Abstract
Purpose
The aim of the study was to describe and characterize a novel small spleen phenotype with splenic lymphopenia in the Ednrb-null (Ednrb−/−) mouse with aganglionosis known to also develop enterocolitis.
Methods
We compared spleen weight as a percent of body weight from Ednrb+/+, Ednrb+/−, and Ednrb−/− mice to quantify our initial observation. Splenic microarchitecture of Ednrb+/+ and Ednrb−/− mice was assessed using both H and E staining and immunofluorescence staining for CD45R+ (B cells) and CD3+ (T cells) on tissue sections. To identify and quantify cell type, flow cytometry for CD19+ (mature B cells), CD4+ and CD8+ (T cells) was performed on the splenocytes of Ednrb+/+ and Ednrb−/− mice and compared with student’s t test. A separate cohort of Ednrb+/+ and Ednrb−/− mice was killed and splenocytes were analyzed by flow cytometry, and proximal colon was histopathologically graded for enterocolitis. Spearman’s rank correlations comparing total splenocyte and CD19+ cell counts with enterocolitis scores were performed.
Results
We found that the mean spleen weight expressed as a percent of body weight for Ednrb+/+ and Ednrb−/− mice was 0.72 and 0.25%, respectively (P < 0.001), at 25 days of age. In addition, the Ednrb−/− spleens also had markedly abnormal splenic microarchitecture with lymphopenia, and relative reduction of B cells compared to T cells. FACS of splenocytes revealed a 5 to 20-fold reduction in total cell number, CD19+, CD4+, and CD8+ of the Ednrb−/− mice compared to the Ednrb+/+ littermates (P < 0.01). We also found a strong inverse correlation of total spleen and CD19+ cell counts with histopathological enterocolitis scores (rs = −0.43, P = 0.02), showing that mice with reduced cell counts also had increased severity of enterocolitis.
Conclusion
The small spleen immunophenotype in the Ednrb−/− mouse suggests that Ednrb-dependent signaling may be required for normal spleen development. These results raise the possibility that primary immune abnormalities may contribute at least in part to some enterocolitis. At present, our data suggest intriguing new potential explanations for HAEC in Hirschsprung patients.
Keywords: Endothelin receptor B gene, Ednrb-null mouse, Hirschsprung disease, Hirschsprung-associated enterocolitis, Lymphopenia, Small spleen
Background
Hirschsprung disease (HD), also known as aganglionic megacolon, occurs because of an absence of ganglion cells primarily in the colon. Treatment consists of a surgical pull-through operation that resects the aganglionic portion of colon, and is curative in most cases. However, 20–38% of HD patients may develop enterocolitis despite technically successful surgery, a condition referred to as Hirschsprung-associated enterocolitis (HAEC). HAEC may be mild to severe, and can lead to bacteremia, sepsis, and even death. HAEC is difficult to study clinically in children due to small numbers of patients and variable timing of presentation; hence most studies have been observational and correlational in nature, from which little insight into molecular mechanisms have emerged.
In response to this problem, our group developed an animal model of post-pull-through HAEC as one approach to study the genetic and molecular mechanisms underlying this disorder. We employed the endothelin receptor B targeted-null mouse (Ednrb−/−), a well-known mouse model of HD that has short-segment aganglionosis of the rectum and distal colon as well as enterocolitis [1, 2]. We performed a single-stage microsurgical pull-through operation to resect the aganglionic segment and establish fecal continuity [3, 4]. We have shown that approximately 40% of the Ednrb−/− mice developed post-pull-through enterocolitis, closely resembling key features of post-pull-through HAEC in children.
During our characterization of the enterocolitis in Ednrb mouse, we observed that the spleens of the Ednrb−/− mice were markedly smaller than their wild type littermates. Herein, we report these novel findings, and discuss how these observations may inform future clinical studies on patients with HAEC. Our results suggest for the first time the intriguing notion that HAEC may be driven by primary immune abnormalities such as abnormal splenic architecture, size, and presumably function, and that these abnormalities might well be independent of colonic aganglionosis per se.
Materials and methods
Animals
The research protocol and all animal care procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Cedars-Sinai Medical Center. Mice (Ednrbtm1Ywa/J on a hybrid C57BL/6J-129Sv background; JAX stock # 003295) were originally imported from Jackson Laboratory (Bar Harbor, ME, USA). The breeding scheme, animal care and genotyping were performed as previously described [2]. The homozygotes (we refer to these as Ednrb−/− in this paper) are easily identified by the white coat color and progressively enlarging abdomens due to aganglionosis leading to megacolon. The wild type and heterozygote littermates (we refer to these as Ednrb+/+ and Ednrb+/−, respectively), are phenotypically normal.
Preparation of cells
Ednrb+/+ and Ednrb−/− animals were killed at 23–27 days after birth. Spleens were dissected free of fat, weighed and single cell suspension was prepared using mechanical disruption as described [5]. Cells were counted on the hemocytometer by trypan blue staining and adjusted to 1 × 106/100 μl with staining buffer (Dulbecco’s PBS, 1% FCS, 0.1% sodium azide, and 1 mM EDTA).
Flow cytometry
Single cell suspensions of the spleen were evaluated by fluorescence activated cell sorting (FACS). 5 × 105–1 × 106 cells were spread in 96-well plate and incubated with purified rat anti-mouse CD16/CD32 blocking antibody (BD Pharmingen) for 5 min. Cells were incubated on ice for 30 min with an antibody panel for CD4 (clone GK1.5; CD4 APC, 1/200), CD8 (clone 53–6.7; CD8a PE, 1/200) and CD19 (clone 6D5; CD19 FITC, 1/200) detection (Biolegend, San Diego, CA, USA). Cells were then washed twice with staining buffer and fixed in 1% para-formaldehyde in Dulbecco’s PBS overnight at 4°C. Data were acquired on Beckman Coulter flow cytometer. All data were analyzed using Summit v4.3 software. The number of CD19+, CD4+ and CD8+ cells was calculated from the frequency of each positive staining against total cell number of spleen.
Immunofluorescence staining
Spleens were embedded in OCT compound (Tissue-Tek), snap frozen on dry ice and stored at −80°C. Sections were cut on a cryostat at 14 μm, collected to gelatin-coated slides, then fixed in ice-cold acetone for 20 min and air dried for a further 20 min. After washing with PBS, sections were blocked in 5% goat serum and subsequently incubated with primary antibodies: rat anti-CD3e (1/50) and hamster anti-CD45R (1/100) (both from Ebioscience), rat anti-Madcam-1(BD Pharmingen, 1/100) diluted in 5% goat serum. Sections were incubated with primary antibodies at 4°C overnight. Anti-Armenian hamster-FITC (Santa Cruz, 1/200) or anti-rat-Alexa Fluor 594 (Invitrogen, 1/200) were then applied at room temperature for 1 h. Sections were washed and mounted with prolong anti-fade reagent with DAPI (Invitrogen). For detection of immunofluoresence, slides were analyzed using a Leica SP5 confocal microscope (Leica Microsystems).
Histologic preparation of colon and spleen
Colon tissue from Ednrb+/+ and −/− mice were prepared, sectioned, hematoxylin and eosin stained (H and E), and histopathologically graded for enterocolitis as described previously by Cheng et al. [2] Spleens were formalin fixed, paraffin embedded, sectioned longitudinally at 6-lm and H and E stained.
Statistical analysis
The data collected from the study animals were compared using student’s t test, unpaired, two-tailed, 95% confidence interval. Comparisons between groups of enterocolitis score data were made using a two-sided non-parametric Wilcoxon rank-sum test. Correlations between variables were calculated as a Spearman’s rank correlation [6]. Data are presented as means ± standard deviation, unless otherwise stated. Statistical analysis was performed using SAS computer software (SAS Institutes, Inc., Cary, NC, USA) and GraphPad Prism 5 software (GraphPad, Inc., San Diego, CA, USA).
Results
Reduced spleen size in the Ednrb−/− mouse
We observed that around the time of weaning (postnatal day 21) that the Ednrb−/− mice had markedly dilated proximal colon as previously reported [1] and significantly reduced spleen size which was not anticipated (Fig. 1). To study this novel observation further, we compared spleen weight as a percent of body weight for each genotype in 25-day-old mice. We found that the Ednrb+/+ and +/− animals were 0.72 ± 0.07 and 0.67 ± 0.06%, respectively, but that the spleens of the Ednrb−/− animals were 0.25 ± 0.04% and was statistically significant P < 0.001 (student’s t test) (Fig. 2). To assess the possibility that Ednrb−/− mice were born with smaller spleens compared to their wild type and heterozygote littermates, we assessed spleen weight as a percent of body weight at post-natal day 5 for all three genotypes and found no difference between the genotypes (data not shown).
Fig. 1.
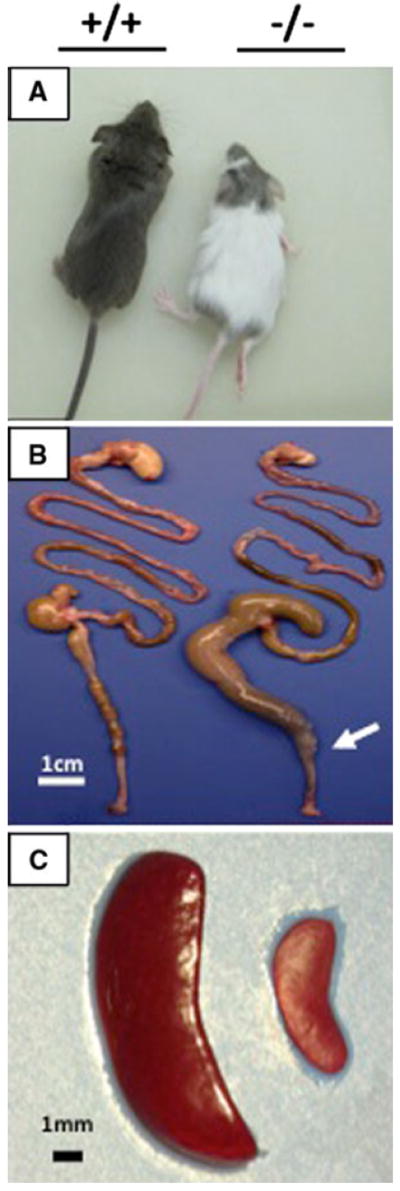
Photographs of the Ednrb+/+ and Ednrb−/− mice. a Ednrb+/+ mouse and −/− littermate at 24 days of age. b Representative picture of entire gut showing megacolon and aganglionic segment of Ednrb−/− mouse compared to Ednrb+/+ mouse. Arrow marks the transition zone. c Representative picture showing markedly sizereduced spleen of Ednrb−/− mouse by comparing to Ednrb+/+ littermate
Fig. 2.
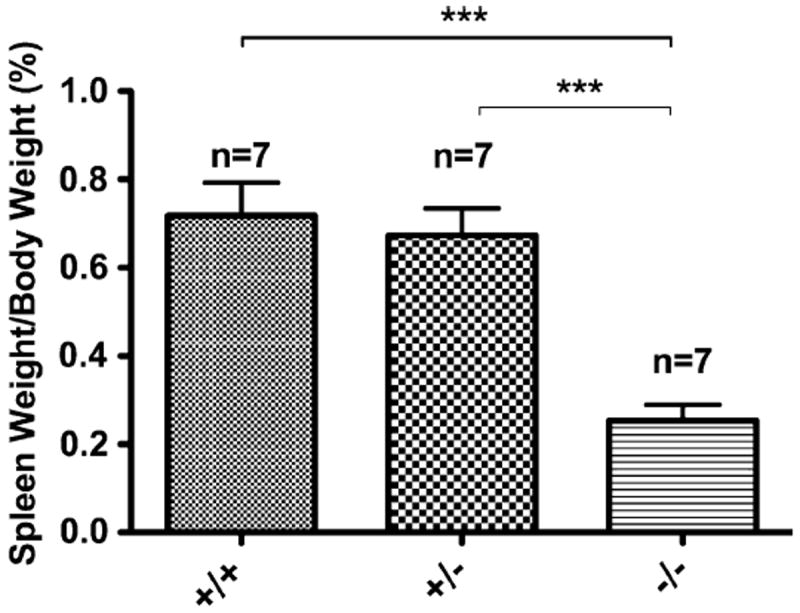
Spleen weight as a percent of body weight for each genotype of Ednrb mice. Seven Ednrb mice of each genotype Ednrb+/+, +/−, and −/− at post-natal day 25 had body weight and wet weight of the spleen measured. The mean Ednrb−/− spleen weight was significantly reduced compared with Ednrb+/+ and +/− (P < 0.0001); there was no difference between the Ednrb+/+ and +/− spleen weight. Groups were compared using student’s t test, data were expressed as mean ± SD. ***P < 0.001
Abnormal splenic architecture in the Ednrb−/− mouse
To evaluate the splenic architecture, we performed hematoxylin and eosin staining of the Ednrb+/+ and −/− mouse spleens and we found significant lymphopenia of the Ednrb−/− spleens along with poorly defined marginal zone (Fig. 3). Immunofluorescence staining for B cells (CD45R) and T cells (CD3) shows markedly abnormal microarchitecture in the Ednrb−/− spleens with a relative reduction of B cells compared with T cells.
Fig. 3.
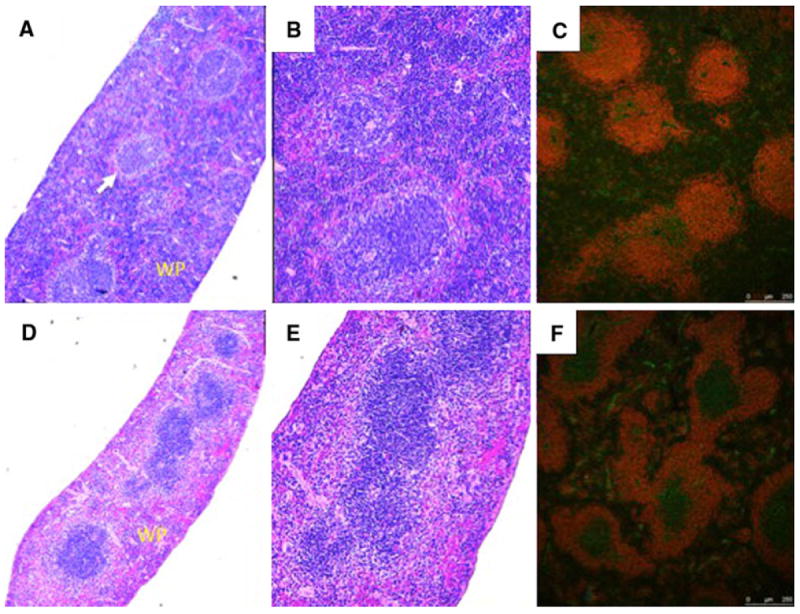
Histology and immunofluorescence staining of Ednrb+/+ and Ednrb−/− spleens. Ednrb+/+ a, b, c (upper panel) and Ednrb−/− d, e, f (lower panel). H and E staining (a, b and d, e); a and d (40×), b and e (100×) show significantly reduced white pulp (WP) with lymphopenia, fewer primary follicles and poorly defined marginal zone (arrow) in the Ednrb−/− (d and e) compared to Ednrb+/+ (a and b). Immunofluorescence staining for CD45R and CD3 of Ednrb+/+ (c) and Ednrb−/− mice (f). CD45R labels B cells red; CD3 labels T cells green
Splenic lymphopenia in the Ednrb−/− mouse
To identify the cell types affected and quantify these differences between wild type and homozygote spleens, FACS was performed on Ednrb+/+ and −/− spleens (n = 4 for each genotype) for CD19+ (mature B cells), CD4+ and CD8+ T cells (Fig. 4). The mean total cell number of the Ednrb+/+ spleens was 43.2 ± 7.6 × 106 compared with a strikingly reduced 1.2 ± 0.2 × 106 for the Ednrb−/− spleens (P < 0.01). FACS analysis showed CD19+, CD4+ and CD8+ cell counts in the Ednrb+/+ animals of 17.8 ± 4.9 × 106, 7.9 ± 1.3 × 106, and 5.8 ± 0.8 × 106, respectively compared with similarly remarkable reductions in the Ednrb−/− animals with 1.5 ± 0.1 × 105, 4 ± 0.5 × 105, 3.1 ± 0.4 × 105, respectively (P < 0.001).
Fig. 4.
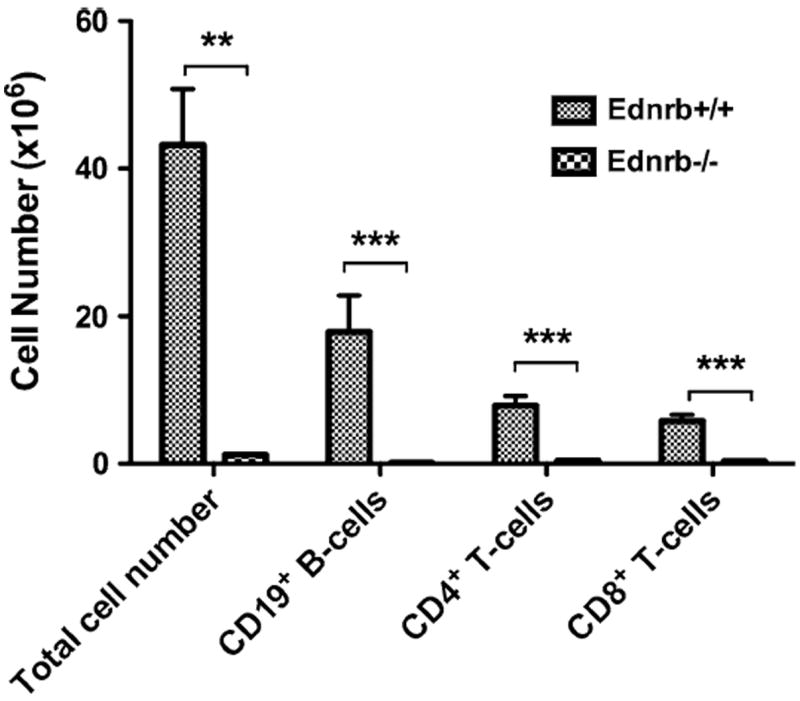
FACS analysis of splenocytes from Ednrb+/+ and −/− mice. Flow cytometry for CD19 (mature B cells), CD4 and CD8 (T cells) from spleens of Ednrb mice (n = 4 per genotype) at 23–25 days of age. Data presented as Mean ± SEM. Comparison between Ednrb+/+ and −/− were compared by using student’s t test. ** and *** represent P < 0.01 and P < 0.001, respectively
Enterocolitis and splenic lymphopenia
We compared total splenocyte counts, CD19+ (mature B cells) and total enterocolitis scores of Ednrb+/+ and Ednrb−/− mice using the Wilcoxon rank-sum test. We found markedly reduced total cell counts and CD19+ cell counts in the Ednrb−/− mice (5.2 ± 7.9 × 106 and 2.3 ± 4.8 × 106, respectively), compared to Ednrb+/+ (32.7 ± 11.8 × 106 and 14.5 ± 7.4 × 106, respectively). However, we also found significantly elevated enterocolitis scores in the Ednrb−/− animals (2.5 ± 2) compared with Ednrb+/+ animals (0.4 ± 0.8). All of the comparisons were significant to at least the P = 0.001 level Table 1. To evaluate if total splenocyte and CD19+ cell count correlated with total enterocolitis scores, Spearman’s rank correlations were performed. We found significant (and identical) inverse correlations of both total cell count and CD19+ cell count with enterocolitis score (rs = −0.43; P = 0.02) (Fig. 5).
Table 1.
Comparison of Ednrb+/+ and Ednrb−/− total splenocyte counts, CD19+ counts and enterocolitis scores
| Ednrb+/+ (n = 11)
|
Ednrb−/− (n = 17)
|
P value | |||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Total cell count (×106) | 32.7 ± 11.8 | 33.6 | 5.2 ± 7.9 | 1.6 | <0.001 |
| CD19 (×106) | 14.5 ± 7.4 | 14.0 | 2.3 ± 4.8 | 0.6 | <0.001 |
| Enterocolitis score | 0.4 ± 0.8 | 0 | 2.5 ± 2.0 | 3 | 0.001 |
The data are expressed as mean ± SD and median. The Wilcoxon rank-sum test was used for tests of significant difference
Fig. 5.
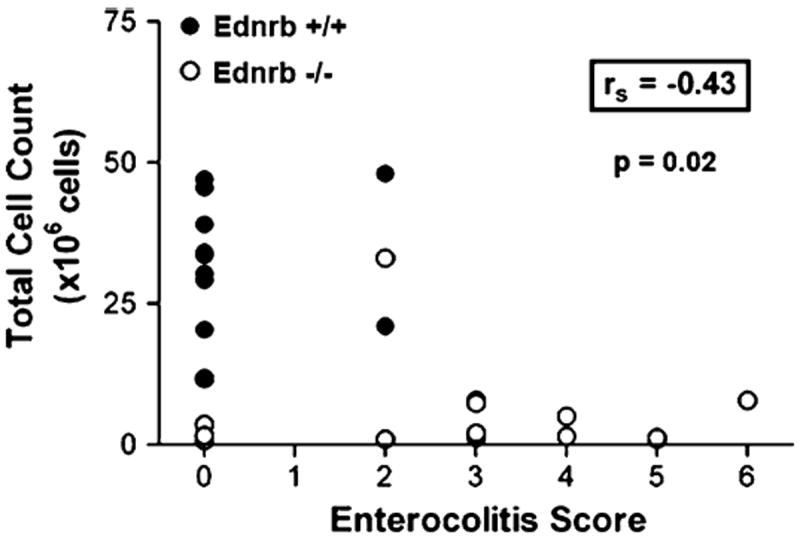
Spearman Rank correlation plot between total enterocolitis score and total splenocyte number of Ednrb+/+ and Ednrb−/− mice. The Spearman Rank correlation is −0.43, significant at P = 0.02
Discussion
Here, we report that mice with genetic deficiency of the endothelin receptor type B display not just colonic aganglionosis that is very similar to clinical HD, but also manifest a profoundly abnormal immunophenotype that is characterized by markedly small spleens, B and T cell splenic lymphopenia, and disordered splenic microarchitecture. These findings suggest that intact Ednrb-dependent signaling is required for normal development of the spleen and perhaps other lymphoid organs. It has been previously shown that even when HD is corrected with a technically successful surgical pull-through procedure, unexplained enterocolitis may occur intermittently or persist for extended periods of time, and can even lead to bacteremia and sepsis [4, 7-9]. Our results now raise the possibility that primary immune abnormalities may contribute to at least some of this enterocolitis. To date, no systematic examination of spleens and other aspects of the immune system have been performed in patients with colonic aganglionoses, but our results provide a basis to now pursue such studies.
A previous study with the piebald lethal spontaneous Ednrb gene mutant mouse report splenitis [10], which would typically be associated with splenomegaly rather than reduced spleen size. Unfortunately, no systematic histopathologic analyzes of spleens accompanied this report. Studies from our laboratory document that in most Ednrb−/− mice, bacteremia occurs, and often progresses to sepsis, which proves lethal [2]. Typical gut species can be cultured from infected kidneys, spleens, and other organs, but despite overwhelming bacteremia, the spleens of the Ednrb−/− mice are markedly reduced in size. This further underscores the importance of the spleen phenotype and the as yet undetermined primary causes that are associated with multiple immune abnormalities, and likely contribute to mortality.
One other genotype that displays a similar reduced spleen size phenotype is the Nkx2-3 knockout mouse [11, 12]. Genetic studies have strongly linked variants of Nkx2-3 with inflammatory bowel disease [13-15], and strong upregulation of Nkx2-3 occurs in Crohn’s disease [16]. Recent studies indicate that Nkx2-3 regulates expression of at least 50 inflammatory and immune response genes. Among the genes regulated by Nkx2-3 is MadCAM, an “addressin” gene that is important for lymphocyte trafficking [17]. However, our studies indicate that there are no differences in gut or spleen expression of MadCAM between Ednrb−/− mice and their wild type littermates (data not shown). Furthermore, colonic aganglionosis does not occur in the Nkx2-3 knockout, suggesting that there may be two independent pathophysiologic processes at work in HAEC: colonic aganglionosis per se, and the dysregulated homeostasis that causes enterocolitis despite successful surgical correction of aganglionosis. Clearly, considerable work is needed to uncover genetic and molecular mechanisms underlying reduced spleen size, immune abnormalities, and enterocolitis. In particular, it will be important to ascertain the clinical relevance of these studies with the Ednrb−/− mice and other genetically altered mouse models. For now, our data suggest intriguing new potential explanations for HAEC.
In conclusion, we have demonstrated that the Ednrb-null mutation manifests not only colonic aganglionsis similar to Hirschsprung’s disease, but also profound immune abnormalties that feature a marked reduction in spleen size that paradoxically persists even when bacteremia and outright sepsis develops. Further studies with the Ednrb−/− and related mutant mice are underway in our lab that may shed important new light on the causes of this phenotype and perhaps, by extension, the factors contributing to clinical HAEC.
Contributor Information
Zhi Cheng, The Pediatric Surgery Laboratory, Division of Pediatric Surgery, Department of Surgery, Cedars-Sinai Medical Center, 8635 W. Third Street Suite 650 W, Los Angeles, CA 90048, USA.
Xiao Wang, The Pediatric Surgery Laboratory, Division of Pediatric Surgery, Department of Surgery, Cedars-Sinai Medical Center, 8635 W. Third Street Suite 650 W, Los Angeles, CA 90048, USA.
Deepti Dhall, Department of Pathology, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Lifu Zhao, The Pediatric Surgery Laboratory, Division of Pediatric Surgery, Department of Surgery, Cedars-Sinai Medical Center, 8635 W. Third Street Suite 650 W, Los Angeles, CA 90048, USA.
Catherine Bresee, The Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Terence M. Doherty, Los Angeles Biomedical Research Institute, Torrance, CA, USA
Philip K. Frykman, Email: Philip.Frykman@cshs.org, The Pediatric Surgery Laboratory, Division of Pediatric Surgery, Department of Surgery, Cedars-Sinai Medical Center, 8635 W. Third Street Suite 650 W, Los Angeles, CA 90048, USA.
References
- 1.Hosoda K, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Z, et al. Murine model of Hirschsprung-associated enterocolitis. I: phenotypic characterization with development of a histopathologic grading system. J Pediatr Surg. 2010;45(3):475–482. doi: 10.1016/j.jpedsurg.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, et al. A novel corrective pullthrough surgery in a mouse model of Hirschsprung’s disease. J Pediatr Surg. 2009;44(4):759–766. doi: 10.1016/j.jpedsurg.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao L, et al. Murine model of Hirschsprung-associated enterocolitis II: surgical correction of aganglionosis does not eliminate enterocolitis. J Pediatr Surg. 2010;45(1):206–211. doi: 10.1016/j.jpedsurg.2009.10.035. discussion 211–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters W, et al. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol. 2004;172(12):7647–7653. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]
- 6.Spearman C. The proof and measurement of association between two things. Amer J Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
- 7.Teitelbaum DH, Coran AG. Enterocolitis. Semin Pediatr Surg. 1998;7(3):162–169. doi: 10.1016/s1055-8586(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 8.Murphy F, Puri P. New insights into the pathogenesis of Hirschsprung’s associated enterocolitis. Pediatr Surg Int. 2005;21(10):773–779. doi: 10.1007/s00383-005-1551-1. [DOI] [PubMed] [Google Scholar]
- 9.Elhalaby EA, et al. Enterocolitis associated with Hirsch-sprung’s disease: a clinical histopathological correlative study. J Pediatr Surg. 1995;30(7):1023–1026. doi: 10.1016/0022-3468(95)90334-8. discussion 1026–1027. [DOI] [PubMed] [Google Scholar]
- 10.Caniano DA, et al. The piebald-lethal murine strain: investigation of the cause of early death. J Pediatr Surg. 1989;24(9):906–910. doi: 10.1016/s0022-3468(89)80593-7. [DOI] [PubMed] [Google Scholar]
- 11.Pabst O, Zweigerdt R, Arnold HH. Targeted disruption of the homeobox transcription factor Nkx2–3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development. 1999;126(10):2215–2225. doi: 10.1242/dev.126.10.2215. [DOI] [PubMed] [Google Scholar]
- 12.Wang CC, et al. Homeodomain factor Nkx2–3 controls regional expression of leukocyte homing coreceptor MAdCAM-1 in specialized endothelial cells of the viscera. Dev Biol. 2000;224(2):152–167. doi: 10.1006/dbio.2000.9749. [DOI] [PubMed] [Google Scholar]
- 13.Parkes M, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39(7):830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher SA, et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet. 2008;40(6):710–712. doi: 10.1038/ng.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke A, et al. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40(6):713–715. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 16.Yu W, et al. Genes regulated by Nkx2–3 in siRNA-mediated knockdown B cells: implication of endothelin-1 in inflammatory bowel disease. Mol Genet Metab. 2010;100(1):88–95. doi: 10.1016/j.ymgme.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Yu W, et al. Genes regulated by Nkx2-3 in sporadic and inflammatory bowel disease-associated colorectal cancer cell lines. Dig Dis Sci. 2010;55(11):3171–3180. doi: 10.1007/s10620-010-1138-0. [DOI] [PubMed] [Google Scholar]


