Abstract
Background
In this study, we hypothesized that higher level of comorbidity and greater body mass index (BMI) may mediate the association between diabetes and access to transplantation.
Methods
We used data from the United States Renal Data System (01/01/2000–24/09/2007) (n=619,151). We analyzed two outcomes using Cox model: 1) time to being placed on the waiting list or transplantation without being listed; 2) time to transplantation after being listed. Two primary Cox models were developed based on different levels of adjustment.
Results
In Cox models adjusted for priori defined potential confounders, history of diabetes was associated with reduced transplant access (compared with non-diabetic population) - both for wait-listing/transplant without being listed [HR 0.80, p < 0.001] and for transplant after being listed [HR 0.72, p < 0.001]. In Cox models adjusted for BMI and comorbidity index along with the potential confounders, history of diabetes was associated with shorter time to wait-listing or transplantation without being listed [HR 1.07, p < 0.001] and there was no significant difference in time to transplantation after being listed [HR 1.01, p = 0.42].
Conclusion
We demonstrated that higher level of comorbidity and greater BMI mediate the association between diabetes and reduced access to transplantation.
Keywords: Body Mass Index, Comorbidities, Diabetes, Pre-transplant evaluation, Renal transplantation access
INTRODUCTION
Diabetes mellitus is the leading cause of end-stage renal disease (ESRD) worldwide[1]. In 2008, nearly 48,000 people with diabetes related ESRD were started on dialysis in the US [2]. Apart from diabetes related ESRD, there is also a high prevalence of associated diabetes in the ESRD population [3, 4]. For suitable ESRD patients, renal transplantation is accepted as the optimal modality of renal replacement therapy, conferring both better quality of life and better life expectancy [5, 6]. This is also true for ESRD patients with diabetes [7, 8].
With a limited supply of donor organs available for transplantation and an increasing ESRD population requiring renal transplantation, there is a need to ensure a fair and equitable system of organ allocation. In order to achieve this, it is important to identify the objective barriers, bias, and disparities among different population groups in regard to transplant access. There is evidence to suggest that African Americans [9–11], females [12–14], and elderly [11, 15] have inferior access to transplantation. Literature also suggests that history of diabetes is associated with inequitable access to transplantation [15–21]. Most of these studies however looked at access to transplantation in population with diabetes mellitus as the primary cause of ESRD (as opposed to diabetes being present in ESRD patients, but not necessarily the cause of the renal failure).
In this large retrospective study, based on a nationally representative sample of US ESRD patients, we tested the hypothesis that diabetes is associated with inferior access to transplantation. We studied all diabetic patients with ESRD, whether or not diabetes caused renal failure. We further aimed to evaluate the factors mediating this association. Specifically, we hypothesized that higher level of comorbidities and greater body mass index (associated with diabetes and not the diabetic status per se) may be the barriers in placement of patients with diabetes on the waiting list and subsequent transplantation.
METHODS
Data Source and Study Population
We analyzed data from the United States Renal Data System (USRDS) including the data directly provided to USRDS by United Network for Organ Sharing (UNOS). Data was used from the Txunos_ki, Waitlist_ki, Rxhist60, Case mix, Adequacy, Patient and Medevid files. The information regarding the diabetic status was obtained from the Patient and Medevid files. Incident and prevalent ESRD patients, with known diabetic status, who were started on dialysis during or after January 1, 2000, through September 24, 2007 were included in the study (n=793,106). Patients of age < 18 years and ≥80 years were not included in the study population. Patients with acute kidney failure who were on dialysis initially but then recovered renal function were excluded from the analysis. With the above exclusions, the final study cohort consisted of 619,151 ESRD patients. Median income based on zip codes and stratified by race was obtained from the US Census Bureau data source and linked to our study cohort.
Primary and Outcome Variables of interest
Diabetic status at the time of onset of ESRD was the primary variable of interest. We analyzed two outcomes using Cox model: (1) time to being placed on the waiting list or transplantation without being listed (whichever occurred first) from the time of dialysis initiation. Some candidates were transplanted without being listed (e.g., recipients of living donor kidneys); in that case we used time to transplantation instead of listing time. (2) time to transplantation (the waiting time between dialysis initiation and transplantation) in the group of patients who were initially placed on the waiting list. For recipients of multiple transplants, the first transplantation was considered to be the transplant of interest.
Cox proportional hazards models and Covariates
Two primary Cox models were developed based on different levels of adjustment. The first model estimated the impact of history of diabetes, standard socio-demographic and ESRD-related covariates on access to transplantation. For this model, covariates included are a priori defined potential confounding factors (Figure 1) - patient age at onset of ESRD, race, sex, geographic location, hemoglobin, serum albumin, eGFR, median income, and duration of pre-ESRD nephrology care. Of the covariates included in the Cox model, some of the patient records had missing information for duration of pre-ESRD nephrology care (n=448,265), serum albumin (n=157,883), hemoglobin (n=54,485), and estimated glomerular filtration rate (eGFR) (n=9,906) at the time of initiation of dialysis. Continuous variables (albumin, hemoglobin, and eGFR) with missing information were converted into categorical variables and missing category was analyzed separately. Similarly, missing information for pre-ESRD nephrology care was analyzed as a separate category.
Figure 1.
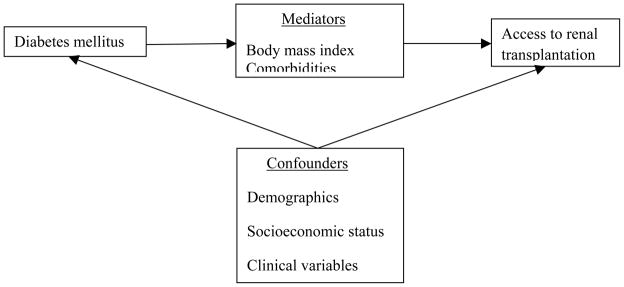
Directed acyclic graph representing association between diabetes and access to transplantation.
To evaluate the mechanism of potential association between the diabetes and access to transplantation, our second model was also adjusted for potential mediators: BMI and comorbidity index (described below). To further dissect the role of BMI and comorbidities as potential mediators, we performed two additional Cox models adjusting for only one of these two factors each time along with the previously defined covariates.
To adjust for patient comorbidities, we formed a comorbidity coefficient similar to the Charlson comorbidity index [22]. Each of the comorbidity conditions available in the dataset (from the CMS form 2728) contributed one point towards the composite index with additional point given for older age. However, since our primary variable of interest is diabetes, we removed diabetes from the comorbidity index calculation to eliminate potential co-linearity. We previously used similar approaches to describe comorbidities using abbreviated comorbidity indices of Davies et al. [23] and Charlson et al.[22] using information available in the data. These abbreviated indices were validated by strong association with clinical outcomes [24, 25].
Statistical analysis
Means and standard deviations were used to summarize the distributions of continuous variables. Categorical variables were summarized as percent of total. To compare between groups, we used analysis of variance for continuous variables and Chi-square for the categorical variables. Cox model was used for time to outcome analysis. As measures of association between diabetic status and outcome variables, we estimated hazard ratios (HR) and 95% confidence intervals. All analyses were performed with SAS software version 9.2 (SAS Institute, Cary, NC)
RESULTS
Descriptive Statistics
We identified 619,151 ESRD patients with available information regarding diabetic status. The study cohort had a mean age of ESRD onset of 60.2±13.7 years. Of the entire study population, 62.3 % were White, 54.9 % were male, and 59.2 % had diabetes mellitus. Of the patients who were eventually transplanted, 42.6 % received it from a living donor. Among the diabetic population who were transplanted, 37.7% received it from a living donor, while it was 45.4% in the non-diabetic population. Distribution of other baseline characteristics of the study population was presented in Table 1.
Table 1.
Baseline characteristics of the study population at the time of ESRD onset.
| Entire study population (n=619,151) | Non-diabetic population (n=252,789) | Diabetic population (n=366,362) | P | |
|---|---|---|---|---|
|
| ||||
| Age at ESRD onset | 60.2(13.7) | 57.9(15.9) | 61.8(11.7) | <0.001 |
|
| ||||
| Age categories (yr) | ||||
|
| ||||
| 18–40 | 9.5 | 15.7 | 5.2 | <0.001 |
| 41–65 | 47.6 | 43.6 | 50.4 | |
| 66–79 | 42.9 | 40.7 | 44.4 | |
|
| ||||
| Race/ethnicity | ||||
| White | 62.3 | 61 | 63.2 | <0.001 |
| African American | 31.3 | 33.3 | 29.8 | |
| Native American | 1.3 | 0.6 | 1.8 | |
| Asian | 3.9 | 3.8 | 3.9 | |
| Other | 1.2 | 1.2 | 1.2 | |
|
| ||||
| Sex | ||||
| Male | 54.9 | 58.3 | 52.5 | <0.001 |
| Female | 45.1 | 41.7 | 47.5 | |
|
| ||||
| Body mass index (kg/m2) | 28.3(7.3) | 26.4(6.7) | 29.4(7.4) | <0.001 |
|
| ||||
| Co-morbidity index | 5.9(2.0) | 5.1(2.1) | 6.4(1.8) | <0.001 |
|
| ||||
| Donor type | ||||
| Cadaveric | 4.8 | 7.1 | 3.2 | <0.001 |
| Living | 3.6 | 5.9 | 2.0 | |
| Missing (i.e. Patients not transplanted) | 91.6 | 87 | 94.8 | |
|
| ||||
| Hemoglobin categories (g/dl) | ||||
| ≤ 10 | 48.5 | 48.2 | 48.7 | <0.001 |
| > 10 | 42.7 | 42.7 | 42.7 | |
| Missing | 8.8 | 9.1 | 8.6 | |
|
| ||||
| GFR categories (ml/min/1.73m2) | ||||
| < 15 | 86.5 | 90.0 | 84.2 | <0.001 |
| 15 to 30 | 11.8 | 8.2 | 14.3 | |
| Missing | 1.7 | 1.8 | 1.5 | |
|
| ||||
| Albumin Categories (g/dl) | ||||
| < 3.5 | 49.4 | 44 | 53.2 | <0.001 |
| ≥ 3.5 | 25.1 | 30.7 | 25.6 | |
| Missing | 25.5 | 25.3 | 21.2 | |
|
| ||||
| Duration of Pre-ESRD nephrology care | ||||
| No nephrology care | 9.3 | 10.4 | 8.5 | <0.001 |
| Less than 6 months | 3.2 | 3.0 | 3.4 | |
| 6 to 12 months | 7.8 | 6.6 | 8.6 | |
| More than 12 months | 7.3 | 6.8 | 7.5 | |
| Missing | 72.4 | 73.1 | 72.0 | |
|
| ||||
| Geography | ||||
| Rural | 19.3 | 18.1 | 20.2 | <0.001 |
| Urban | 78.3 | 79.9 | 77.1 | |
| Unknown | 2.4 | 2.0 | 2.7 | |
|
| ||||
| Median income (US $ per annum) | 39914.1 (16680) | 40591.35 (17317.85) | 39445.35 (16207.34) | <0.001 |
Continuous variables presented as mean (standard deviations) and categorical variables presented as percent of total. ESRD- End Stage Renal Disease.
Transplant access in the entire study population
In the model adjusted for the priori defined potential confounding factors, but not for comorbidities and BMI (Model 1), history of diabetes was associated with reduced transplant access (compared with non-diabetic population): both for wait-listing/transplant without being listed [HR 0.80, p < 0.001] and for transplant after being listed [HR 0.72, p < 0.001]. In a separate analysis, when proportional hazard models were adjusted for BMI and comorbidity index along with the potential confounding factors (Model 2), patients with diabetes had better access to waiting list or transplantation without being listed [HR 1.07, p < 0.001] but there was no significant difference in time to transplantation after being listed compared to non-diabetics [HR 1.01, p = 0.42] (Table 2). This trend was same in most of the subgroups studied.
Table 2.
The association of diabetic status in ESRD patients with getting listed/transplanted in the entire study population by Cox models1,2
| Outcome: listed/transplanted without being listed | Outcome: Transplant for those who got listed | |||
|---|---|---|---|---|
|
| ||||
| HAZARD RATIO (95% CI) | HAZARD RATIO (95% CI) | |||
| MODEL 1 | MODEL 2 | MODEL 1 | MODEL 2 | |
| Diabetes (compared to non- diabetes) | 0.80(0.79–0.81) | 1.07(1.05–1.09) | 0.72(0.70–0.73) | 1.01(0.99–1.03)* |
|
| ||||
| Age at ESRD onset | 0.95(0.95–0.95) | 0.98(0.98–0.98) | 0.95(0.95–0.95) | 0.98(0.98–0.98) |
|
| ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.83(0.82–0.84) | 0.83(0.82–0.84) | 0.84(0.82–0.85) | 0.84(0.83–0.86) |
|
| ||||
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.77(0.76–0.78) | 0.75(0.74–0.76) | 0.50(0.49–0.51) | 0.50(0.49–0.51) |
| Native American | 0.82(0.77–0.87) | 0.81(0.77–0.86) | 0.60(0.55–0.65) | 0.59(0.54–0.64) |
| Asian | 1.29(1.26–1.33) | 1.21(1.18–1.24) | 0.70(0.67–0.72) | 0.63(0.61–0.66) |
| Other | 0.68(0.65–0.72) | 0.66(0.63–0.70) | 0.46(0.42–0.49) | 0.44(0.41–0.47) |
|
| ||||
| Hemoglobin (g/dl) | ||||
| ≤ 10 | Reference | Reference | ||
| > 10 | 1.15(1.14–1.17) | 1.14(1.13–1.16) | 1.30(1.28–1.32) | 1.29(1.27–1.32) |
| Missing | 1.02(1.00–1.04)* | 1.02(1.00–1.04)* | 1.17(1.14–1.21) | 1.17(1.13–1.20) |
|
| ||||
| Albumin (g/dl) | ||||
| < 3.5 | Reference | Reference | ||
| ≥ 3.5 | 1.46(1.44–1.49) | 1.41(1.38–1.43) | 1.49(1.46–1.53) | 1.44(1.41–1.47) |
| Missing | 1.14(1.13–1.17) | 1.11(1.09–1.13) | 1.14(1.12–1.17) | 1.11(1.09–1.14) |
|
| ||||
| GFR (ml/min/ 1.73m2) | ||||
| < 15 | Reference | Reference | ||
| 15 – 30 | 0.59(0.57–0.61) | 0.62(0.61–0.64) | 0.50(0.57–0.62) | 0.62(0.60–0.65) |
| Missing | 0.71(0.67–0.75) | 0.73(0.70–0.77) | 0.68(0.63–0.74) | 0.71(0.65–0.76) |
|
| ||||
| Duration of pre-ESRD nephrology care | ||||
| No nephrology care | Reference | Reference | ||
| Less than 6 months | 1.82(1.73–1.92) | 1.83(1.74–1.92) | 2.23(2.03–2.45) | 2.22(2.02–2.44) |
| 6 to 12 months | 1.51(1.45–1.57) | 1.52(1.46–1.58) | 1.92(1.77–2.08) | 1.93(1.78–2.08) |
| More than 12 months | 1.91(1.83–1.99) | 1.95(1.87–2.03) | 2.96(2.75–3.19) | 3.02(2.80–3.25) |
| Missing | 1.35(1.31–1.40) | 1.38(1.34–1.42) | 2.03(1.91–2.16) | 2.05(1.92–2.18) |
|
| ||||
| Geographic location | ||||
| Rural | Reference | Reference | ||
| Urban | 0.99(0.97–1.01)* | 0.97(0.95–0.99)* | 0.86(0.84–0.88) | 0.84(0.82–0.86) |
| Unknown | 0.82(0.77–0.86) | 0.81(0.76–0.85) | 0.77(0.71–.0.83) | 0.75(0.70–0.81) |
|
| ||||
| Median income (US $ per annum) | 1.00(1.00–1.00) | 1.00(1.00–1.00) | 1.00(1.00–1.00) | 1.00(1.00–1.00) |
|
| ||||
| Body mass index (kg/m2) | - | 1.00(0.99–1.00) | - | 0.98(0.98–0.99) |
|
| ||||
| Comorbidity index | - | 0.73(0.73–0.74) | - | 0.72(0.71–0.73) |
Cox model 1 was adjusted for age at ESRD onset, sex, race, history of diabetes, hemoglobin, GFR, Serum albumin, geographic location, median income, and duration of pre-ESRD nephrology care.
Cox model 2 was adjusted for BMI and Comorbidity index in addition to the covariates listed above in model 1.
Note: P value was <0.001 for all the above variables in both the models except i) for diabetes variable for the outcome transplant for those who got listed in the 2nd model (P=0.42), ii) missing category in hemoglobin for the outcome listed/ transplanted without being listed (P=0.12 and 0.18 for model 1 and 2 respectively), and iii) for urban category in geography for the outcome listed/ transplanted without being listed (P=0.22 and 0.002 for model 1 & 2 respectively)
To further dissect the role of BMI and comorbidities as potential mediators, we analyzed two additional Cox models adjusting for only one of these two factors each time. When BMI was included along with the previously defined covariates, the association did not change appreciably compared to Model 1 (HR 0.81, p < 0.001 and HR 0.75, p < 0.001 for listing/transplant without being listed and for transplant after being listed respectively). On the other hand, when comorbidity index was included along with the previously defined covariates, the hazard ratios changed to 1.05 (p < 0.001) for listing / transplant without being listed and to 0.96 (p = 0.004) for transplant after being listed.
Transplant access in Subgroups
We performed subgroup analysis to evaluate the effect of diabetic status in different population subgroups based on age, sex, and race. The results indicate a strong association of diabetic status with transplant access in most of the subgroups studied. When compared to non-diabetic population, diabetic individuals had better or equal access to renal transplantation in most of the sub groups studied after adjusting for the potential mediators (i.e. Cox models were adjusted for BMI and comorbidity index in addition to the potential confounding factors - Model 2). In particular, history of diabetes was associated with better or equal transplant access compared to non-diabetics in males (HR 1.14, p < 0.001 for listing/transplanted without being listed and HR 1.07, p < 0.001 for transplant after being listed), whites (HR 1.02, p = 0.02 for listing/transplanted without being listed and HR 0.97, p = 0.01 for transplant after being listed), and blacks (HR 1.14, p < 0.001 for listing/transplanted without being listed and HR 1.16, p < 0.001 for transplant after being listed). Results for other subgroup analysis were given in Table 3.
Table 3.
Association of diabetic status in ESRD patients with getting listed/transplanted in the entire study population and study groups by Cox model1
| Outcome: listed/transplanted without being listed. | Outcome: transplant for those who got listed. | |||
|---|---|---|---|---|
|
| ||||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Diabetes in the entire study population | 1.07(1.05–1.09) | <0.001 | 1.01(0.99–1.03) | 0.42 |
| Diabetes by age group (yr) | ||||
| 18–40 | 0.94(0.91–0.97) | 0.003 | 1.18(1.13–1.24) | <0.001 |
| 41–65 | 0.96(0.94–0.97) | <0.001 | 0.84(0.82–0.87) | <0.001 |
| 66–80 | 1.21(1.16–1.26) | <0.001 | 0.98(0.92–1.04) | 0.46 |
| Diabetes in males | 1.14(1.11–1.16) | <0.001 | 1.07(1.04–1.10) | <0.001 |
| Diabetes in females | 0.98(0.96–1.01) | 0.13 | 0.93(0.89–0.96) | <0.001 |
| Diabetes in Whites | 1.02(1.00–1.04) | 0.02 | 0.97(0.94–1.00) | 0.01 |
| Diabetes in African Americans | 1.14(1.10–1.17) | <0.001 | 1.16(1.11–1.22) | <0.001 |
| Diabetes in Asians | 1.02(0.95–1.09) | 0.67 | 0.85(0.76–0.95) | 0.003 |
| Diabetes in Native Americans | 1.07(0.92–1.25) | 0.38 | 0.87(0.70–1.08) | 0.20 |
| Diabetes in Others | 1.25(1.09–1.43) | 0.001 | 1.21(0.99–1.49) | 0.06 |
The results shown in the table were derived from 22 separate proportional hazard models, each of them adjusted for the following covariates: age at ESRD onset, race, sex, diabetic status, body mass index, comorbidity index, geographic location, duration of pre-ESRD nephrology care, eGFR, serum albumin, hemoglobin, and median income.
DISCUSSION
Renal transplantation is currently considered the optimal modality of renal replacement therapy for patients with ESRD [26, 27]. Current data indicate better outcomes in patients who receive a transplant early in the course of renal replacement therapy; with each additional year of dialysis therapy, survival is compromised, particularly in the diabetic population [8, 24, 27–30].
In this context, it is important to identify the factors that affect access to transplantation and understand the underlying mechanisms of existing disparities, differences and bias, in order to propose potential interventions to overcome them. One of the critical steps of the transplantation process is the pre-transplant evaluation, which identifies patient’s transplant candidacy based on medical guidelines. However, studies of factors affecting access to renal transplantation, have reported a number of disparities, that may not be totally explained on the basis of medical criteria, including African American patients [9–11], and females [13, 14, 31], having inferior access. Age [11, 32], nephrology referral [33], primary renal disease [11, 34] body mass index [35], and comorbidities [20] have been shown to affect access to renal transplantation. Our team has previously shown that social adaptability index is also associated with access to transplantation [36].
Existing literature suggests that ESRD patients with diabetes are disadvantaged in terms of access to renal transplantation [13, 37]. Alexander et al, in a prospective study on 7125 patients demonstrated that diabetes-related ESRD population had inferior access to wait-listing (OR: 0.73) [18]. Similarly, Wolfe et al studied 228,552 ESRD patients using US data from 1991 to 1997 and demonstrated that the relative rates for wait-listing and transplantation associated with diabetes as a cause of ESRD (compared to glomerulonephritis) were 0.52 and 0.98 respectively [21]. Villar et al., in a study on 549 subjects in France, demonstrated that patients with Type-2 diabetes had inferior rates for pre-transplant evaluation (33.0%) and wait-listing for transplantation (24.2%) compared to the non-diabetic population (65.8 and 60.6%, respectively). The duration of pre-transplant evaluation was significantly longer in patients with type-2 diabetes (12.7±11.0 months) compared to patients without (7.5±7.1). Also, among patients without apparent clear reasons for exclusion from pre-transplant evaluation, patients with type-2 diabetes were twice as likely to be excluded compared to patients without [16]. Similarly, Bayat et al. reported that diabetic patients had less access to wait-listing for transplantation compared to the non-diabetic population (OR 2.52, 95% CI 1.44–4.43) in a French community-based network of care[19]. In addition, the report of a national conference in the U.S on wait-listing for kidney transplantation by Gaston et al documented diabetes as a variable that might delay referral of ESRD patients for renal transplantation [15]. Our results are consistent with the previous reports demonstrating an association between presence of diabetes and inferior access to transplantation. Similarly, we also demonstrated inferior access to transplantation in females, blacks, and elderly.
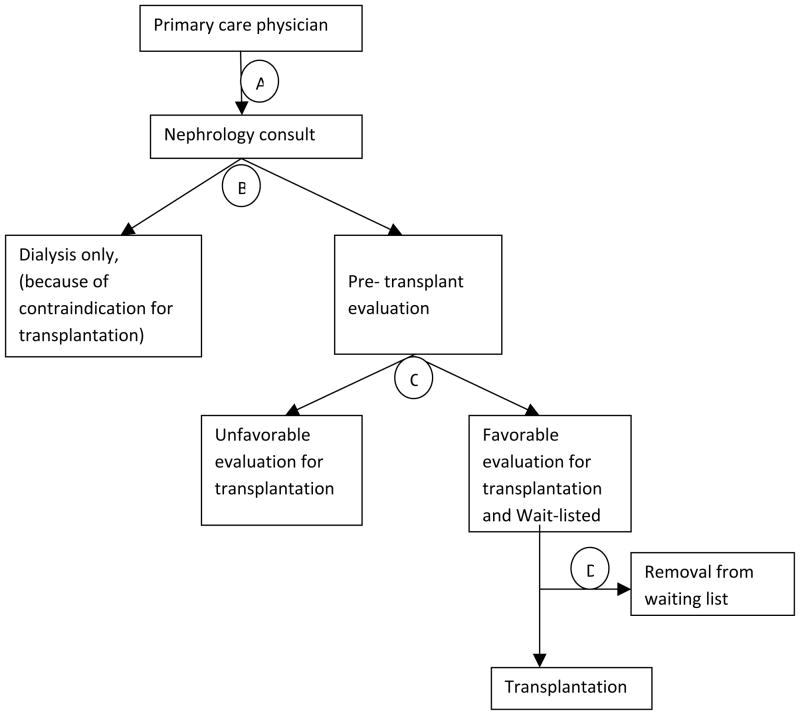
The general process of the nephrology referral and evaluation for transplantation in ESRD patients is illustrated in Figure 2. Diabetic patients are subjected to exclusion from the evaluation process at any of the three decision points B, C or D as in the Figure 2. Thus, due to higher comorbidity and BMI they may never be referred for transplant evaluation by primary nephrologist (B). Furthermore, patients with diabetes might be declined by the transplant program after initial evaluation (C) due to higher level of comorbidity and concern that the recipient’s diabetic status might lead to poor graft and recipient survival post-transplant [38–40]. Alternatively, the evaluation itself may be prolonged due to the various cardiovascular investigations such as angiography that might be needed for evaluation of associated coronary disease in a diabetic patient. Once placed on the waiting list candidates may be later removed or become “temporarily unavailable” due to their comorbid conditions or events, predominantly cardiovascular disease (D) [41–44]. The number of diabetic patients who eventually get transplanted therefore tends to be limited [45].
Figure 2.
The general process of the nephrology referral and evaluation for transplantation in ESRD patients.
Comorbidities and greater BMI are more prevalent in the diabetic population compared to the non-diabetic population (Table 1). It has been previously shown that patients with high co-morbidity [17, 20], less access to living donors [46], and greater BMI [35] have delayed access to transplantation. We hypothesized that higher level of co-morbidity and greater BMI may be mediating the observed affect. When we control for these factors in our model, we anticipated that access to transplantation in patients with diabetes would not be worse than in the general population. In fact, when adjusted for comorbidities and BMI, history of diabetes was associated shorter time to wait-listing or transplantation without being listed but there was no significant difference for time to transplantation after being listed compared to non-diabetics. Furthermore when BMI and comorbidity index were included individually in two separate Cox models along with the priori defined potential confounders, the results demonstrated that majority of the effect was mediated specifically by comorbidities. Also, we noted that there was a significant difference in the donor type (cadaveric vs living) between the ESRD patients with diabetes and without diabetes and who had received a transplant (Table 1). Since donor type was viewed as a “post-baseline” event, we did not include it in the Cox model. Still, we hypothesized that along with comorbidities and BMI it could be another potential mediator between diabetes and inferior access to transplantation.
The association of diabetes mellitus with shorter time to listing after adjustment for comorbidities and BMI might potentially be explained by greater level of exposure to healthcare system in the diabetic population, potentially more frequent follow-up visits with the primary care physician, involvement of the specialists, and potentially higher awareness and earlier referral for renal disease [47]. In general, more frequent surveillance of renal status likely increases opportunity to identify need for nephrology referral and transplantation evaluation. Referring back to Figure 2, we would anticipate the quicker and earlier referral of diabetic ESRD patients to nephrologist by the primary care physician (point A in Figure 2) when compared to non-diabetic patients with similar comorbidity and BMI leading to relatively shorter time to being listed. As patients being listed, the time to transplantation is not different between diabetic and non-diabetic patients. These results make sense since that time period is determined by administrative regulations of transplant list (rather than “human decision”) and should not depend on diabetic status, once adjusted for comorbidities, BMI, and other patient characteristics.
There are some limitations to this study that deserve mentioning. First, because our study was a retrospective analysis, we could not assess causality, but only the association between diabetic status and transplant access. However, the fact that association changed after comorbidity index and BMI were included in the models suggest that these factors mediate the effect of diabetes. A second limitation may have been our inability to stratify by the type and severity of diabetic status (as opposed to simple binary designation of presence or absence of diabetes) because of the limitations of the data. Another limitation is - we were not able to censor the patient records that were removed from the waiting list as we do not have information regarding these events in our data set.
In conclusion, in our study cohort the association between the presence of diabetes and reduced access to renal transplantation seems to be mediated by comorbidities and BMI. Adjusted for these factors the access to transplantation in diabetic population is not worse (or even better) than in other groups.
Acknowledgments
This study was funded from the departmental funds and did not have any outside sponsor or funding agency. All authors had full access to the data used in the study and take responsibility for the integrity of the data and accuracy of the data analysis. The data reported here have been supplied by the USRDS. The results presented in this paper have not been published previously in whole or part (even in abstract format). The interpretation and reporting of the data is the responsibility of the authors and in no way should be seen as official policy or interpretation of the U.S. government. This work was conducted with support from Harvard Catalyst / The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Footnotes
None of the authors of the manuscript have any conflict of interest to declare.
References
- 1.N. I. o. H. 2010 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. [Accessed June, 2010]. Available at: http://www.usrds.org/adr.htm. [Google Scholar]
- 2.A. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3.Williams ME. Diabetic CKD/ESRD 2010: a progress report? Semin Dial. 2010 Mar-Apr;23:129–33. doi: 10.1111/j.1525-139X.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 4.Villar E, Zaoui P. Diabetes and chronic kidney disease: lessons from renal epidemiology. Nephrologie & therapeutique. 2010 Dec;6:585–90. doi: 10.1016/j.nephro.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Neipp B Karavul, Jackobs S, Meyer zu Vilsendorf A, Richter N, Becker T, Schwarz A, Klempnauer J. Quality of life in adult transplant recipients more than 15 years after kidney transplantation. Transplantation. 2006 Jun 27;81:1640–4. doi: 10.1097/01.tp.0000226070.74443.fb. [DOI] [PubMed] [Google Scholar]
- 6.Eggers PW. Effect of transplantation on the Medicare end-stage renal disease program. The New England journal of medicine. 1988 Jan 28;318:223–9. doi: 10.1056/NEJM198801283180406. [DOI] [PubMed] [Google Scholar]
- 7.Brunkhorst R, Lufft V, Dannenberg B, Kliem V, Tusch G, Pichlmayr R. Improved survival in patients with type 1 diabetes mellitus after renal transplantation compared with hemodialysis: a case-control study. Transplantation. 2003 Jul 15;76:115–9. doi: 10.1097/01.TP.0000070225.38757.81. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999 Dec 2;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 9.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM. Racial Disparities in Access to Renal Transplantation — Clinically Appropriate or Due to Underuse or Overuse? New England Journal of Medicine. 2000;343:1537–1544. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navaneethan SD, Singh S. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clinical transplantation. 2006;20:769–775. doi: 10.1111/j.1399-0012.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 11.Ravanan R, Udayaraj U, Ansell D, Collett D, Johnson R, O’Neill J, Tomson CRV, Dudley CRK. Variation between centres in access to renal transplantation in UK: longitudinal cohort study. BMJ. 2010 Jan 1;341 doi: 10.1136/bmj.c3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg PP, Furth SL, Fivush BA, Powe NR. Impact of gender on access to the renal transplant waiting list for pediatric and adult patients. Journal of the American Society of Nephrology : JASN. 2000 May;11:958–64. doi: 10.1681/ASN.V115958. [DOI] [PubMed] [Google Scholar]
- 13.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR, Montgomery RA. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. Journal of the American Society of Nephrology : JASN. 2009 Mar;20:621–8. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, Fenton SS. Sex inequality in kidney transplantation rates. Archives of internal medicine. 2000 Aug 14–28;160:2349–54. doi: 10.1001/archinte.160.15.2349. [DOI] [PubMed] [Google Scholar]
- 15.Gaston RS, Danovitch GM, Adams PL, Wynn JJ, Merion RM, Deierhoi MH, Metzger RA, Cecka JM, Harmon WE, Leichtman AB, Spital A, Blumberg E, Herzog CA, Wolfe RA, Tyan DB, Roberts J, Rohrer R, Port FK, Delmonico FL. The report of a national conference on the wait list for kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003 Jul;3:775–85. doi: 10.1034/j.1600-6143.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 16.Villar E. A multicentre study of registration on renal transplantation waiting list of the elderly and patients with type 2 diabetes. Nephrology Dialysis Transplantation. 2004;19:207–214. doi: 10.1093/ndt/gfg500. [DOI] [PubMed] [Google Scholar]
- 17.Gaylin DS, Held PJ, Port FK, Hunsicker LG, Wolfe RA, Kahan BD, Jones CA, Agodoa LYC. The Impact of Comorbid and Sociodemographic Factors on Access to Renal Transplantation. JAMA: The Journal of the American Medical Association. 1993 Feb 3;269:603–608. [PubMed] [Google Scholar]
- 18.Alexander GC, Sehgal AR. Variation in access to kidney transplantation across dialysis facilities: Using process of care measures for quality improvement. American Journal of Kidney Diseases. 2002;40:824–831. doi: 10.1053/ajkd.2002.35695. [DOI] [PubMed] [Google Scholar]
- 19.Bayat S, Frimat L, Thilly N, Loos C, Briançon S, Kessler M. Medical and non-medical determinants of access to renal transplant waiting list in a French community-based network of care. Nephrology Dialysis Transplantation. 2006 Oct;21:2900–2907. doi: 10.1093/ndt/gfl329. [DOI] [PubMed] [Google Scholar]
- 20.Stel VS, van Dijk PC, van Manen JG, Dekker FW, Ansell D, Conte F, Kramar R, Leivestad T, Vela E, Briggs JD, Jager KJ. Prevalence of co-morbidity in different European RRT populations and its effect on access to renal transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005 Dec;20:2803–11. doi: 10.1093/ndt/gfi099. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK. Differences in access to cadaveric renal transplantation in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000 Nov;36:1025–33. doi: 10.1053/ajkd.2000.19106. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Davies SJ, Russell L, Bryan J, Phillips L, Russell GI. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1995 Aug;26:353–61. doi: 10.1016/0272-6386(95)90657-6. [DOI] [PubMed] [Google Scholar]
- 24.Goldfarb-Rumyantzev A, Hurdle JF, Scandling J, Wang Z, Baird B, Barenbaum L, Cheung AK. Duration of end-stage renal disease and kidney transplant outcome. Nephrology, dialysis, transplantation. 2005 Jan;20:167–75. doi: 10.1093/ndt/gfh541. [DOI] [PubMed] [Google Scholar]
- 25.Tang H, Chelamcharla M, Baird BC, Shihab FS, Koford JK, Goldfarb-Rumyantzev AS. Factors affecting kidney-transplant outcome in recipients with lupus nephritis. Clinical transplantation. 2008 May-Jun;22:263–72. doi: 10.1111/j.1399-0012.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 26.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of Survival Probabilities for Dialysis Patients vs Cadaveric Renal Transplant Recipients. JAMA: The Journal of the American Medical Association. 1993 Sep 15;270:1339–1343. [PubMed] [Google Scholar]
- 27.Locatelli F, Pozzoni P, Del Vecchio L. Renal Replacement Therapy in Patients with Diabetes and End-Stage Renal Disease. Journal of the American Society of Nephrology. 2004 Jan 1;15:S25–S29. doi: 10.1097/01.asn.0000093239.32602.04. [DOI] [PubMed] [Google Scholar]
- 28.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002 Nov 27;74:1377–81. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 29.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, Leichtman AB, Kaplan B. Effect of waiting time on renal transplant outcome. Kidney international. 2000 Sep;58:1311–7. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 30.Becker BN, Rush SH, Dykstra DM, Becker YT, Port FK. Preemptive transplantation for patients with diabetes-related kidney disease. Archives of internal medicine. 2006 Jan 9;166:44–8. doi: 10.1001/archinte.166.1.44. [DOI] [PubMed] [Google Scholar]
- 31.GARG PP, FURTH SL, FIVUSH BA, POWE NR. Impact of Gender on Access to the Renal Transplant Waiting List for Pediatric and Adult Patients. Journal of the American Society of Nephrology. 2000 May 1;11:958–964. doi: 10.1681/ASN.V115958. [DOI] [PubMed] [Google Scholar]
- 32.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche H-U. Barriers to Evaluation and Wait Listing for Kidney Transplantation. Clinical Journal of the American Society of Nephrology. doi: 10.2215/CJN.08620910. [DOI] [PubMed] [Google Scholar]
- 33.Cass A, Cunningham J, Snelling P, Ayanian JZ. Late referral to a nephrologist reduces access to renal transplantation. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003 Nov;42:1043–9. doi: 10.1016/j.ajkd.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Dudley CRK, Johnson RJ, Thomas HL, Ravanan R, Ansell D. Factors That Influence Access to the National Renal Transplant Waiting List. Transplantation. 2009;88:96–102. doi: 10.1097/TP.0b013e3181aa901a. [DOI] [PubMed] [Google Scholar]
- 35.Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA. Obesity Impacts Access to Kidney Transplantation. Journal of the American Society of Nephrology. 2008 Feb 1;19:349–355. doi: 10.1681/ASN.2007050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfarb-Rumyantzev AS, Sandhu GS, Baird BC, Khattak M, Barenbaum A, Hanto DW. Social adaptability index predicts access to kidney transplantation. Clinical transplantation. 2011 Jan 27; doi: 10.1111/j.1399-0012.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 37.Kianda MN, Wissing KM, Broeders NE, Lemy A, Ghisdal L, Hoang AD, Mikhalski D, Donckier V, Vereerstraeten P, Abramowicz D. Ineligibility for renal transplantation: prevalence, causes and survival in a consecutive cohort of 445 patients. Clinical transplantation. 2010 doi: 10.1111/j.1399-0012.2010.01317.x. pp. no-no. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann F, Haastert B, Koch M, Giani G, Glaeske G, Icks A. The effect of diabetes on incidence and mortality in end-stage renal disease in Germany. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 May;26:1634–40. doi: 10.1093/ndt/gfq609. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Cheigh JS. Kidney transplantation in patients with type 1 diabetes mellitus: long-term prognosis for patients and grafts. The Korean journal of internal medicine. 2001 Jun;16:98–104. doi: 10.3904/kjim.2001.16.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Revanur VK, Jardine AG, Kingsmore DB, Jaques BC, Hamilton DH, Jindal RM. Influence of diabetes mellitus on patient and graft survival in recipients of kidney transplantation. Clinical transplantation. 2001;15:89–94. doi: 10.1034/j.1399-0012.2001.150202.x. [DOI] [PubMed] [Google Scholar]
- 41.Rice M, Martin J, Hathaway D, Tolley E. Prevalence of cardiovascular risk factors before kidney transplantation. Progress in transplantation. 2002 Dec;12:299–304. doi: 10.1177/152692480201200411. [DOI] [PubMed] [Google Scholar]
- 42.Tomaszuk-Kazberuk A, Bachorzewska-Gajewska H, Malyszko J, Mysliwiec M, Musial WJ. Impact of diabetes mellitus on survival in patients with end-stage renal disease: a three-year follow-up. Kidney & blood pressure research. 2011;34:83–6. doi: 10.1159/000323894. [DOI] [PubMed] [Google Scholar]
- 43.Wang AY. Cardiovascular risk in diabetic end-stage renal disease patients. Journal of diabetes. 2011 Jun;3:119–31. doi: 10.1111/j.1753-0407.2011.00113.x. [DOI] [PubMed] [Google Scholar]
- 44.Ramanathan V, Goral S, Tanriover B, Feurer ID, Kazancioglu R, Shaffer D, Helderman JH. Screening asymptomatic diabetic patients for coronary artery disease prior to renal transplantation. Transplantation. 2005 May 27;79:1453–8. doi: 10.1097/01.tp.0000164147.60036.67. [DOI] [PubMed] [Google Scholar]
- 45.Zivcic-Cosic S, Fucak M, Orlic P, Vujaklija-Stipanovic K, Orlic L, Racki S, Grzetic M, Matic-Glazar D, Zelic M, Mavric Z. Evaluation and selection of candidates for renal transplantation at the Clinical Hospital Center in Rijeka. Acta medica Croatica : casopis Hravatske akademije medicinskih znanosti. 2003;57:65–8. [PubMed] [Google Scholar]
- 46.Walker RG, Kanellis J, Robertson AJ, Saunder AC. Living donor transplantation: is there inequality of access? ANZ Journal of Surgery. 2011;81:2–3. doi: 10.1111/j.1445-2197.2010.05609.x. [DOI] [PubMed] [Google Scholar]
- 47.Association AD. Standards of Medical Care in Diabetes—2011. Diabetes Care. 2011 Jan 1;34:S11–S61. [Google Scholar]