Abstract
Background
Drug-related adverse events remain an important cause of morbidity and mortality and impose huge burden on healthcare costs. Routinely collected electronic healthcare data give a good snapshot of how drugs are being used in ‘real-world’ settings.
Objective
To describe a strategy that identifies potentially drug-induced acute myocardial infarction (AMI) from a large international healthcare data network.
Methods
Post-marketing safety surveillance was conducted in seven population-based healthcare databases in three countries (Denmark, Italy, and the Netherlands) using anonymised demographic, clinical, and prescription/dispensing data representing 21,171,291 individuals with 154,474,063 person-years of follow-up in the period 1996–2010. Primary care physicians’ medical records and administrative claims containing reimbursements for filled prescriptions, laboratory tests, and hospitalisations were evaluated using a three-tier triage system of detection, filtering, and substantiation that generated a list of drugs potentially associated with AMI. Outcome of interest was statistically significant increased risk of AMI during drug exposure that has not been previously described in current literature and is biologically plausible.
Results
Overall, 163 drugs were identified to be associated with increased risk of AMI during preliminary screening. Of these, 124 drugs were eliminated after adjustment for possible bias and confounding. With subsequent application of criteria for novelty and biological plausibility, association with AMI remained for nine drugs (‘prime suspects’): azithromycin; erythromycin; roxithromycin; metoclopramide; cisapride; domperidone; betamethasone; fluconazole; and megestrol acetate.
Limitations
Although global health status, co-morbidities, and time-invariant factors were adjusted for, residual confounding cannot be ruled out.
Conclusion
A strategy to identify potentially drug-induced AMI from electronic healthcare data has been proposed that takes into account not only statistical association, but also public health relevance, novelty, and biological plausibility. Although this strategy needs to be further evaluated using other healthcare data sources, the list of ‘prime suspects’ makes a good starting point for further clinical, laboratory, and epidemiologic investigation.
Introduction
Drug-related adverse events remain an important cause of morbidity and mortality and impose a burden on healthcare costs. [1], [2], [3] There is continuous influx of new drugs into the worldwide market, but pre-approval clinical trials are unable to detect rare adverse events and to provide a complete picture of a drug’s safety profile, which evolves over its lifetime on the market. [4], [5], [6] Once a drug is made available outside the limited study population of clinical trials, there are bound to be changes in the circumstances of the drug’s actual clinical use (including exposure of broader population than was included in the clinical trials, off-label indications, concomitant use with other drugs, and dosing regimen changes) which may give rise to previously unobserved adverse effects. Post-marketing surveillance has traditionally been carried out by systematic manual review of spontaneous reports of adverse drug reactions (ADRs). Enormous improvements in computing capabilities have provided opportunities to partially automate detection of potentially drug-induced adverse events and various international initiatives are exploring new approaches to do this, primarily through data mining of electronic healthcare records. [7], [8], [9].
Electronic healthcare data, collected in the course of actual clinical practice by physicians or of healthcare utilisation by insurers and health maintenance organisations, give a good snapshot of how drugs are being used in ‘real-world’ settings. Being routine by-products of the healthcare delivery system, the use of such data offers the advantage of efficiency in terms of time, manpower, and financial costs needed to investigate patient safety issues. While the advantages of automated surveillance are obvious, there are growing concerns that such data mining may generate more signals than can be followed up effectively with currently available resources. This concern is not entirely unfounded, considering that the annual volume of reports received in spontaneous reporting systems (SRS), database systems primarily designed for signal detection, has become enormous and unmanageable. [10], [11] The problem is likely to be worse with the use of EHR data which have been intended for other purposes and which can be mined for associations without routine human evaluation of potential alternative explanations.
Detection of safety signals is only the initial step in the long and complex process of post-marketing safety surveillance. The evaluation of a signal may take years, from the earliest suspicion of a potential risk to an established mechanism of causation and fully understood phenomenon. [12] While signals derived from EHR data may give a good snapshot of how drugs are being used in real-world settings, there remains the need to establish guidelines as to when - and how - to consider a safety signal likely to be substantial enough to warrant verification and follow-up. Various strategies for signal prioritisation have been proposed in many publications, although most of these refer to signals derived from SRS. [12], [13], [14], [15], [16] These strategies focus consistently on signals with serious adverse effect, strong supporting evidence, and greatest public health impact.
In this paper we describe findings from post-marketing surveillance using healthcare data of over 20 million individuals from three European countries within the EU-ADR network (http:\www.euadr-project.org). We look at primary care physicians’ medical records which comprise detailed clinical information including patients’ symptoms, physical examination findings, diagnostic test results, and prescribed medications or other interventions. We also look at administrative claims that document reimbursements for filled prescriptions, laboratory and ancillary tests, as well as hospitalisations. Taking the adverse event acute myocardial infarction (AMI) as an example, we describe a strategy for combining evidence from different data sources to identify associations that may represent genuine risk and, hence, necessitate further investigation through formal hypothesis testing studies or action from drug regulatory agencies.
Methods
Data Sources
Identification of ‘prime suspects’ was performed in seven databases of the EU-ADR network [8] for the period 1996–2010: (1) Health Search/CSD LPD (HSD, Italy); (2) Interdisciplinary Processing of Clinical Information (IPCI, Netherlands); (3) Pedianet (Italy); (4) PHARMO Network (PHARMO, Netherlands); (5) Aarhus University Hospital Database (Aarhus, Denmark); (6) Lombardy database (Lombardy, Italy); and (7) Tuscany database (Tuscany, Italy). HSD, IPCI, and Pedianet are primary care/general practitioner (GP) databases, where clinical information and drug prescriptions are recorded. Aarhus, PHARMO, Lombardy, and Tuscany are comprehensive record-linkage systems where drug dispensing data are linked to registries containing hospitalisation and other services. Table 1 provides an overview of the characteristics of each database. All of the databases in EU-ADR have been widely used for pharmacoepidemiologic research, have well-developed safeguard mechanisms ensuring patient data protection, and have been validated for a variety of drug exposures and clinical outcomes. [17], [18], [19], [20], [21], [22], [23] Most healthcare services, including pharmaceutical services, are provided for, or subsidised by, the state in Italy and Denmark and covered by obligatory health insurance in the Netherlands and turnover is low. In all of the countries with GP databases, GPs function as ‘gatekeepers’ of the healthcare system. A more detailed description of the database network can be found in earlier publications. [8], [24] Healthcare data used in this study represent anonymised demographic and healthcare information from 21,171,291 individuals with 154,474,063 person-years of follow-up.
Table 1. Characteristics of the databases in the EU-ADR network.
| CHARACTERISTICS | Pedianet (Italy) | HSD (Italy) | Lombardy Regional (Italy) | Tuscany Regional (Italy) | IPCI (Netherlands) | PHARMO (Netherlands) | QRESEARCH* (UK) | Aarhus (Denmark) |
| Current source population | 160,000 children | 1,500,000 | 9,000,000 | 3,500,000 | 1,500,000 | 3,000,000 | 4,000,000 | 1,800,000 |
| Years covered for this study | 2003–2007 | 2003–2007 | 2003–2005 | 2003–2006 | 1996–2006 | 1998–2007 | 2000–2007 | 2001–2006 |
| Type of database | General Practice pediatric database | General Practice database | Administrative | Administrative | General Practice database | Hybrid(administrativeand medicalrecord/registries) | General Practice database | Administrative |
| Age range | 0–14 | From 15 onwards | All ages | All ages | All ages | All ages | All ages | All ages |
| % Males | 52.2 | 47.2 | 48.8 | 48.1 | 49.6 | 45.8 | 49.6 | 49.9 |
| Demographic information available | ||||||||
| Date of registration | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Date of transferring out | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Date of birth | MM-YY | MM-YY | DD-MM-YY | DD-MM-YY | MM-YY | DD-MM-YY | YY | MM-YY |
| Gender | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ethnicity/Race | No | No | No | No | No | No | No | No |
| Drug information available | ||||||||
| Product coding | MINSAN | MINSAN | MINSAN | MINSAN | HPK | Z index | EMIS | VAerets |
| Active international principlecoding system | ATC | ATC | ATC | ATC | ATC | ATC | BNF | ATC |
| Date of prescription/dispensing | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dosing regimen | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Quantity | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Indication of use | Yes | Yes | No | No | Yes | Yes for in-hospital | No | Yes |
| Outcome information available | ||||||||
| Symptoms (Yes/No) | Yes, as free text/codes | Yes, as freetext/codes | No | No | Yes, as freetext/codes | Yes for some | Yes, as codes | No |
| Outpatient primarycare diagnoses | Yes, as free text/codes | Yes Freetext/codes | No | No | Yes, as freetext/codes | No | Yes | No |
| Outpatient specialistcare diagnoses | Yes, as free text/codes | Yes | No | No | Yes | No | Yes | No |
| Hospital discharge diagnoses | Yes, as free text/codes | Yes, as freetext/codes | Yes | Yes | Yes, as freetext/codes | Yes | Yes | Yes |
| Diagnosis coding scheme | ICD-9CM | ICD-9CM | ICD-9CM | ICD-9CM | ICPC | ICD-9CM | RCD | ICD-10 |
| Diagnostic procedures | Yes | Yes | Yes | Yes | No | Yes for in-hospital interventions | Yes | Yes, in-hospital only |
| Laboratory tests | Yes | Yes | No | No | Yes | Yes subset | Yes | Yes, in-hospital only |
Legend:
ICPC: International Classification of Primary Care.
ICD9-CM: International Classification of Diseases –9th revision Clinical Modification.
RCD: READ CODE Classification.
ICD-10: International Classification of Diseases –10th revision.
MINSAN: Italian Ministry of Health.
NOTE: * QRESEARCH did not contribute data for the analyses described in this paper.
Ethical Approval
The respective Scientific and Ethics committees of each database approved the use of the data for this study. All of the databases in the EU-ADR network adhere to local governance rules regarding the storage of patient data and its use for research and have well-developed safeguard mechanisms ensuring compliance with the European directives and national regulations; no individual written informed consent was required for this study.
Distributed Data Processing
A distributed database network approach was chosen in EU-ADR, allowing database custodians to maintain local control of their data, while reaching the goal of sharing data in a standardised manner. Input data files are created locally and are subsequently managed by purpose-built software called Jerboa©, written entirely in Java™ to ensure that it will run in a wide variety of computational environments. The software queries patient-level data in the different databases, which are later aggregated, de-identified and sent in encrypted format to a central repository for evaluation and further analysis. This repository is managed by the Department of Medical Informatics at Erasmus Medical Center in the Netherlands, the project’s coordinating centre.
Identification and Validation of Cases of Acute Myocardial Infarction
Each of the databases in the EU-ADR network has unique characteristics depending on its primary objective and local function (i.e. administrative claims or medical records) and contains medical information coded according to different (natural) languages and disease terminologies. Potential cases of AMI were identified using search queries that utilised three disease coding terminologies: (1) International Classification of Primary Care (ICPC) for IPCI; (2) International Classification of Diseases 9th revision-Clinical Modification (ICD-9CM) for ARS, HSD, Lombardy, and PHARMO; and (3) ICD-10th revision for Aarhus. To extract the same event across databases, these different terminologies were mapped using the Unified Medical Language System, a biomedical terminology integration system handling more than 150 terminologies. The mapping ensured that AMI was described using a common language. We identified AMI from the databases using an iterative process that included harmonising definitions based on clinical criteria established from literature, using diagnosis codes and free text as well as laboratory findings when available. We inspected differences in event ascertainment by comparing data queries and benchmarking age-specific and standardised incidence rates of the events (direct standardisation was carried out using the WHO World Standard Population). The incidence rates we obtained in EU-ADR are consistent with what has been cited in previous literature. The multi-step process of terminology mapping, harmonisation and benchmarking for the data extraction for AMI (and for four other events) has been described in more detail in earlier publications. [25], [26] We reproduce in Figure S1 (available as supplementary file online) the schematic diagram summarising the harmonisation process of event identification across the databases in EU-ADR.
Case validation by manual review of hospitalisation records and GP records was done in a random subset of the cases. The overall positive predictive value (PPV) for identifying AMI was good, ranging from 75% (ICPC) to 95% (ICD9-CM) to 100% (ICD-10). These findings are consistent with PPV estimates for ICD9-CM and ICD-10 cited in the literature (To date there is no study describing the PPV of ICPC codes or free text search for identifying AMI). [27].
Only the first occurrence of AMI (i.e. incident case) was considered in the analyses; patient time after an AMI was censored.
Drug Exposure
Drug prescription/dispensing data were used to estimate event rates during drug exposure and were assessed according to the Anatomical Therapeutic Chemical (ATC) classification system of the World Health Organization (WHO) (http://www.whocc.no/atc/structure_and_principles/). The duration covered by each prescription or dispensing was estimated according to legend duration (if dosing regimen is available), or otherwise based on the defined daily dose (http://www.whocc.no/ddd/definition_and_general_considera/). Overlapping treatment episodes with the same drug were combined into a single episode of drug use that starts when the first prescription begins and stops when the last prescription ends. When a patient uses more than one drug at a time, the corresponding person-time is labelled accordingly. Events are assigned to the episodes (drug use/non-use) in which they occurred.
Screening for ‘Prime Suspects’
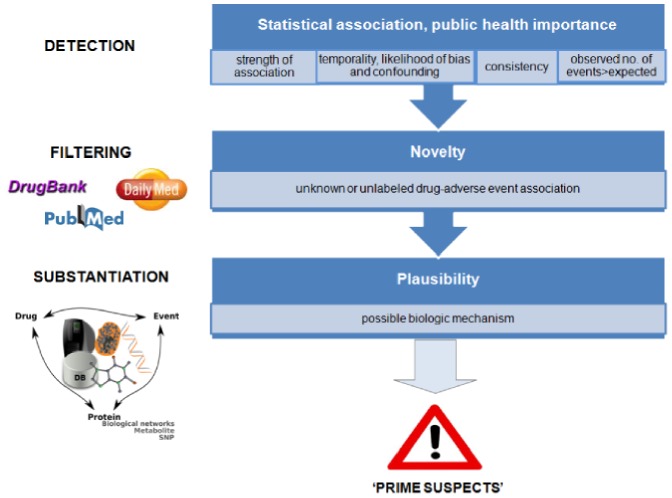
We developed a three-tier triage system (detection, filtering, and substantiation) that generated a list of drugs potentially associated with AMI ( Figure 1 ).
Figure 1. Three-tier triage system (detection, filtering, and substantiation) for detecting ‘prime suspects’.
Strength of statistical association
In the EU-ADR Project we have applied a wide range of statistical methods, including case-based methods (e.g., case control and self-controlled case series), cohort methods, as well as methods developed initially for use in spontaneous ADR reporting systems. We have previously evaluated the relative performance of these methods for detecting known ADRs from EHR data and our findings showed that combinations of methods demonstrate good performance in distinguishing known ADRs from negative controls. [28] Among these methods, the Longitudinal Gamma Poisson Shrinker (LGPS, an adaptation of the GPS, a data mining technique widely used in spontaneous reporting systems to detect potential ADRs) [29] was the best-performing among the methods. We calculated the relative risk, RRLGPS and used this to rank the initial list of ‘prime suspects.’ The results from the different databases were combined to generate a single risk estimate per drug as if the databases together form one large database. We did not perform any meta-analyses. A value of RRLGPS≥2.0 and a lower 95% CI of RRLGPS>1 were used as threshold values for further processing. A more detailed description of LGPS and how the RRLGPS is calculated is given in Appendix S1 (available as supplementary file online).
Alternative explanations for the identified associations: protopathic bias and confounding
Another method, LEOPARD (Longitudinal Evaluation of Observational Profiles of Adverse events Related to Drugs), developed in EU-ADR, attempts to single out associations that may be detected because the drug is used to treat the event, or a prodrome of the event, rather than cause it (protopathic bias). [29] For every suspect drug, LEOPARD compares the rates of prescription starts within a fixed window (±25 days) before and after the event. An increase in the number of prescriptions after an event relative to number of prescriptions before the event is taken to be an indication of protopathic bias. All drug-related AMI flagged by LEOPARD as possibly due to protopathic bias were eliminated from the list. To account for possible confounding, we further sorted out the list and considered only associations that had significant increased risk estimates based on the matched case-control method (lower 95% CI of exposure odds ratio (OR)>1) or the self-controlled case series (SCCS) (lower 95% CI of incidence rate ratio (IRRSCCS)>1). In the case control method, each case was matched to two controls of same age, sex, and index date (i.e. date of AMI). To adjust for co-morbidity and global patient health status, we used as proxy the number of different drugs an individual was exposed to within the period one year and one month prior to index date. We also employed the SCCS method which controls for time-fixed confounders such as genetic factors, socio-economic status, individual frailty, and severity of underlying disease.
Public health importance
To quantify the public health impact of potentially drug-induced AMI, we used as surrogate the number of excess cases of patients exposed to the drug relative to the background unexposed population (observed – expected).
Automated Filtering and Substantiation of Signals
We have developed in the EU-ADR Project a web-based platform that allows systematic analysis of potential safety signals through several distributed software, streamlined into a single computational workflow (https://bioinformatics.ua.pt/euadr). The entry point of the system is a potential drug safety signal, which is composed of a drug and its associated adverse event (in this case AMI). Both signal filtering and substantiation are carried out using dedicated bioinformatics methods integrated into processing pipelines by means of Taverna, an open source workflow management system used to design and execute scientific workflows and aid in silico experimentation. We provide in Figure S2 (available as supplementary file online) a schematic representation of the web platform set up. A more comprehensive description of the EU-ADR web platform can be found in other publications. [30], [31].
Novel associations
The interest in drug safety surveillance is discovery of phenomenon describing a ‘new potentially causal association, or a new aspect of a known association.’ [32] To discriminate among potentially relevant new and already known associations, we used the abovementioned web platform to assess previous reporting of such drugs with AMI in the biomedical literature and eliminated from the list of ‘prime suspects’ drugs previously reported to be associated with AMI in more than one of three biomedical databases: MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed); DrugBank (http://www.drugbank.ca/); or DailyMed (http://dailymed.nlm.nih.gov/dailymed/about.cfm). The Medline ADR signal filtering workflow automates literature analysis by assessing a list of publications regarding AMI. The algorithm adopts a semantics-based approach that processes Medline annotations looking for particular MeSH terms. This workflow’s output is a direct relationship between AMI and its descriptions in Medline, if present. In addition, there is a signal filtering that identifies co-occurrence of the drug and the event (in this case AMI) in Medline literature (Medline Co-occurrence) or drug databases such as DailyMed (http://dailymed.nlm.nih.gov/) or DrugBank (http://www.drugbank.ca/). The workflows use statistical and text-mining techniques to evaluate drug names, ATC codes and AMI co-occurrences in the indexed resources.
Substantiation for biological plausibility
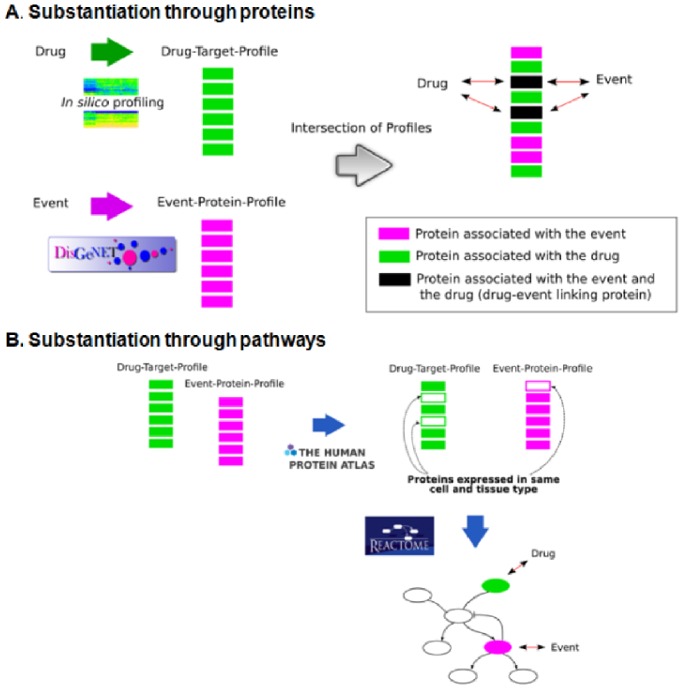
As a final assessment procedure, we retained in the ‘prime suspects’ list only those associations for which a possible biologic mechanism could be found. Automatic linkage of biomedical entities (drugs, proteins and their genetic variants, biological pathways and clinical events) via customised bioinformatics methods was done to find supporting evidence for the ‘prime suspects’ (see Figure 2 ). The associations that passed the screening described above were processed by a computational framework that identifies pair-wise relationships between the drug and AMI based on in silico prediction of drug targets, analysis of drug metabolites and gene-disease associations. [30].
Figure 2. Schematic representation of the process of substantiation of suspected drug-induced adverse events via proteins (A) and via pathways (B).
(From Bauer-Mehren A, van Mulligen EM, Avillach P, Carrascosa Mdel C, Garcia-Serna R, et al. (2012) Automatic filtering and substantiation of drug safety signals. PLoS Comput Biol 8: e1002457. Reproduced with permission from the authors).
Using the above substantiation requirement may preclude finding drugs that induce AMI with mechanisms that cannot be predicted from the drug’s pharmacological action. To account for this type of ADRs, we determined which drugs would remain if we keep those for which the substantiation workflow did not find anything, but passed the novelty requirement. A manual literature search was further performed to determine a logical explanation for these associations.
Results
Identifying ‘Prime Suspects’
Overall, we found 235,283 cases of AMI (both drug-related and non drug-related) during the period 1996–2010, with a background incidence rate of 153.7 per 100,000 person-years. We initially identified 163 drugs possibly associated with AMI. We subsequently flagged, and discarded from the list, 72 drugs as likely being used to treat prodromal symptoms of AMI rather than cause it (i.e. due to protopathic bias). Systemic antibiotics comprised about one-fourth of the suspect drugs (22 drugs out of 91), with the rest involving 14 other therapeutic classes. Adjustment for confounding reduced the number of suspect drugs to 39. The number of excess cases attributable to drug exposure ranged from 18 (for the antibiotic rokitamycin) to 2,445 (for metformin fixed-dose combinations). Table 2 shows the list of suspect drugs that passed preliminary screening, ranked according to a surrogate of public health importance: the number of excess cases.
Table 2. Drugs potentially associated with acute myocardial infarction†.
| Therapeutic class | Drug | RRLGPS(95% CI) | OR(95% CI) | IRRSCCS(95% CI) | No. ofexcess cases |
| Oral hypoglycemic agent | Metformin and sulfonamides | 2.5 (2.4, 2.6) | 1.9 (1.8, 2.0) | 1.5 (1.4, 1.6) | 2,445 |
| Antihypertensive | Nifedipine | 2.1 (2.0, 2.2) | 1.6 (1.6, 1.7) | 1.8 (1.7, 2.0) | 2,097 |
| Systemic corticosteroid | Prednisone | 2.5 (2.4, 2.6) | 1.5 (1.4, 1.6) | 2.2 (1.9, 2.6) | 1,261 |
| β-adrenergic agonist | Salbutamol (systemic) | 2.1 (2.0, 2.2) | 1.2 (1.2, 1.3) | 1.9 (1.6, 2.2) | 1,017 |
| Systemic corticosteroid | Methylprednisolone | 2.3 (2.2, 2.4) | 1.5 (1.3, 1.6) | 2.0 (1.7, 2.3) | 832 |
| Opioid analgesic | Tramadol | 2.1 (2.0, 2.2) | 1.3 (1.2, 1.4) | 2.2 (1.7, 2.8) | 736 |
| Oral hypoglycemic agent | Glibenclamide | 2.2 (2.1, 2.4) | 1.6 (1.6, 1.8) | 1.3 (1.1, 1.6) | 686 |
| Antihypertensive | Clonidine | 2.9 (2.7, 3.1) | 1.8 (1.6, 1.9) | 2.5 (1.9, 3.2) | 650 |
| Systemic antibiotic | Clarithromycin | 3.5 (3.2, 3.7) | 2.4 (2.2, 2.6) | 3.3 (2.8, 3.8) | 645 |
| β-adrenergic agonist | Fenoterol (inhaled) | 2.5 (2.3, 2.6) | 1.4 (1.3, 1.5) | 1.6 (1.1, 2.3) | 588 |
| β-adrenergic agonist | Salbutamol (inhaled) | 2.4 (2.2, 2.6) | 1.3 (1.2, 1.4) | 1.7 (1.4, 2.2) | 510 |
| Systemic antibiotic | Amoxicillin | 2.2 (2.0, 2.3) | 1.6 (1.5, 1.8) | 2.0 (1.8, 2.4) | 497 |
| Systemic corticosteroid | Betamethasone | 2.9 (2.7, 3.2) | 1.7 (1.5, 2.0) | 3.3 (2.6, 4.3) | 365 |
| Antacid | Magaldrate | 2.8 (2.5, 3.0) | 1.9 (1.7, 2.2) | 4.8 (3.9, 5.9) | 365 |
| Systemic antibiotic | Phenoxymethylpenicillin | 3.6 (3.3, 4.0) | 2.6 (2.3, 2.9) | 3.8 (3.0, 4.9) | 335 |
| Systemic corticosteroid | Dexamethasone | 3.2 (2.9, 3.5) | 1.9 (1.7, 2.2) | 5.4 (4.1, 7.2) | 285 |
| Antacid | Combinations of aluminum,magnesium, or calcium salts | 3.1 (2.8, 3.5) | 1.9 (1.6, 2.2) | 4.4 (3.3, 5.7) | 265 |
| Opioid analgesic | Fentanyl | 2.5 (2.3, 2.8) | 1.2 (1.1, 1.4) | 2.1 (1.2, 3.9) | 249 |
| Antiemetic/gastric prokinetic | Metoclopramide | 5.7 (5.1, 6.4) | 2.6 (2.2, 3.1) | 8.9 (5.1, 15.6) | 236 |
| Antiemetic/gastric prokinetic | Domperidone | 2.8 (2.5,3.1) | 1.6 (1.4, 1.8) | 3.1 (2.4, 4.0) | 229 |
| Systemic antibiotic | Azithromycin | 2.8 (2.5, 3.2) | 1.7 (1.5, 2.1) | 2.5 (1.8, 3.5) | 159 |
| Systemic antibiotic | Pivampicillin | 4.5 (3.9, 5.2) | 3.1 (2.6, 3.7) | 3.6 (2.1, 6.1) | 156 |
| Systemic antibiotic | Ceftriaxone | 8.2 (7.0, 9.4) | 5.2 (2.1, 13.1) | 5.6 (2.8, 11.0) | 154 |
| Nonsteroidalanti-inflammatory drug | Ketorolac | 4.6 (3.9, 5.3) | 2.7 (2.1, 3.4) | 2.8 (1.7, 4.7) | 135 |
| Other anti-anemic | Darbepoetin alfa | 3.3 (2.8, 3.8) | 1.7 (1.4, 2.1) | 3.2 (1.8, 5.6) | 126 |
| Systemic antibiotic | Cefixime | 3.1 (2.6, 3.6) | 2.4 (1.9, 3.1) | 4.4 (3.1, 6.2) | 104 |
| Systemic antibiotic | Roxithromycin | 3.3 (2.8, 3.9) | 2.4 (1.9, 3.0) | 2.9 (1.8, 4.9) | 89 |
| Opioid analgesic | Ketobemidone and antispasmodics | 2.2 (1.9, 2.6) | 1.2 (1.0, 1.5) | 2.6 (1.2, 5.7) | 78 |
| Systemic antibiotic | Dicloxacillin | 2.6 (2.2, 3.1) | 1.8 (1.5, 2.2) | 2.5 (1.2, 5.2) | 73 |
| Antiemetic/gastric prokinetic | Cisapride | 2.1 (1.8, 2.5) | 1.2 (1.0, 1.5) | 2.4 (1.6, 3.6) | 69 |
| Antineoplastic/immunomodulator | Azathioprine | 2.1 (1.7, 2.4) | 1.2 (1.1, 1.5) | 3.4 (1.9, 6.1) | 69 |
| Oral hypoglycemic agent | Gliquidone | 2.7 (2.2, 3.2) | 2.1 (1.6, 2.7) | 2.2 (1.2, 4.0) | 66 |
| Systemic antibiotic | Erythromycin | 3.7 (3.0, 4.6) | 2.6 (1.9, 3.4) | 2.4 (1.1, 5.1) | 63 |
| Systemic antifungal | Fluconazole | 2.7 (2.2, 3.3) | 1.5 (1.2, 2.0) | 2.2 (1.2, 4.4) | 53 |
| Phosphate binder | Polystyrene sulfonate | 4.8 (3.6, 6.4) | 2.2 (1.5, 3.1) | 3.3 (1.1, 10.3) | 48 |
| Antiemetic/gastric prokinetic | Butylscopolamine | 5.8 (4.2, 7.7) | 2.1 (1.4, 3.4) | 11.3 (5.0, 25.8) | 45 |
| Antineoplastic/immunomodulator | Megestrol | 3.2 (2.5, 4.0) | 2.5 (1.8, 3.4) | 4.0 (1.8, 9.3) | 44 |
| Systemic antibiotic | Ceftibuten | 2.3 (1.8, 3.0) | 1.9 (1.3, 2.7) | 3.0 (1.7, 5.2) | 31 |
| Systemic antibiotic | Rokitamycin | 2.6 (1.8, 3.7) | 1.8 (1.1, 3.0) | 4.3 (2.3, 8.0) | 18 |
Filtering and Substantiation to Determine Novelty and Plausibility of Associations
Out of the 39 drugs that passed initial screening, only 11 are previously known from literature to be associated with AMI. After applying criteria for both novelty and plausibility, we arrived at nine ‘prime suspects’: the systemic macrolide antibiotics erythromycin roxythromycin, and azithromycin; the gastric prokinetic agents metoclopramide, cisapride, and domperidone; the antifungal fluconazole; and the steroidal drugs betamethasone and megestrol acetate (see Table 3 ).
Table 3. Table 3. ‘Prime suspects’: drugs potentially associated with increased risk of acute myocardial infarction which passed the filtering (i.e. novelty) and substantiation (i.e. biological plausibility) criteria.
| Drugs that satisfied both novelty and plausibility criteria | Drugs that satisfied only novelty criterion |
| Metoclopramide | Combinations of aluminum, magnesium, and calcium salts |
| Cisapride | Magaldrate |
| Domperidone | Butylscopolamine |
| Betamethasone | Gliquidone |
| Erythromycin | Metformin combinations with sulfonamides |
| Roxithromycin | Methylprednisolone |
| Azithromycin | Pivampicillin |
| Fluconazole | Phenoxymethylpenicillin |
| Megestrol acetate | Dicloxacillin |
| Ceftriaxone | |
| Cefixime | |
| Ceftibuten | |
| Rokitamycin | |
| Azathioprine | |
| Ketobemidone and antispasmodics | |
| Fenoterol (inhaled) | |
| Salbutamol (inhaled) | |
| Polystyrene sulfonate |
Second Look at ‘Prime Suspects’: Idiosyncratic Reactions
Consideration of associations not substantiated by a known biologic mechanism increased the number of ‘prime suspects’ to 27 ( Table 3 ). Butylscopolamine is another prokinetic drug; methylprednisolone is another corticosteroid; while pivampicillin, phenoxymethylpenicillin, dicloxacillin, ceftriaxone, cefixime, ceftibuten, and rokitamycin are all β-lactam antibiotics except for the last one, which is a macrolide. Other drugs include the bronchodilators fenoterol and salbutamol, antacids, the opioid ketobemidone, and the phosphate binder polysterene sulfonate.
Discussion
We have described a strategy that identifies and prioritises potentially drug-induced acute myocardial infarction from routinely collected healthcare data. We attempted to simulate how a physician or drug regulator would go about evaluating suspected drug-induced events. This is the first triage strategy for safety surveillance developed for use – and tested – in data from electronic healthcare records. In this strategy, we take into account public health relevance, novelty, and biological plausibility in addition to statistical association. Stepwise exclusion of alternative causes is part of an etiology-based approach for the assessment of ADRs. [33], [34] While usually inherent in physician-reported ADRs, such is not the case with associations obtained from secondary healthcare data (particularly with insurance/administrative claims), which are inferred outside the actual physician-patient encounter. We tried to offset this limitation by adjusting for bias and confounding. The mechanisms behind most ADRs are still not completely understood, but accumulating evidence over the years indicate the interplay of various factors and increasing role of inter-individual genetic variants in genes encoding drug-metabolising enzymes and drug target genes. [35] The triage strategy we developed takes into account various pathways that can lead to a plausible explanation of the identified associations.
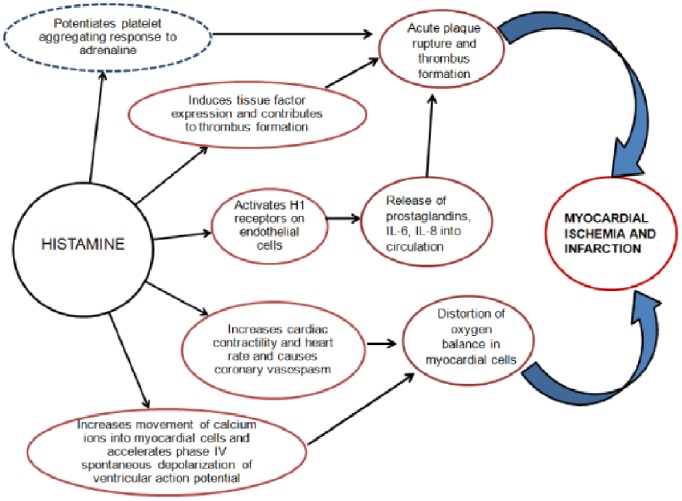
Because drugs belonging to the same class often have a similar pharmacological mechanism of therapeutic action and adverse effects, [36], [37] we assumed that associations involving drugs of the same class may require more thorough investigation: systemic antibiotics comprised about 25% of the initial list of suspect drugs. The proposed mechanism underlying this association is via allergic angina progressing to AMI. The occurrence of chest pain and allergic-anaphylactic reaction, accompanied by clinical and laboratory findings of classical angina pectoris, is caused by inflammatory mediators released during an allergic insult and constitutes the so-called Kounis syndrome. [38], [39] Several studies have shown that β-lactam antibiotics may cause allergic reactions and initiate acute coronary syndrome in hypersensitive individuals. Clinical manifestations of Kounis syndrome, including electrocardiographic findings, are similar to AMI. Kounis syndrome is largely attributed to the action of cardiac mast cells found in the coronary artery intimal layer and atherosclerotic plaques; it has been demonstrated that the density of mast cells in the culprit atheroma of patients who died from AMI was 200 times higher than the density in normal coronary vessels from the same patients. [40] These mast cells become activated during the allergic reaction and release endogenous mediators, including histamine, leukotrienes, thromboxane, platelet activation factor, tryptase, chymase, and rennin - all of which affect different receptors on the coronary vessel wall that may result in AMI. [41] Histamine, the main amine released during allergic reactions, plays a central role in the development of allergic AMI (see Figure 3 ). The effects of histamine on cardiac function, including increased cardiac contractility and heart rate as well as coronary vasospasm, are mediated via H1- and H2- receptors situated on the cardiac chambers and coronary arteries. In addition to direct coronary vasoconstriction and thrombus generating effects, histamine also potentiates the platelet aggregating response to adrenaline. Kounis syndrome has previously been described with use of penicillin, ampicillin, amoxicillin, cefuroxime, cefoperazone, and cefoxitin. [41] To date, there have been no reports in the literature associating macrolide antibiotics with the Kounis syndrome. It is, possible, however, that macrolides induce coronary vasopasm via the same mechanism as that of the β-lactams. [42], [43] Immediate-type hypersensitivity (i.e. anaphylaxis), non-immediate reactions like fixed drug eruptions, toxic epidermal necrolysis and leukocytoclastic vasculitis have been reported with the use of macrolides. [42], [44] Oral contraceptive use in women and recreational drug use with cocaine are the main culprits usually implicated when AMI occurs in a young patient with no clinically evident coronary artery disease (CAD) or other known cardiovascular risk factors. [45] With recent literature implicating Kounis syndrome in drug-eluting stent thrombosis, [46] there is good reason to believe that antibiotic-associated Kounis syndrome is a condition that clinicians need to be more aware of. Although the possibility of channeling bias in the association between macrolides and AMI cannot be discounted (i.e. preferential use of macrolide antibiotics in those patients who may be at higher risk for developing hypersensitivity to β-lactams and, consequently, at risk for developing Kounis syndrome), this association deserves further investigation.
Figure 3. Central role of histamine in drug-induced acute myocardial infarction via Kounis syndrome.
Aside from its direct vasoconstricting and thrombus-generating effects, histamine also potentiates the platelet aggregating response to adrenaline (dotted outline).
Among the gastric prokinetic drugs, cisapride has the most well characterised cardiac adverse effect profile, which includes ventricular arrhythmia, QT prolongation and torsades de pointes. [47], [48], [49] Both metoclopramide and domperidone have also been reported to have arrythmogenic potential. [50] The effects of these drugs on the cardiovascular system are related to their action on dopaminergic and 5-HT receptors; this could be the same mechanism that predisposes to myocardial ischemia or infarction, although how this may happen is yet unclear. [51].
Long-term use of some drugs may increase risk for AMI by accelerating development of atherosclerosis and CAD. Any drug that alters the modifiable risk factors for CAD (e.g., cigarette smoking, elevated plasma low-density lipoprotein cholesterol, reduced plasma high-density lipoprotein cholesterol, hypertension, obesity, and diabetes) [52] has the potential to increase the risk of AMI. Lipodystrophy, weight gain, and hypertension are known corticosteroid-induced adverse effects. [53] Hyperlipidemia is usually associated with long-term corticosteroid use and cases of AMI with use of systemic corticosteroids have also been documented. [54] In a Danish study of patients with out-of-hospital cardiac arrest, use of corticosteroids, bronchodilators, and antipsychotics were found to have the strongest association up to 30 days before the event. [55] Moreover, corticosteroids are used in patients with systemic lupus erythematosus (SLE), psoriasis, and other rheumatologic diseases - accelerated atherosclerosis and premature CAD are recognised complications of these disorders, although the exact etiology remains unclear and is likely to be multifactorial. [56], [57] Megestrol acetate, a progesterone derivative used for hot flushes and for palliative treatment of hormone-dependent malignant neoplasms, may predispose to AMI via its effects on known cardiovascular risk factors: weight gain, hypertension, and hyperglycemia or diabetes mellitus occur with use of megestrol via glucocorticoid action-mediated increased peripheral insulin resistance, especially with long-term use. [58], [59], [60] Fluconazole has been associated with cardiac adverse effects including QT prolongation and torsades de pointes, [61], [62] but not with myocardial ischemia or infarction. Another drug belonging to the same class, itraconazole, has been described as causing a negative inotropic effect resulting in hypertension, hypokalemia, and edema (congestive heart failure). [63], [64] The product label of itraconazole has been changed to include a warning to avoid administration to patients with evidence, or history, of heart failure (http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a4d555fa-787c-40fb-bb7d-b0d4f7318fd0). Azole antifungals may trigger AMI in those already at risk by modifying lipid profile, an important determinant of cardiovascular risk. The product label of fluconazole indicates that there have been post-marketing reports of both hypercholesterolemia and hypertriglyceridemia with fluconazole use (http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f694c617-3383-416c-91b6-b94fda371204). Drug-drug interactions may also play a role in the development of AMI, especially in high-risk patients who are taking multiple cardiac drugs: all the azole antifungals inhibit CYP450 enzymes to some degree and may predispose to adverse cardiac complications, including rhythm problems and ischemia or infarction. [65], [66].
There are many recognised ADRs which cannot be predicted from a drug’s pharmacological action and whose mechanisms remain unclear and have yet to be elucidated. [67], [68] We looked at novel associations which were not obviously explained by the drug’s pharmacology. Doing away with the substantiation requirement, however, yielded drugs that are similar to those already described.
Strengths and Limitations
We took into account global health status and co-morbidities, but residual confounding cannot be ruled out. Dose-response relationships, carryover effects, and effect of concomitant use of other drugs (including drug-drug interactions) were not considered in this triage strategy. Many new molecular entities are introduced into the market every year and databases that catalog the pharmacology and toxicology of these drugs (including information on molecular targets and gene associations) need to be continually updated. Furthermore, many of these bioinformatics databases may not be publicly available and hence not easily verifiable. Automated filtering and substantiation streamlined the triage and greatly reduced manual work, but full automation is still not possible at this time. Manual verification of the output produced by these workflows, in terms of both accuracy and completeness, remains a crucial step. Finally, safety surveillance for ‘prime suspects’ in electronic healthcare data is, by definition, a hypothesis-generating exercise. Formal clinical and epidemiologic studies to investigate the associations identified by the triage system as necessitating follow-up are obvious and necessary next steps.
Conclusions
We have proposed a strategy to identify potentially drug-induced acute myocardial infarction using electronic healthcare records that takes into account not only statistical association, but also public health relevance, novelty, and biological plausibility. Although this strategy needs to be further evaluated using other healthcare data sources, the list of ‘prime suspects’ makes a good starting point for further clinical, laboratory, and epidemiologic investigation.
Supporting Information
Iterative process of harmonising event definitions and queries across the different databases in EU-ADR.
(DOC)
EU-ADR web platform set up.
(DOC)
Description of Longitudinal Gamma Poisson Shrinker (LGPS).
(DOC)
Acknowledgments
The contributions of all partners of the EU-ADR Consortium during the many feedback sessions and discussions about this paper are hereby acknowledged.
Funding Statement
This research has been funded by the European Commission’s Seventh Framework Programme (FP7/2007–2013) under grant no. 215847–The EU-ADR Project. The funding agency had no role in the design and conduct of the study; or in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- 1. Budnitz DS, Lovegrove MC, Shehab N, Richards CL (2011) Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 365: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 2. Hug BL, Keohane C, Seger DL, Yoon C, Bates DW (2012) The costs of adverse drug events in community hospitals. Jt Comm J Qual Patient Saf 38: 120–126. [DOI] [PubMed] [Google Scholar]
- 3. Wu TY, Jen MH, Bottle A, Molokhia M, Aylin P, et al. (2010) Ten-year trends in hospital admissions for adverse drug reactions in England 1999–2009. J R Soc Med 103: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black N (1996) Why we need observational studies to evaluate the effectiveness of health care. BMJ 312: 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papanikolaou PN, Christidi GD, Ioannidis JP (2006) Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ 174: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ray WA (2003) Population-based studies of adverse drug effects. N Engl J Med 349: 1592–1594. [DOI] [PubMed] [Google Scholar]
- 7. Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, et al. (2009) The new Sentinel Network–improving the evidence of medical-product safety. N Engl J Med 361: 645–647. [DOI] [PubMed] [Google Scholar]
- 8. Coloma PM, Schuemie MJ, Trifiro G, Gini R, Herings R, et al. (2011) Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf 20: 1–11. [DOI] [PubMed] [Google Scholar]
- 9. Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, et al. (2010) Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med 153: 600–606. [DOI] [PubMed] [Google Scholar]
- 10.The Uppsala Monitoring Centre. WHO Global Database Reaches 7 Million.
- 11.US Food and Drug Administration. Reports received and entered into AERS by year.
- 12. Meyboom RH, Lindquist M, Egberts AC, Edwards IR (2002) Signal selection and follow-up in pharmacovigilance. Drug Saf 25: 459–465. [DOI] [PubMed] [Google Scholar]
- 13. Waller P, Heeley E, Moseley J (2005) Impact analysis of signals detected from spontaneous adverse drug reaction reporting data. Drug Saf 28: 843–850. [DOI] [PubMed] [Google Scholar]
- 14. Heeley E, Waller P, Moseley J (2005) Testing and implementing signal impact analysis in a regulatory setting: results of a pilot study. Drug Saf 28: 901–906. [DOI] [PubMed] [Google Scholar]
- 15. Levitan B, Yee CL, Russo L, Bayney R, Thomas AP, et al. (2008) A model for decision support in signal triage. Drug Saf 31: 727–735. [DOI] [PubMed] [Google Scholar]
- 16. Lindquist M (2007) Use of triage strategies in the WHO signal-detection process. Drug Saf 30: 635–637. [DOI] [PubMed] [Google Scholar]
- 17. Mazzaglia G, Mantovani LG, Sturkenboom MC, Filippi A, Trifiro G, et al. (2005) Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens 23: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 18. Vlug AE, van der Lei J, Mosseveld BM, van Wijk MA, van der Linden PD, et al. (1999) Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med 38: 339–344. [PubMed] [Google Scholar]
- 19. Sturkenboom M, Nicolosi A, Cantarutti L, Mannino S, Picelli G, et al. (2005) Incidence of mucocutaneous reactions in children treated with niflumic acid, other nonsteroidal antiinflammatory drugs, or nonopioid analgesics. Pediatrics 116: e26–33. [DOI] [PubMed] [Google Scholar]
- 20. Christensen S, Riis A, Norgaard M, Thomsen RW, Sorensen HT (2007) Introduction of newer selective cyclo-oxygenase-2 inhibitors and rates of hospitalization with bleeding and perforated peptic ulcer. Aliment Pharmacol Ther 25: 907–912. [DOI] [PubMed] [Google Scholar]
- 21. Goettsch WG, de Jong RB, Kramarz P, Herings RM (2007) Developments of the incidence of osteoporosis in The Netherlands: a PHARMO study. Pharmacoepidemiol Drug Saf 16: 166–172. [DOI] [PubMed] [Google Scholar]
- 22. Corrao G, Zambon A, Conti V, Nicotra F, La Vecchia C, et al. (2008) Menopause hormone replacement therapy and cancer risk: an Italian record linkage investigation. Ann Oncol 19: 150–155. [DOI] [PubMed] [Google Scholar]
- 23. Barchielli A, Balzi D, Marchionni N, Carrabba N, Margheri M, et al. (2007) Early discharge after acute myocardial infarction in the current clinical practice. Community data from the AMI-Florence Registry, Italy. Int J Cardiol 114: 57–63. [DOI] [PubMed] [Google Scholar]
- 24. Coloma PM, Trifiro G, Schuemie MJ, Gini R, Herings R, et al. (2012) Electronic healthcare databases for active drug safety surveillance: is there enough leverage? Pharmacoepidemiol Drug Saf 21: 611–621. [DOI] [PubMed] [Google Scholar]
- 25. Avillach P, Joubert M, Thiessard F, Trifiro G, Dufour JC, et al. (2010) Design and evaluation of a semantic approach for the homogeneous identification of events in eight patient databases: a contribution to the European EU-ADR project. Stud Health Technol Inform 160: 1085–1089. [PubMed] [Google Scholar]
- 26. Avillach P, Coloma PM, Gini R, Schuemie M, Mougin F, et al. (2013) Harmonization process for the identification of medical events in eight European healthcare databases: the experience from the EU-ADR project. J Am Med Inform Assoc 20: 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coloma PM, Valkhoff VE, Mazzaglia G, Nielsson MS, Pedersen L, et al.. (2013) Identification of acute myocardial infarction from electronic healthcare records using different disease coding systems: a validation study in three European countries. BMJ Open 3. [DOI] [PMC free article] [PubMed]
- 28.Schuemie MJ, Coloma PM, Straatman H, Herings RM, Trifiro G, et al.. (2012) Using Electronic Health Care Records for Drug Safety Signal Detection: A Comparative Evaluation of Statistical Methods. Med Care. [DOI] [PubMed]
- 29. Schuemie MJ (2011) Methods for drug safety signal detection in longitudinal observational databases: LGPS and LEOPARD. Pharmacoepidemiol Drug Saf 20: 292–299. [DOI] [PubMed] [Google Scholar]
- 30. Bauer-Mehren A, van Mullingen EM, Avillach P, Carrascosa Mdel C, Garcia-Serna R, et al. (2012) Automatic filtering and substantiation of drug safety signals. PLoS Comput Biol 8: e1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliveira JL, Lopes P, Nunes T, Campos D, Boyer S, et al. (2013) The EU-ADR Web Platform: delivering advanced pharmacovigilance tools. Pharmacoepidemiol Drug Saf 22: 459–467. [DOI] [PubMed] [Google Scholar]
- 32. Hauben M, Aronson JK (2009) Defining ‘signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf 32: 99–110. [DOI] [PubMed] [Google Scholar]
- 33. Hill AB (1965) The Environment and Disease: Association or Causation? Proc R Soc Med 58: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caster O, Edwards IR (2010) Reflections on attribution and decisions in pharmacovigilance. Drug Saf 33: 805–809. [DOI] [PubMed] [Google Scholar]
- 35. Gurwitz D, Motulsky AG (2007) ‘Drug reactions, enzymes, and biochemical genetics’: 50 years later. Pharmacogenomics 8: 1479–1484. [DOI] [PubMed] [Google Scholar]
- 36. Stricker BH, Psaty BM (2004) Detection, verification, and quantification of adverse drug reactions. BMJ 329: 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Evans M, Rees A (2002) Effects of HMG-CoA reductase inhibitors on skeletal muscle: are all statins the same? Drug Saf 25: 649–663. [DOI] [PubMed] [Google Scholar]
- 38. Kounis NG, Zavras GM (1991) Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract 45: 121–128. [PubMed] [Google Scholar]
- 39. Biteker M (2010) Current understanding of Kounis syndrome. Expert Rev Clin Immunol 6: 777–788. [DOI] [PubMed] [Google Scholar]
- 40. Kovanen PT, Kaartinen M, Paavonen T (1995) Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 41. Ridella M, Bagdure S, Nugent K, Cevik C (2009) Kounis syndrome following beta-lactam antibiotic use: review of literature. Inflamm Allergy Drug Targets 8: 11–16. [DOI] [PubMed] [Google Scholar]
- 42. Araujo L, Demoly P (2008) Macrolides allergy. Curr Pharm Des 14: 2840–2862. [DOI] [PubMed] [Google Scholar]
- 43. Thong BY (2010) Update on the management of antibiotic allergy. Allergy Asthma Immunol Res 2: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demoly P, Gomes ER (2005) Drug hypersensitivities: definition, epidemiology and risk factors. Eur Ann Allergy Clin Immunol 37: 202–206. [PubMed] [Google Scholar]
- 45. Rubin JB, Borden WB (2012) Coronary heart disease in young adults. Curr Atheroscler Rep 14: 140–149. [DOI] [PubMed] [Google Scholar]
- 46. Chen JP, Hou D, Pendyala L, Goudevenos JA, Kounis NG (2009) Drug-eluting stent thrombosis: the Kounis hypersensitivity-associated acute coronary syndrome revisited. JACC Cardiovasc Interv 2: 583–593. [DOI] [PubMed] [Google Scholar]
- 47. Keller GA, Di Girolamo G (2010) Prokinetic agents and QT prolongation: a familiar scene with new actors. Curr Drug Saf 5: 73–78. [DOI] [PubMed] [Google Scholar]
- 48. Quigley EM (2011) Cisapride: what can we learn from the rise and fall of a prokinetic? J Dig Dis 12: 147–156. [DOI] [PubMed] [Google Scholar]
- 49. Hennessy S, Leonard CE, Newcomb C, Kimmel SE, Bilker WB (2008) Cisapride and ventricular arrhythmia. Br J Clin Pharmacol 66: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Collins KK, Sondheimer JM (2008) Domperidone-induced QT prolongation: add another drug to the list. J Pediatr 153: 596–598. [DOI] [PubMed] [Google Scholar]
- 51. Tonini M, De Ponti F, Di Nucci A, Crema F (1999) Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment Pharmacol Ther 13: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 52. Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, et al. (2007) Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol 49: 1230–1250. [DOI] [PubMed] [Google Scholar]
- 53. Stone N (1994) Secondary causes of hyperlipidemia. Med Clin North Am 78: 117–141. [DOI] [PubMed] [Google Scholar]
- 54. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, et al. (2011) Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther 33: 1413–1432. [DOI] [PubMed] [Google Scholar]
- 55. Weeke P, Folke F, Gislason GH, Lippert FK, Olesen JB, et al. (2010) Pharmacotherapy and hospital admissions before out-of-hospital cardiac arrest: a nationwide study. Resuscitation 81: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 56. Gisondi P, Girolomoni G (2009) Psoriasis and atherothrombotic diseases: disease-specific and non-disease-specific risk factors. Semin Thromb Hemost 35: 313–324. [DOI] [PubMed] [Google Scholar]
- 57. Szekanecz Z, Kerekes G, Der H, Sandor Z, Szabo Z, et al. (2007) Accelerated atherosclerosis in rheumatoid arthritis. Ann N Y Acad Sci 1108: 349–358. [DOI] [PubMed] [Google Scholar]
- 58. Panwalker AP (1992) Hyperglycemia induced by megestrol acetate. Ann Intern Med 116: 878. [DOI] [PubMed] [Google Scholar]
- 59. Henry K, Rathgaber S, Sullivan C, McCabe K (1992) Diabetes mellitus induced by megestrol acetate in a patient with AIDS and cachexia. Ann Intern Med 116: 53–54. [DOI] [PubMed] [Google Scholar]
- 60. Kilby JM, Tabereaux PB (1998) Severe hyperglycemia in an HIV clinic: preexisting versus drug-associated diabetes mellitus. J Acquir Immune Defic Syndr Hum Retrovirol 17: 46–50. [DOI] [PubMed] [Google Scholar]
- 61. Khazan M, Mathis AS (2002) Probable case of torsades de pointes induced by fluconazole. Pharmacotherapy 22: 1632–1637. [DOI] [PubMed] [Google Scholar]
- 62. McMahon JH, Grayson ML (2008) Torsades de pointes in a patient receiving fluconazole for cerebral cryptococcosis. Am J Health Syst Pharm 65: 619–623. [DOI] [PubMed] [Google Scholar]
- 63. Sharkey PK, Rinaldi MG, Dunn JF, Hardin TC, Fetchick RJ, et al. (1991) High-dose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother 35: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ahmad SR, Singer SJ, Leissa BG (2001) Congestive heart failure associated with itraconazole. Lancet 357: 1766–1767. [DOI] [PubMed] [Google Scholar]
- 65. Groll AH, Piscitelli SC, Walsh TJ (1998) Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol 44: 343–500. [DOI] [PubMed] [Google Scholar]
- 66. Brüggemann RJ AJ, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, et al. (2009) Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 48: 1441–1458. [DOI] [PubMed] [Google Scholar]
- 67. Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 68. Park BK, Kitteringham NR, Powell H, Pirmohamed M (2000) Advances in molecular toxicology-towards understanding idiosyncratic drug toxicity. Toxicology 153: 39–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Iterative process of harmonising event definitions and queries across the different databases in EU-ADR.
(DOC)
EU-ADR web platform set up.
(DOC)
Description of Longitudinal Gamma Poisson Shrinker (LGPS).
(DOC)