Abstract
Analyses of mature adipocytes have shown that they possess a reprogramming ability in vitro, which is associated with dedifferentiation. The subsequent dedifferentiated fat cells (DFAT cells) are multipotent and can differentiate into adipocytes and other cell types as well. Mature adipocytes can be easily obtained by biopsy, and the cloned progeny cells are homogeneous in vitro. Therefore, DFAT cells (a new type of stem cell) may provide an excellent source of cells for tissue regeneration, engineering and disease treatment. The dedifferentiation of mature adipocytes, the multipotent capacity of DFAT cells and comparisons and contrasts with mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPS) are discussed in this review.
Keywords: adipocytes, DFAT cells, stem cells, MSCs, iPS
Introduction
Since obesity and metabolic-related diseases continue to be significant health issues, adipose tissue and/or adipocytes have been a keen research focus for many years.1,2 Although a number of cell types exist in fat/adipose tissue, adipocytes are the prominent cell type within adipose tissue and are responsible for performing its main function—lipid metabolism.3 Recently, the ability of mature adipocytes to dedifferentiate (DFAT) has been demonstrated in several species using in vitro approaches.4 In addition, several studies indicate DFAT cells may redifferentiate into lipid-filled adipocytes5-9 or trans-differentiate into chondrocytes, osteoblasts, myocytes, or other possible cell types under the proper conditions.7,10-13 The multipotent capacity of DFAT cells makes these cells attractive for new stem cell research, especially since they are relatively easy to isolate from prospective patients. Moreover, reports suggest that implantation of DFAT cells in the body can regenerate fat pads, ectopic osteoid tissue, or muscle tissue;7-10,12,13 DFAT cells contribute to sphincter function,14 participate in infarcted cardiac tissue repair10 and even central nervous system recovery.15 Therefore, DFAT cells may be a good resource for tissue engineering and certain cell replacement therapies for diseases.
Adipose Tissue
BAT (brown adipose tissue) and WAT (white adipose tissue) are two types of adipose tissue in mammals. BAT is generally abundant in newborns and decreases with age, whereas WAT exists widely, especially in the intra-abdominal and subcutaneous tissue.16 WAT is an important energy transit station involved in storing or releasing triglycerides and it is the biggest endocrine organ of the body. This review will mainly focus on WAT, which is easy to access and widely distributed. Adipose tissue is dynamic, and influenced by dietary regimen, exercise, and drugs.17 Dynamic variations in adipose depots mainly result from changes in the numbers and volumes of adipocytes, accompanied by a series of processes including gene expression and regulation, protein synthesis and degradation, cytokine secretion, and lipid synthesis and metabolism.18 Excessive fat tissue results in human obesity and is a comorbidity with insulin resistance, type 2 diabetes, and metabolic syndrome.1 In addition, the amount and locations of fat deposits directly influence economic profitability in agricultural animal production.19 For example, people prefer to buy beef with more intramuscular fat, owing to its tenderness and palatability; and unwanted subcutaneous fat tissue always leads to undesirable and eventually wasted feed and money.20-23 Adipose tissue attracts wide attention by both human and animal scientists.
Adipose tissue contains a number of cell types. In primary cell culture after digestion of dissected adipose tissue, fibroblasts, endothelial cells, blood cells, macrophages, and mesenchymal stem cells have been found co-isolatable with adipocytes.24 By using the traditional ceiling culture (an established adipocyte culture method), the floating lipid-filled adipocytes together with some fibroblast-like cells, possessing the similar buoyancy, attach to the ceiling of the flask.6,25,26 However, these lipid-laden adipocytes, which are the most abundant cells in any adipose tissue, may be purified from other cells as described25 and be used in further studies.
The identity of the adipocyte precursor has been pursued for over one hundred and thirty years and continues to be pursued by many labs with state-of-the-art approaches.27 A large body of evidence demonstrates that the onset of adipocyte development is tightly coupled with the expanding and elaborating vascularity during adipocyte tissue development.3 This is true throughout development such that adipocytes never develop in the absence of vasculature. In the developing embryo the vasculature is first formed by originating from proliferating MSCs that give rise to clusters of spherical basophilic cells. The peripheral cells of these clusters form the endothelium of primitive blood vessels while the central cells represent blast cells for various blood cells. Recent studies indicate that either the endothelial or perivascular cells of these clusters represent adipocyte stem cells and differentiate into adipocytes.28 Adipocyte differentiation occurs in concert with blood vessel angiogenesis which progressively leads to the formation of a functional circulatory system. The elaboration of the vasculature then enhances both adipocyte hypertrophy and adipocyte recruitment. Therefore, adipocytes appear at either the site of vasculogenesis or angiogenesis. Very recent studies demonstrate that adipocytes arise from a perivascular location or are directly derived from endothelial cells.29-31 Regardless, the origin of adipocytes is linked to the origin of differentiating or functional vascular endothelial cells.
Dedifferentiation of Mature Adipocytes
Adipocytes have been viewed as a terminally differentiated cell type for over 30 years. However, Adebonojo32,33 first showed mature adipocytes might proliferate into a large number of daughter cells in vitro. This process was ascribed to dedifferentiation and the daughter cells were termed dedifferentiated fat cells (DFAT). By using flow cytometry, immunostaining and western blot, CD34 (a stem cell marker) was confirmed being expressed in fully differentiated adipocytes together with other mature adipocyte specific markers.34 In addition, end-point PCR showed Sca1, CD90, CD45 (stem cell markers), as well as Oct3/4, Sox2, c-Myc, and Klf4 (which were used to induce the formation of pluripotent stem cells) were expressed in mature adipocytes.35 These surface membrane proteins and/or the transcription factors help to broaden our understanding on the functions of mature adipocytes.
Ceiling culture method was designed for collecting mature fat cells due to the buoyancy of lipid-filled adipocytes. However, contaminating cells could attach to the flasks together with mature fat cells.6,25,26 The dedifferentiation of lipid-filled fat cells was not widely accepted under the traditional notion about mature adipocytes. Some contaminating cells co-isolated together with lipid-filled adipocytes might confuse the observation for mature fat cells. However, by differential plating, isopycnic density gradient centrifugation, cell surgery and cloning techniques, and purified ceiling culture and single cell culture approaches added support for the notion that dedifferentiation does indeed occur, and have enabled us to access the physiology of purified mature fat cells.25 In this method, mature adipocytes were first acquired through traditional ceiling culture, and effective procedures are now performed to insure that there are no contaminating cells attached on the ceiling culture. In short, differential plating, cell surgery, and cloning techniques are crucial steps involved in the process combined with careful observations.
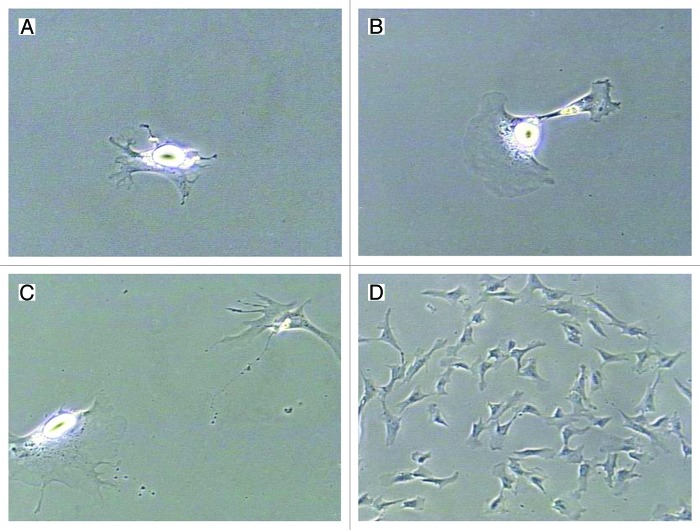
Until now, numerous studies have indicated that the reverse adipogenesis (lipid loss and re-entry into the cell cycle) occurs widely.4 Recently, the dedifferentiation potential has become increasingly accepted by more and more researchers, especially when cultured cells did not need to lose 100% of lipid prior to observing proliferative-competent progeny cells (Fig. 1). During dedifferentiation, lipids were divided into daughter cells symmetrically (equal amount of lipids) or asymmetrically (different amount of lipids);36,37 or lipids would be expelled into medium directly prior to cell division.38-40 The reason for the different dedifferentiation pathways might be due to species variation or even individual variation40 but the mechanisms underlying the phenomenon were not clear. Yet, both of the routes of dedifferentiation formed strongly proliferative DFAT cells.
Figure 1. Dedifferentiation and proliferation of mature adipocytes in vitro. (A) Three days after cell isolation, mature adipocytes attached on the cell culture flask and stretched cell membrane. (B) Four days after cell isolation, the same fat cell was dividing. (C) Six hours later, this fat cell divided into two daughter cells, containing different amount of lipids in the cytoplasm. (D) A colony of DFAT cells formed on d 7, which were derived from the single mature adipocyte. The DFAT cells possessed fibroblast-like appearance and lipids were not detectable. All the photographs were taken by a Sony RGB digital camera (3/4-inch chip) married to a Nikon Diaphot phase contrast microscope and Image Pro Plus® image analysis software. (A–C) 200× magnification; (D) 100× magnification.
Multipotent Capacity of DFAT Cells
Mature adipocyte-derived DFAT cells possess the fibroblast-like appearance and strong proliferative ability (Fig. 1). The potential capacities of DFAT cells attract numerous attentions. A number of researchers have reported DFAT cells could redifferentiate into adipocytes again. Mouse DFAT cells possessed the ability to redifferentiate into lipid-assimilating cells after over 20 passages in vitro and fat pads formed by implanting the DFAT cells into the sternum of ddY mice.9 Similar findings were reported by Nobusue et al.8 In addition to mice, the redifferentiation potential of the cells has been proved in humans,41 ovine,42 bovine,6 and porcine species,5,7 triggering studies on the possible ability of DFAT cells to function in a wide range of species.
DFAT cells exhibit robust proliferation and redifferentiation capacities, and they also exhibit additional stem cell characteristics including multipotency. Matsumoto et al.7 showed that mouse DFAT cells differentiated into chondrocytes and osteoblasts under appropriate culture conditions in vitro and formed osteoid matrix when implanted into subcutaneous tissue of nude mice. Oki et al.12 indicated DFAT cells transdifferentiated into osteoblasts in the presence of all-trans retinoic acid in vitro. Furthermore, subcutaneous transplanted DFAT cells formed ectopic osteoid tissue in C57BL/6N mice in vivo only with all-trans retinoic acid. Kishimoto et al.43 demonstrated rabbit derived DFAT cells differentiated into osteoblasts in a rigid scaffold consisting of titanium fiber mesh, providing an experimental basis for bone regeneration therapy.
Furthermore, myogenic induction of DFAT cells was examined by several researchers. Kazama et al.11 showed that myogenic induction of DFAT cells resulted in the expression of MyoD and myogenin, followed by cell fusion and formation of syncytial cells expressing sarcomeric myosin heavy chain, indicating that DFAT cells can be induced to form skeletal myotubes in vitro. Sakuma et al.13 found 50% of the human adipocyte derived DFAT cells differentiated into α-actin-positive smooth muscle. Moreover, DFAT cells contributed to the regeneration of bladder smooth muscle after DFAT cell injection. A recent study14 examined the effects of DFAT cell transplantation on urethral tissue regeneration and sphincter function. Results showed that transplanted DFAT cells converted into smooth muscle cells, promoting sphincter muscle regeneration and improving leak point pressure in the rat vaginal distension model.14 Jumabay et al.10 found the DFAT cells expressed cardiac markers when co-cultured with cardiomyocytes and also when grown in stem cell methylcellulose medium with the absence of cardiomyocytes. In a rat acute myocardial infarction model, transplanted DFAT cells accumulated efficiently in infarcted myocardium and expressed cardiac sarcomeric actin at 8 weeks after the cell transplantation. The transplantation of DFAT cells significantly increased capillary density in the infarcted area when compared with hearts from saline-injected control rats.10 In addition, transplantation of DFAT cells led to neovascularization in rats with myocardial infarction.10
The conditions for DFAT cell transdifferentiation into chondrocytes, osteoblasts, skeletal myocytes, smooth muscle cells, and cardiomyocytes are listed in Table 1.7,10-13,41 Recently, studies showed myeloid, lymphoid, and epithelial cell CD marker genes were upregulated during dedifferentiation of mature adipocytes.44 Besides, DFAT cells could contribute to central nervous system recovery.15 All of these indicate that the multilineage potential of DFAT cells may not be limited to the above cell types. A recent review showed that changes in culture conditions might alter the fate and/or potency of stem cells or reprogram adult stem/progenitor cells to assume a broader range of multipotency.45 The examination of microenvironment (including the cell density, the oxygen demand, the amount and type of serum, the basic medium, and proper inducer) of DFAT cells might allow a better understanding of the range of cellular potential. And if the corresponding changes of the differentiation fate are induced by the culture condition itself, it may be that epigenetic events affected by particular media need to be assessed.45
Table 1. Multilineage potential of mouse/human derived DFAT cells.
| Multilineage potential | Species | Inducement reagents/conditions | References |
|---|---|---|---|
| Adipocytes |
Mice/human |
Dexamethasone, isobutylmethylxanthine, insulin-transferrin-selenium-X |
7 and 13 |
| Human |
Insulin, triiodothyronine, biotin, pantothenate, transferrin, hydrocortisone |
41 |
|
| Chondrocytes |
Mice/human |
l-ascorbic acid-2-phosphate, proline, pyruvate, transforming growth factor-β3, ITS |
7 and 13 |
| Osteoblasts |
Mice/human |
Dexamethasone, β-glycerophosphate, l-ascorbic acid-2-phosphate |
7 and 13 |
| Mice |
All-trans retinoic acid |
12 |
|
| Skeletal myocytes |
Mice |
5-azacytidine |
11 |
| Smooth muscle cells |
Human |
Transforming growth factor-β1 |
7 and 13 |
| Cardiomyocytes | Mice | Co-cultured with cardiomyocytes or stem cell methylcellulose medium (containing methylcellulose, 2-mercaptoethanol, l-glutamine, recombinant human insulin, human transferring, recombinant murine interleukin 3, recombinant human IL-6, and recombinant mouse stem cell factor) | 10 |
Similarities and Differences between DFAT and MSCs/iPS Cells
Mesenchymal stem cells (MSCs) were first isolated from bone marrow (BM) by Friedenstein et al.46 and have been found to exist in adipose depots and many other tissues. These MSCs adhere to plastic when cultured with strong proliferative ability and fibroblast-like appearance. Moreover, MSCs possess the potential ability to differentiate into various lineages, including adipocytes, chondrocytes, osteoblasts, cardiomyocytes, neural precursors, and other possible cell types.47 Mature adipocyte-derived DFAT cells are similar to MSCs as evidenced by comparisons of multilineage potentiality, proliferative ability, and cellular morphology as compared with MSCs. Moreover, during the dedifferentiation process of mature adipocytes, changes in the epigenetic status led DFAT cells to display a similar DNA methylation condition to BM-derived MSCs.48 Like MSCs, DFAT cells inhibited the proliferation of stimulated lymphocytes in co-culture while mature adipocytes stimulated their growth.48 Therefore, during the dedifferentiation process, mature adipocytes lost their lineage characters, assumed the typical MSCs immunophenotype and gene expression profile is an interesting notion. Recent research on DFAT cells showed that human-derived DFAT cells were positive for CD90, CD105, CD73, CD44, and CD29, and negative for CD34, CD117, CD133, CD271, CD45, HLA-DR, and CD14, which was consistent with previous findings for BM-derived MSCs.48 These CD (cluster of differentiation) makers are cell surface molecules commonly used for identifying specific cell types by immunophenotyping. Mature adipocytes acquired some specific characters of MSCs after dedifferentiation process. Gao et al.49 reported similar findings about CD105 and CD34 with the mouse-derived DFAT cells. Currently, numerous studies on MSCs indicate that murine-derived MSCs are positive for CD105 and negative for CD31.50 No consistent conclusion has been made regarding CD34 for MSCs.50 However, CD 31 was found to be widely positive in mouse-derived DFAT cells,49 indicating the differences between DFAT cells and MSCs. In addition, CD marker antibodies are not available for all species, including meat animals. Extensive examination of surface antigens on DFAT cells and MSCs in human, mice, and other species would provide a better understanding about this new type of stem cell. It is clear, however, that DFAT cells are distinct from MSCs. Except for the surface antigens, MSCs could be isolated from bone marrow, adipose tissue, and other tissues as well, while DFAT cells are only derived from mature adipocytes in vitro. Owing to the ease to access significant amounts of DFAT cells and the high homogeneity as compared with MSCs, the DFAT cells possess a unique dominance in clinical applications.
Induced pluripotent stem cells (iPS cells) are made from adult somatic cells by various reprogramming schemes that produce cells that are functionally identical to embryonic stem cells. iPS cells are potentially useful for a wide range of human disease treatments and tissue replacement therapies without the controversial use of embryos. iPS cells are generally formed by forcing the expression of embryonic stem cell markers (Oct4, Sox2, c-Myc, and Klf4, or other combinations) often using viral vectors, which become silenced during the course of the iPS procedure. Like embryonic stem cells, iPS cells possess the pluripotent ability to generate a variety of tissues from all three germ layers.51 More recently, it has become possible to produce iPS cells by methods that do not require the use of integrating (and potentially mutagenic) virus. For instance, iPS cells have been made with non-integrating episomal systems,52,53 continued transfection with mRNAs encoding pluripotency factors,54 and recently, by expressing microRNAs only.55 These new approaches yield human iPS cells as good as those produced by the original method, and in ways that leave the genome unharmed. In addition, since iPS cells can be produced from easily accessible skin and nucleated blood cells, they can be made to be exact genetic matches for specific patients.
Adipose tissue is also readily accessible in the patient, and therefore, represents a potential source of patient-matched cells. DFAT cells have been found to express embryonic stem cell markers extensively such as Oct4, Sox2, c-Myc, and Nanog, which are key factors to maintain the pluripotent embryonic stem cell-like state.49 In this report, higher alkaline phosphatase and telomerase activity than in adipose tissue-derived stromal cells showed that DFAT cells had similarities to undifferentiated pluripotent stem cells.49 Ono et al.44 showed significant changes in gene expression by comparing the porcine-derived mature adipocytes and DFAT cells. In the dedifferentiation process, lipid metabolism-related gene expression declined significantly with a concomitant increase in cell proliferation, cell morphogenesis, cell differentiation, and developmental process of tissue, indicating the loss of the functional mature adipocyte phenotype and the acquisition of multipotent capacity.44 In addition, several CD marker genes of other cell types were upregulated, including myeloid, lymphoid, endothelial, epithelial, and stromal cells.44 The comparison of the gene expression profiles of DFAT cells with iPS cells showed that 194 genes associated with mitosis, M phase, cell division process, and cell cycle progression were highly expressed in both cell types.44 One hundred and twenty-eight genes of these 194 genes were considered for functional analysis of iPS cells.44 In the end, the utility of DFAT cells as compared with iPS cells for cell replacement therapies will depend upon their multipotentiality. It seems unlikely that DFAT cells will have the degree of pluripotency as iPS cells; however, the propensity of DFAT cells to form mesoderm cell types such as hematopoietic and osteogenic lineages and the close relationship between adipocytes and vasculature may render them more useful than iPS cells, since it has been difficult to achieve differentiation culminating in mature hematopoietic cell types in iPS cells. Like iPS cells, DFAT cells can be made to match the patient, thus opening the door to cell replacement therapies with autologous transplantation considerations.
As stated previously in this review, DFAT cells exhibit similarities with MSCs. Since DFAT cells can be derived from autologous WAT samples, they offer a potential source of autologous transplantable cells after differentiation to non-fat cells of interest. It should also be noted that MSC-like cells can be produced from iPS cells.56,57 Though the route by which MSCs are produced from iPS cells is more indirect than is the case for DFAT cells, the outcome (autologous transplantable cells) is similar, and it should be noted that since iPS cells proliferate indefinitely in culture in the undifferentiated state, and can be genetically modified, this route to MSCs offers the option of performing gene therapy ex vivo (at the iPS stage) followed by differentiation to MSCs and subsequent terminally differentiated cell types.
Conclusions
In our research based on purified ceiling culture and single cell systems, not every mature fat cell possesses the spontaneous ability to dedifferentiate in vitro.22 Whether the dedifferentiation takes place under physiological or pathological conditions in vivo and mechanisms involved in the transition from the mature adipocyte to a more immature-type cell need to be explored.22,26 If one removes a cell from the in vivo environment, will the cell spontaneously revert to a stem cell phenotype? Certainly the culture conditions for maintaining the mature adipocyte phenotype, as well as inducing the formation of DFAT cells in vitro need to be examined further. Until now, research involved in DFAT cells is limited to white adipocytes, even though different compositions in brown adipocytes and white adipocytes and different regulatory mechanisms exist between these two types of fat cells.58 Similar studies using brown adipocytes would broaden this area of adipocyte physiology. Will it be possible to compare progeny from adult mature adipocytes, which undergo dedifferentiation, and adipocytes from fetal or younger animals? If so, the difference in the rate of dedifferentiation and the potency of DFAT cells would become clearer. Moreover, since the DFAT cells express embryonic stem cell markers and are similar with iPS cells in some physiological aspects, it may be more efficient to make DFAT cells express embryonic stem cell characteristics. DFAT cells possess unique merits as compared with iPS cells since they have a propensity to form mesoderm-type cells. With easy access from patients per se and homogeneous traits of progeny cells, multipotent DFAT cells may provide an excellent source of cells for tissue regeneration and disease treatment, expanding the stem cell repertoire used in research and clinical applications.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/23784
References
- 1.Dodson MV, Mir PS, Hausman GJ, Guan LL, Du M, Jiang Z, et al. Obesity, metabolic syndrome, and adipocytes. J Lipids. 2011;2011:721686. doi: 10.1155/2011/721686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodson MV, Hausman GJ. Metabolic syndromes: Resolving a malady that involves numerous tissues, cells, regulators and regulatory pathways. J Metabol Syndro. 2012;1:e101. [Google Scholar]
- 3.Hausman GJ, Dodson MV. Stromal vascular cells and adipogenesis: Cells within adipose depots regulate adipogenesis. J Genomics. 2012;1:56–66. doi: 10.7150/jgen.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei S, Duarte MS, Zan L, Du M, Jiang Z, Guan L, et al. Cellular and molecular implications of mature adipocyte dedifferentiation. J Genomics. 2012;1:5–12. doi: 10.7150/jgen.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Dodson MV, Jiang Z. Cellular and molecular comparison of redifferentiation of intramuscular- and visceral-adipocyte derived progeny cells. Int J Biol Sci. 2010;6:80–8. doi: 10.7150/ijbs.6.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. Cells Tissues Organs. 2008;188:359–72. doi: 10.1159/000134007. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–22. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 8.Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008;332:435–46. doi: 10.1007/s00441-008-0593-9. [DOI] [PubMed] [Google Scholar]
- 9.Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–74. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565–75. doi: 10.1016/j.yjmcc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780–5. doi: 10.1016/j.bbrc.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct. 2008;33:211–22. doi: 10.1247/csf.08038. [DOI] [PubMed] [Google Scholar]
- 13.Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, et al. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355–65. doi: 10.1016/j.juro.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 14.Obinata D, Matsumoto T, Ikado Y, Sakuma T, Kano K, Fukuda N, et al. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model. Int J Urol. 2011;18:827–34. doi: 10.1111/j.1442-2042.2011.02865.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohta Y, Takenaga M, Tokura Y, Hamaguchi A, Matsumoto T, Kano K, et al. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877–86. doi: 10.3727/096368908786576516. [DOI] [PubMed] [Google Scholar]
- 16.Billon N, Monteiro MC, Dani C. Developmental origin of adipocytes: new insights into a pending question. Biol Cell. 2008;100:563–75. doi: 10.1042/BC20080011. [DOI] [PubMed] [Google Scholar]
- 17.Dodson M, Jiang Z, Du M, Hausman GJ. Adipogenesis: It is not just lipid that comprises adipose tissue. J Genomics. 2012;1:1–4. doi: 10.7150/jgen.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodson MV, Fernyhough ME, Vierck JL, Hausman GJ. Adipocytes may not be a terminally differentiated cell type: implications for animal production. Anim Sci. 2005;80:239–40. [Google Scholar]
- 19.Du M, Dodson MV. Advanced techniques to enhance marbling in meat. In: Joo S, ed. Control of Meat Quality. Kerala: Research Signpost, 2011:105-15. [Google Scholar]
- 20.Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, et al. Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int J Biol Sci. 2010;6:691–9. doi: 10.7150/ijbs.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodson MV, Jiang Z, Chen J, Hausman GJ, Guan L, Novakofski J, et al. Allied industry approaches to alter intramuscular fat content and composition in beef animals. J Food Sci. 2010;75:R1–8. doi: 10.1111/j.1750-3841.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- 22.Dodson MV, Hausman GJ, Guan L, Du M, Jiang Z. Potential impact of mature adipocyte dedifferentiation in terms of cell numbers. Intl J Stem Cells. 2011;4:76–8. doi: 10.15283/ijsc.2011.4.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, et al. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 2009;87:1218–46. doi: 10.2527/jas.2008-1427. [DOI] [PubMed] [Google Scholar]
- 24.Dodson MV, Wei S, Duarte M, Du M, Jiang Z, Hausman GJ, et al. Cell supermarket: Adipose tissue as a source of stem cells. J Genomics. 2012;1:39–44. doi: 10.7150/jgen.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernyhough ME, Vierck JL, Hausman GJ, Mir PS, Okine EK, Dodson MV. Primary adipocyte culture: adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnology. 2004;46:163–72. doi: 10.1007/s10616-005-2602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernyhough ME, Vierck JL, Dodson MV. Assessing a non-traditional view of adipogenesis: adipocyte dedifferentiation--mountains or molehills? Cells Tissues Organs. 2006;182:226–8. doi: 10.1159/000093970. [DOI] [PubMed] [Google Scholar]
- 27.Hausman GJ, Campion DR, Martin RJ. Search for the adipocyte precursor cell and factors that promote its differentiation. J Lipid Res. 1980;21:657–70. [PubMed] [Google Scholar]
- 28.Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–22. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 29.Cai X, Lin Y, Hauschka PV, Grottkau BE. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103:435–47. doi: 10.1042/BC20110033. [DOI] [PubMed] [Google Scholar]
- 30.Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–9. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–9. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adebonojo FO. Monolayer cultures of disaggregated human adipocytes. In Vitro. 1975;11:50–4. doi: 10.1007/BF02615322. [DOI] [PubMed] [Google Scholar]
- 33.Adebonojo FO. Studies on human adipose cells in culture: relation of cell size and multiplication to donor age. Yale J Biol Med. 1975;48:9–16. [PMC free article] [PubMed] [Google Scholar]
- 34.Festy F, Hoareau L, Bes-Houtmann S, Péquin AM, Gonthier MP, Munstun A, et al. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol. 2005;124:113–21. doi: 10.1007/s00418-005-0014-z. [DOI] [PubMed] [Google Scholar]
- 35.De Matteis R, Zingaretti MC, Murano I, Vitali A, Frontini A, Giannulis I, et al. In vivo physiological transdifferentiation of adult adipose cells. Stem Cells. 2009;27:2761–8. doi: 10.1002/stem.197. [DOI] [PubMed] [Google Scholar]
- 36.Fernyhough ME, Bucci LR, Hausman GJ, Antonio J, Vierck JL, Dodson MV. Gaining a solid grip on adipogenesis. Tissue Cell. 2005;37:335–8. doi: 10.1016/j.tice.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Fernyhough ME, Helterline DL, Vierck JL, Hausman GJ, Hill RA, Dodson MV. Dedifferentiation of mature adipocytes to form adipofibroblasts: More than just a possibility. Adipocytes. 2005;1:17–24. [Google Scholar]
- 38.Chen J, Guridi M, Fernyhough M, Jiang Z, Guan L, Hausman G, et al. Initial differences in lipid processing leading to pig- and beef-derived mature adipocyte dedifferentiation. Basic Appl Myol. 2009;19:243–6. [Google Scholar]
- 39.Chen J, Guridi M, Fernyhough ME, Jiang Z, Guan L, Hausman GJ, et al. Clonal mature adipocyte production of proliferative-competent daughter cells requires lipid export prior to cell division. Inter J Stem Cells. 2009;2:76–9. doi: 10.15283/ijsc.2009.2.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei S, Duarte MS, Du M, Jiang Z, Paulino PVR, Chen J, et al. Like pigs, and unlike other breeds of cattle examined, mature Angus-derived adipocytes may extrude lipid prior to proliferation in vitro. Adipocyte. 2012;1:237–41. doi: 10.4161/adip.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tholpady SS, Aojanepong C, Llull R, Jeong JH, Mason AC, Futrell JW, et al. The cellular plasticity of human adipocytes. Ann Plast Surg. 2005;54:651–6. doi: 10.1097/01.sap.0000158065.12174.40. [DOI] [PubMed] [Google Scholar]
- 42.Vierck JL, McNamara JP, Dodson MV. Proliferation and differentiation of progeny of ovine unilocular fat cells (adipofibroblasts) In Vitro Cell Dev Biol Anim. 1996;32:564–72. doi: 10.1007/BF02722983. [DOI] [PubMed] [Google Scholar]
- 43.Kishimoto N, Momota Y, Hashimoto Y, Ando K, Omasa T, Kotani J. Dedifferentiated fat cells differentiate into osteoblasts in titanium fiber mesh. Cytotechnology. 2013;65:15–22. doi: 10.1007/s10616-012-9456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ono H, Oki Y, Bono H, Kano K. Gene expression profiling in multipotent DFAT cells derived from mature adipocytes. Biochem Biophys Res Commun. 2011;407:562–7. doi: 10.1016/j.bbrc.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 45.Roobrouck VD, Vanuytsel K, Verfaillie CM. Concise review: culture mediated changes in fate and/or potency of stem cells. Stem Cells. 2011;29:583–9. doi: 10.1002/stem.603. [DOI] [PubMed] [Google Scholar]
- 46.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 47.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 48.Poloni A, Maurizi G, Leoni P, Serrani F, Mancini S, Frontini A, et al. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells. 2012;30:965–74. doi: 10.1002/stem.1067. [DOI] [PubMed] [Google Scholar]
- 49.Gao Q, Zhao L, Song Z, Yang G. Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol Biol Rep. 2012;39:5791–804. doi: 10.1007/s11033-011-1371-4. [DOI] [PubMed] [Google Scholar]
- 50.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 52.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–12. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459–61. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teramura T, Onodera Y, Mihara T, Hosoi Y, Hamanishi C, Fukuda K. Induction of mesenchymal progenitor cells with chondrogenic property from mouse-induced pluripotent stem cells. Cell Reprogram. 2010;12:249–61. doi: 10.1089/cell.2009.0086. [DOI] [PubMed] [Google Scholar]
- 57.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 58.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]