response.
Spatial discrimination again produced greater activity in top-down attention areas, such as IPS and FEF, despite the fact that they were also activated during intensity discrimination. This difference is surprising given that the duration of the discrimination period for the spatial task was shorter than that of the intensity task, and fewer brain volumes were included in the analysis of spatial discrimination-related activity.
In addition, spatial discrimination was associated with greater activity in the SPL. This activity likely is associated with the processing of spatial information by the dorsal stream [63, 48, 42]. Finally, intensity discrimination produced greater activation of the left DLPFC. This is consistent with the more bilateral involvement of the DLPFC in intensity compared to spatial discrimination previously noted [43]. Given its role in expectation intensity coding, left DLPFC may be differentially important for processing of intensity features of pain [31].
IPS activity during attention to spatial features of pain
Bilateral activity of IPS was preserved through three periods (cue maintenance, acquisition, discrimination) of the spatial task. Moreover, the right IPS was more active in these three phases of the spatial versus the intensity tasks. From this and previous points in the discussion, IPS plays a differential role in direction of attention to spatial features of pain.
Limitations
A potential limitation of this study is that subjects did not provide a subjective rating of the difficulty of shifting attention to spatial versus intensity features of the noxious stimulus. Accordingly, the alternative explanation that the greater IPS activation reflects greater attentional effort of the spatial versus intensity task cannot be assessed. Similarly, since stimuli were only delivered to the left side of the body, we are not able to assess the degree of right lateralization of the IPS during the spatial versus intensity task.
This study was designed to identify differences in mechanisms supporting attention to spatial versus intensity features of noxious stimuli. To ensure that the meaning of attentional cues learned during the psychophysical training session was maintained during the imaging session, we did not employ a control series in which cues were presented outside of the task context. Such a control condition would have allowed a more definitive assessment of the functional roles of activated brain regions when considered within one task. However, this limitation has limited impact on the comparison of spatial versus intensity attentional mechanisms since nonspecific components would be largely equal between tasks.
Summary and implications
Attentional tuning of nociceptive processing may provide a critical link between pain severity and psychosocial state. Factors such as anxiety, catastrophizing, and somatization are invariably associated with the severity of chronic pain and could potentially engage top-down attentional mechanisms, thereby facilitating the amplification of nociceptive information [13, 12, 38, 55, 14, 15]. Conversely, attention may be harnessed for the treatment of pain. Supraspinal activity during spatial attention can shift information processing away from intensity. Consistent with this observation, training in spatial discrimination tasks reduces pain in patients with complex regional pain syndrome [40]. The present findings confirm that brain regions surrounding the intraparietal sulcus are critically involved in the top-down direction of attention to spatial features of nociceptive stimuli.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (R01 NS39426).
We thank Sharon Warren and the staff of The Center for Biomolecular Imaging for their technical support. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albanese MC, Duerden EG, Rainville P, Duncan GH. Memory traces of pain in human cortex. J Neurosci. 2007;27(17):4612–4620. doi: 10.1523/JNEUROSCI.0695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Topographic maps of multisensory attention. Proc Natl Acad Sci U S A. 2010;107(46):20110–20114. doi: 10.1073/pnas.1011616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson JLR, Jenkinson M, Smith SM. Non-linear optimisation. FMRIB technical report 2007(TR07JA1) [Google Scholar]
- 4.Andersson JLR, Jenkinson M, Smith SM. Non-linear registration, aka Spatial normalisation. FMRIB technical report 2007(TR07JA2) [Google Scholar]
- 5.Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Res. 2009;49(10):1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14(2):212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287(4):393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 9.Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 10.Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- 11.Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13(3):1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. 1998;75(2–3):187–198. doi: 10.1016/s0304-3959(97)00219-4. [DOI] [PubMed] [Google Scholar]
- 13.Drossman DA, McKee DC, Sandler RS, Mitchell CM, Cramer EM, Lowman BC, Burger AL. Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology. 1988;95(3):701–708. doi: 10.1016/s0016-5085(88)80017-9. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RR, Bingham CO, 3rd, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55(2):325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 15.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12(11 Suppl):T46–60. doi: 10.1016/j.jpain.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 17.Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431(7005):188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 19.Friston KJ, Worsley KJ, Frackowiak RJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 20.Giesbrecht B, Weissman DH, Woldorff MG, Mangun GR. Pre-target activity in visual cortex predicts behavioral performance on spatial and feature attention tasks. Brain Res. 2006;1080(1):63–72. doi: 10.1016/j.brainres.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 21.Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19(3):496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 22.Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122 ( Pt 6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci. 2010;30(43):14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci. 2003;18(11):3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- 25.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 26.Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265(3):332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- 27.Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One. 2011;6(4):e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josephs O, Turner R, Friston K. Event-related f MRI. Hum Brain Mapp. 1997;5(4):243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 30.Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27(9):715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102(36):12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauritzen TZ, D’Esposito M, Heeger DJ, Silver MA. Top-down flow of visual spatial attention signals from parietal to occipital cortex. J Vis. 2009;9(13):18, 11–14. doi: 10.1167/9.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legrain V, Bruyer R, Guerit JM, Plaghki L. Nociceptive processing in the human brain of infrequent task-relevant and task-irrelevant noxious stimuli. A study with event-related potentials evoked by CO2 laser radiant heat stimuli. Pain. 2003;103(3):237–248. doi: 10.1016/S0304-3959(02)00451-7. [DOI] [PubMed] [Google Scholar]
- 34.Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99(1–2):21–39. doi: 10.1016/s0304-3959(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 35.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77(1):24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 36.Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain. 2008;138(2):362–374. doi: 10.1016/j.pain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Macaluso E, Frith CD, Driver J. Supramodal effects of covert spatial orienting triggered by visual or tactile events. J Cogn Neurosci. 2002;14(3):389–401. doi: 10.1162/089892902317361912. [DOI] [PubMed] [Google Scholar]
- 38.McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: results of a large population-based study. Arthritis Rheum. 2001;44(4):940–946. doi: 10.1002/1529-0131(200104)44:4<940::AID-ANR151>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 40.Moseley GL, Zalucki NM, Wiech K. Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain. 2008;137(3):600–608. doi: 10.1016/j.pain.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci. 2007;27(13):3388–3394. doi: 10.1523/JNEUROSCI.5128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting discrimination of sensory features of pain: a new model. J Neurosci. 2009;29(47):14924–14931. doi: 10.1523/JNEUROSCI.5538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozaki TJ. Frontal-to-parietal top-down causal streams along the dorsal attention network exclusively mediate voluntary orienting of attention. PLoS One. 2011;6(5):e20079. doi: 10.1371/journal.pone.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oztekin I, McElree B, Staresina BP, Davachi L. Working memory retrieval: contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. J Cogn Neurosci. 2009;21(3):581–593. doi: 10.1162/jocn.2008.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22(8):3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puri AM, Wojciulik E, Ranganath C. Category expectation modulates baseline and stimulus-evoked activity in human inferotemporal cortex. Brain Res. 2009;1301:89–99. doi: 10.1016/j.brainres.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 48.Reed CL, Klatzky RL, Halgren E. What vs. where in touch: an fMRI study. Neuroimage. 2005;25(3):718–726. doi: 10.1016/j.neuroimage.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 49.Reilly CE. Hippocampus selectively supports episodic memory retrieval. J Neurol. 2001;248(11):1014–1015. doi: 10.1007/s004150170064. [DOI] [PubMed] [Google Scholar]
- 50.Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18(4):817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salmi J, Rinne T, Degerman A, Salonen O, Alho K. Orienting and maintenance of spatial attention in audition and vision: multimodal and modality-specific brain activations. Brain Struct Funct. 2007;212(2):181–194. doi: 10.1007/s00429-007-0152-2. [DOI] [PubMed] [Google Scholar]
- 52.Salmi J, Rinne T, Koistinen S, Salonen O, Alho K. Brain networks of bottom-up triggered and top-down controlled shifting of auditory attention. Brain Res. 2009;1286:155–164. doi: 10.1016/j.brainres.2009.06.083. [DOI] [PubMed] [Google Scholar]
- 53.Serences JT, Boynton GM. Feature-based attentional modulations in the absence of direct visual stimulation. Neuron. 2007;55(2):301–312. doi: 10.1016/j.neuron.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 54.Serences JT, Schwarzbach J, Courtney SM, Golay X, Yantis S. Control of object-based attention in human cortex. Cereb Cortex. 2004;14(12):1346–1357. doi: 10.1093/cercor/bhh095. [DOI] [PubMed] [Google Scholar]
- 55.Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17(2):165–172. doi: 10.1097/00002508-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Shibata K, Yamagishi N, Goda N, Yoshioka T, Yamashita O, Sato MA, Kawato M. The effects of feature attention on prestimulus cortical activity in the human visual system. Cereb Cortex. 2008;18(7):1664–1675. doi: 10.1093/cercor/bhm194. [DOI] [PubMed] [Google Scholar]
- 57.Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19(21):9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol. 2006;96(2):941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- 59.Silvanto J, Muggleton N, Lavie N, Walsh V. The perceptual and functional consequences of parietal top-down modulation on the visual cortex. Cereb Cortex. 2009;19(2):327–330. doi: 10.1093/cercor/bhn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94(2):1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27(20):5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- 63.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 64.Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nat Neurosci. 2006;9(9):1156–1160. doi: 10.1038/nn1748. [DOI] [PubMed] [Google Scholar]
- 65.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 66.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 67.Zipser D, Andersen RA. A back-propagation programmed network that simulates response properties of a subset of posterior parietal neurons. Nature. 1988;331(6158):679–684. doi: 10.1038/331679a0. [DOI] [PubMed] [Google Scholar]