Abstract
Obesity and its associated secondary complications are active areas of investigation in search of effective treatments. As a result of this intensified research numerous differences between males and females at all levels of metabolic control have come to the forefront. These differences include not only the amount and distribution of adipose tissue, but also differences in its metabolic capacity and functions between the sexes. Here, we review some of the recent advances in our understanding of these dimorphisms and emphasize the fact that these differences between males and females must be taken into consideration in hopes of obtaining successful treatments for both sexes.
Keywords: sexual dimorphism, leptin, steroids, metabolism, morphology
Introduction
The obesity epidemic is a primary health concern in most industrialized countries, as being obese increases the risk of mortality due to associated comorbidities. Although both males and females are affected, there is a difference among sexes with regards to the propensity to develop obesity and the related metabolic complications.1-4 Substantial differences in the metabolic responses to dietary challenges, weight gain, weight loss, and pharmacological interventions have been reported between males and females;2,5,6 however, the mechanisms underlying this dimorphism remain largely unknown.
Differences in circulating gonadal steroid levels play an important role in many sexually dimorphic features, but they cannot account for all of the metabolic dissimilarities observed between males and females. Indeed, there are clear differences even before the onset of puberty.7,8 Identifying the mechanisms underlying the different metabolic responses in males and females will not only advance our general understanding of metabolic control, but it is fundamental for the development of effective treatments of obesity and its complications in both sexes. Here, we briefly review some of the advances in our understanding of sex differences in white adipose tissue (WAT) and how this may affect the differential responses of males and females to some metabolic challenges.
Adipose Distribution
Sex differences in fat distribution can be observed even before puberty, although they become much more pronounced after pubertal onset.7,8 Females experience a continuous increase in both absolute and percent body fat throughout development and although males as well display a persistent increase in absolute body fat, they have the highest percentage of body fat during puberty.9 Thus, the gradual changes in adipose accrual throughout early development can result in differences between post-pubertal males and females in the percentage of body fat, with a divergent distribution of adipose tissue also becoming clearly apparent.9-12 While females predominantly accumulate subcutaneous fat, males amass significantly more visceral fat.10-13 This sex difference in visceral adipose diminishes at older ages as postmenopausal women have increased visceral fat accrual,14,15 emphasizing the role of gonadal steroids in this phenomenon. Fat distribution is not only influenced by sex, but also by ethnic background, as the reported sexually dimorphic adipose distribution is less apparent in some ethnic groups.13,16 Although sex steroids are involved in determining adipose distribution, other factors are certainly important. Indeed, Chen et al. recently reported that the number of X chromosomes affects adipose tissue function in mice.17 This study suggests that genes expressed on the X chromosome are specifically implicated in the control of adiposity and that dosage or parental imprinting of these genes may be involved in the metabolic differences between males and females.
The differential location of fat has functional implications as adipokine production, development of insulin sensitivity, inflammatory responses, mitochondrial function, lipolysis, and free fatty acid (FFA) release differs between adipose depots.1,12,18-20 One clear example of the importance of adipose distribution vs. total body fat is circulating leptin levels. Leptin is a hormone that is primarily produced by adipose tissue,21 with circulating levels being positively correlated with total body fat in both human adults and children,22,23 as well as in rodents.24 Serum leptin levels are sexually dimorphic in post-pubertal humans22,25 and rodents;26,27 however, women have higher levels than men,22,25,28,29 while male rodents have higher levels than females.26,27 As leptin mRNA expression has been shown to vary between adipose depots,30,31 the difference in absolute amounts of each type of adipose tissue could contribute to the sex differences in circulating leptin levels and hence metabolic functions. Moreover, even within a specific anatomical location, leptin expression levels differ between males and females,30 with differences also being seen prepubertally.32 Thus, although sex steroids modulate leptin levels in an anatomically specific manner,33 factors other than gonadal steroid levels and fat distribution are also involved in determining the difference in leptin levels between males and females.
Information is rapidly accumulating regarding the differential expression of numerous proteins in the distinct adipose depots, leading us closer to understanding the variations in their metabolic responses. Karastergiou and colleagues34 recently reported that a number of genes are differentially expressed in abdominal and gluteal adipose of young adults in a non-BMI-dependent manner. Some of these differences are found in both sexes, while others are specific to either males or females.34 These studies further support the concept that the differences in adipose tissue metabolism between males and females are not only due to the amount of a specific fat depot, but also to the fact that there are numerous differences between the sexes in the functionality of each specific type of adipose tissue.
Sexual Dimorphism in Functional Capacity of WAT
The metabolic rate per kilogram adipose tissue is reported to be higher in woman than in men and this could be due to increased expression of various genes involved in mitochondrial function, including uncoupling protein (UCP)-1.35 Moreover, these authors report that the difference in UCP-1 levels is specific to WAT. Numerous genes are differentially expressed in adipose tissue from obese males and females both before and after weight loss.36 After controlling for fat mass, Viguerie et al.36 report that the expression of 88 genes differs between males and females, with 5 of these genes being located on sex chromosomes. Many of these genes code for proteins involved in immune response as well as lipid, protein, or carbohydrate metabolism. The expression of clock genes (PER2, BMAL1, and CRY1) are also higher in subcutaneous and visceral fat from obese females compared with males, with the differences being greatest in visceral fat.37 As discussed below, dissimilarities in circulating levels of gonadal steroids may underlie some of these sex differences, but others appear to be independent of gonadal hormones.
Sex Differences in Inputs and Signals Affecting WAT
Adipose tissue also responds to hormones and factors produced in the pituitary or hypothalamus and in 2006 Schäffler and colleagues38 proposed that these signals be called adipotropins. Growth hormone (GH) stimulates lipolysis39 and antagonizes insulin’s effects on adipocytes40 and the GH receptor (GHR) has been shown to be reduced in adipose tissue from obese women,41 indicating a physiological implication of this receptor during obesity. Prolactin is reported to modulate adipose tissue development and adipokine production,39 while thyrotropin stimulating hormone (TSH) also affects adipocyte turnover and cytokine production.42-44 Circulating levels of many of these anterior pituitary hormones are also sexually dimorphic and/or influenced by sex steroids,45-47 suggesting that the effects of these hormones on adipose tissue will differ between males and females.
Catecholamines modulate lipolysis in adipose tissue, with this effect being both site- and sex-dependent. Basal lipolysis was shown to be higher in visceral and subcutaneous fat from obese men compared with obese women, with noradrenaline-stimulated lipolysis being greater in visceral compared with subcutaneous fat in both sexes.48 The larger adipocyte size and greater HSL activity in adipose tissue from men could explain the higher basal lipolytic activity compared with women. In both visceral and subcutaneous fat women have higher β1 and β3 adrenergic receptor levels, which would indicate increased capacity of lipid mobilization compared with males.48
Diverse structural or anatomical variations are most likely involved in some of the functional differences between adipose tissues from males and females. For example, adipose tissue is innervated by sympathetic efferents and these innervations are distinct between males and females. Male rats have more neurons projecting to abdominal fat, whereas females have more projections to subcutaneous fat.49 The number of estrogen receptor (ER)-α expressing neurons projecting to subcutaneous fat was shown to be lower in males compared with females.49 In addition, projections from the hypothalamus to WAT also differ between males and females. In both sexes the majority of neurons projecting to WAT from the arcuate nucleus are proopiomelanocortin (POMC) producing cells,49 with these neurons also being sensitive to estradiol.50 Lateral hypothalamic neurons expressing orexin and melanin-concentrating hormone (MCH) project to both retroperitoneal WAT and subcutaneous (inguinal) WAT, but in males the majority of MCH projections are to inguinal WAT.49 Thus, in males and females these adipose depots receive different inputs from neuronal circuits intricately involved in metabolic control.
Sex Steroids and Adipose Tissue
Estrogens have been widely reported to protect against adipose accumulation, particularly that of subcutaneous fat depots in post-menopausal women, and to diminish inflammatory signaling and improve insulin action.51-55 Estrogens act through two nuclear receptors, ERα and ERβ, both of which are expressed in adipose tissue,55,56 although ERα is reported to be more highly expressed in this tissue than ERβ.55 Both receptor isoforms are present in mitochondria of WAT,57 suggesting important influences on cell metabolism. Regulation of these two receptors in adipose tissue by factors such as diet or estrogens themselves is divergent as high fat diet (HFD) intake and ovariectomy decrease ERα in WAT of female rats, with no effect on ERβ.56 This observation may suggest different metabolic roles for these two receptor isoforms.
Many of the protective effects of estrogens have been shown to be mediated through ERα, with reduced ERα action causing increased adiposity in humans and mice of both sexes.55,58,59 Loss of ERα also increases the adverse effects of HFD.55,56 Different mechanisms have been proposed for the protective effects of estrogens. Ribas and colleagues55 reported that the HFD-induced increase in heat shock protein (HSP)72 is not found in ERα KO females.55 As this chaperone protein is protective against inflammation,60 the inability to increase HSP72 in response to HFD in the absence of the ERα may contribute to the increased susceptibility to insulin resistance.56 Likewise, these KO animals did not increase circulating adiponectin levels in response to HFD as found in controls.55 As this adipokine is reported to be protective toward the development of insulin resistance,61 the inability to increase its production may also contribute to increased insulin resistance susceptibility. Activation of ERα not only has an effect on total adiposity, but also on adipocyte size,59 which could indicate that estrogens affect the ability of individual adipocytes to accumulate triglycerides.
Despite the fact that it has been less well studied, ERβ also appears to play a role in adipose tissue physiology. Although ERβ KOs have similar body weight, fat distribution, and insulin sensitivity compared with wild-type (WT) mice,62 their response to a HFD is modified.63 In effect, mice lacking ERβ gain more weight and adipose tissue on a HFD, with liver and muscle accumulation of triglycerides being decreased and insulin sensitivity better compared with WT animals. ERβ impairment of insulin sensitivity may be at least partially due to the negative cross-talk of this receptor with PPAR gamma signaling in adipose tissue.63 Treatment with ERβ agonists have also been reported to reduce visceral fat mass and adipocyte size. Glucose transporter 4 (GLUT4) is the primary regulator of glucose uptake in adipose tissue,64 with levels of this transporter shown to be regulated by both ERα and ERβ activation.65,66 Recent evidence indicates that ERβ may be involved in epigenetic regulation of GLUT4 expression.66 Advances in our understanding of estrogen’s role in adipose tissue development have also been made during the past year. Mesenteric estrogen-dependent adipose (meda)-4 is involved in control of adipocyte maturation, possibly through effects on PPARγ and the expression of this protein is modulated by estrogens.67
Androgens have sex-specific effects on WAT,68 with their actions on adipose possibly differing between rodents and primates. KO mice for the androgen receptor (AR) specifically in WAT do not become obese,69 supporting the reported results on castration of mice.12 Androgen depletion reportedly does not modify obesity or insulin resistance in non-human primates.68 However, castration markedly changes visceral WAT morphology, with the lack of testosterone resulting in smaller adipocytes with an abnormal multilocular appearance.68 Androgens have adipogenic effects and improve insulin sensitivity in male adipose tissue, but are reported to have negative effects on insulin sensitivity in female adipose tissue.68,70
The metabolic response of adipose tissue to progesterone also differs between sexes. Females are reported to increase production of leptin, resistin, and adiponectin in response to this hormone with males having no response.71 This differential response is most likely due, at least in part, to higher levels of progesterone receptors in adipose tissue from females compared with males.71
Differential Response to High Fat Diet Intake
Females tend to be less susceptible to many of the detrimental effects of HFD intake. When subjected to a HFD males have been reported to have both higher absolute and relative weight gain and fat mass indexes than females, and this is associated with more greatly impaired glucose tolerance in males.72-77 However, other authors have reported similar HFD-induced weight/adipose gain between males and females78 or that the percent change in body weight or fat mass relative to baseline is greater in females, with this response being modified by the maternal hormonal or nutritional environments.79,80 The type of diet employed may be a factor in determining these differential outcomes as not all diets are equal in the percentage or types of fat. Another confounding factor may be the time of exposure to HFD as extended exposure appears to equalize the differences between the sexes in weight gain.81 None the less, in all of these studies glucose tolerance or insulin sensitivity was more affected in males.
The HFD-induced weight or adipose tissue gain is also accompanied by sex differences in the changes in gene expression in the different adipose depots.75 Genes involved in inflammatory responses are more highly upregulated in males compared with females and this is only partially explained by circulating sex steroid levels.73,75 Furthermore, regardless of dietary intake genes related to insulin signaling and lipid synthesis were shown to have higher expression levels in females compared with males.75 Some genes are also inversely regulated by HFD, as LPL decreases in females and increases in males,72 while adiponectin and PPARγ mRNA levels decrease in males and increase in females.73 Thus, the differences between the sexes in the development of HFD-induced insulin resistance and the adipose inflammation are most likely interrelated.
The fact that females have often been shown to gain less weight than males when exposed to a HFD could explain the lack of or delay in development of secondary complications reported in many studies. However, even in studies when the same body weight is obtained in response to HFD females have better glucose tolerance than males and, in contrast to males, do not develop hyperglycemia73,76,78 and when females have been shown to gain more relative body weight or adiposity they still remain more glucose-tolerant.80 In the study by Nickleson and colleagues,76 although males and females were studied at the same weight after exposure to a HFD, females took longer to obtain this weight. Females also had more fat mass and larger adipocytes and although gonadal adipose production of leptin was similar between the sexes, males had reduced adiponectin production.76 Males also had increased clusters of proinflammatory immune cells and oxidative stress markers in both gonadal fat and subcutaneous fat.76 Medrikova et al.81 also report that in response to HFD-induced weight gain male mice have greater macrophage infiltration of both gonadal and subcutaneous adipose tissue compared with females, although inflammatory markers in gonadal adipose were similar between the sexes. They also indicate that in general, macrophage infiltration is lower in subcutaneous adipose tissue. Similar results have been reported by Pettersson et al.,78 where males were shown to be more susceptible to increased pro-inflammatory macrophage infiltration after HFD. These authors show that there is also a decrease in anti-inflammatory lymphocytes in males, while this population of T cells increases in adipose of HFD-fed females. Thus, both changes in macrophage subtypes may contribute to females being more resistant to development of further complications.
These differential adipose inflammatory responses could help to explain why females are less prone to developing or have delayed development of insulin insensitivity/diabetes. However, the mechanisms underlying the different responses of males and females remain to be fully elucidated. Indeed, in a study of young obese humans, although macrophage infiltration in subcutaneous adipose tissue was associated with hyperinsulinemia and upregulation of the NFκB stress pathway, this association was not dependent on sex.82 The inflammatory response in adipose tissue most likely depends on numerous factors including not only the degree of obesity, but also its duration. Hence, cross-sectional studies of obese individuals may not detect differences between men and women in the sensitivity to or onset of adipose infiltration.
The mechanisms for sex- and depot-specific adipose tissue formation in response to HFD remain unclear. The sex differences in adipose mitochondrial response to HFD could be involved in this phenomenon. In retroperitoneal adipose, HFD induces mitochondrial proliferation in male rats and mitochondrial differentiation in females.83 In addition, in response to HFD, females have a greater expandability capacity of retroperitoneal WAT, which could help to explain their increased protection from developing insulin insensitivity compared with males.83 Perigonadal adipose from female rats is reported to have a higher expandability capacity, low hypoxic and pro-inflammatory state and no signs of mitochondrial dysfunction compared with males.84 Recent data suggest that visceral fat formation in response to HFD involves sex-specific actions of aldehyde dehydrogenase 1 (Aldh1), a class of retinoic acid (RA) producing enzymes that are involved in sex-specific fat depot formation.85 Silencer of cytokines (SOCS) 3 may also be involved in the differential response to dietary challenges as KO of this protein in adipose tissue protects female mice against insulin resistance, but not male mice.86
Intake of a HFD also modifies circulating sex steroid levels. Serum testosterone levels are reduced in response to a HFD,87 with decreased testosterone being associated to increased abdominal fat.88 In females, estrogens increase in response to HFD,89 which could be protective. Adipose tissue is capable of aromatizing androgens to estrogens and this is modified by both age and obesity.90,91 Thus, not only do sex steroids affect adipose tissue metabolism, but adipose tissue modulates circulating levels of these steroids.
Summary
There are clear differences in the white adipose tissue of males and females at many levels (Fig. 1) including quantity, distribution, metabolic capacity, inputs, etc. Some of these differences are due to direct activational effects of sex steroids or are mediated indirectly through other factors that are modified by sex steroids, while others appear to be inherent to each sex and thus not sex steroid-dependent. Here we have briefly reviewed some of the recent advances in delineating the mechanisms underlying this important aspect of adipose tissue, but much is yet to be learned. Understanding these differences and the mechanisms involved will undoubtedly lead to better therapies for the treatment of obesity and its complications, with some of these treatments surely having different efficaciousness in males and females.
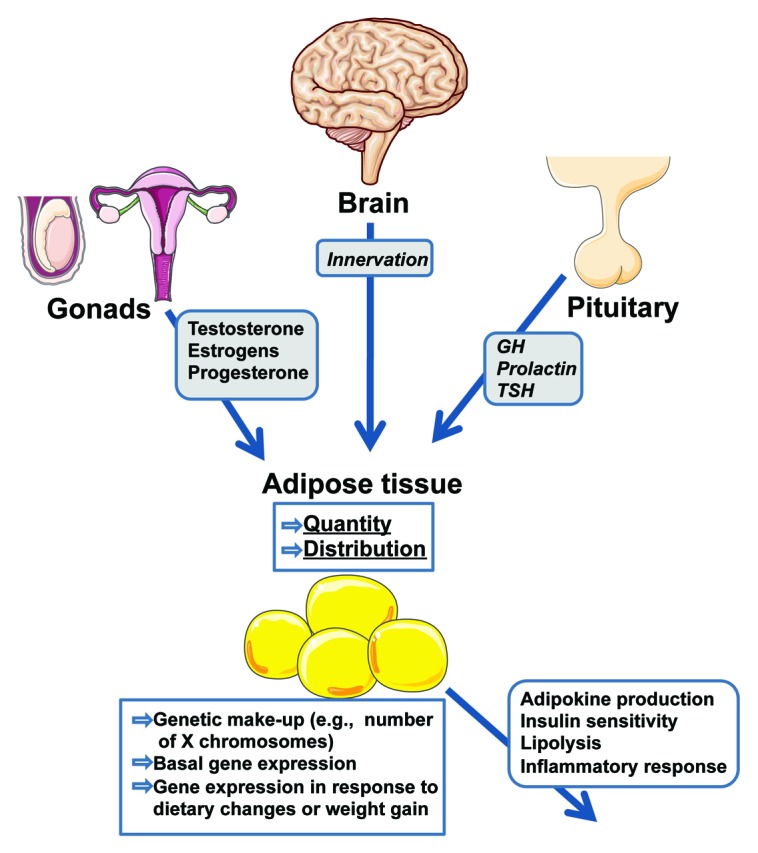
Figure 1. Schematic summary of the differences between adipose tissue from males and females discussed in this review. The genetic make-up and basal gene expression of adipose tissue differs between the sexes. This, in addition to diverse signals and inputs to adipose tissue that differ between males and females, results in differences in the quantity and distribution of adipose tissue, as well as its responses and outputs.
We would also like to emphasize the fact that much of the information in the literature pertaining to metabolism and obesity has been derived from studies performed in only one sex; many of these results may or may not be directly applicable to the opposite sex. Here we have only reviewed some of the sex differences in white adipose tissue, when in fact there are differences at all levels of the metabolic control system. Hence, it would be of great benefit for the scientific community to have more data available that directly compares outcomes or experimental parameters in males and females. Moreover, other factors such as age and sex steroid levels (prepubertal, young adult, post-menopausal, etc.) should be more precisely defined and compared in metabolic studies. Indeed, as childhood obesity has come to the forefront as an important health concern research in this area has augmented considerably and we are beginning to understand that not all mechanisms previously described in obese adults can be directly applied to obese prepubertal children. The concept that we are not dealing with “obesity” but “obesities” needs to become more widely accepted. Understanding this may facilitate the development of specific treatments for the different causes underlying excess weight gain.
Acknowledgments
The authors are funded by grants from Ministerio de Ciencia e Innovación (BFU2011-27492), Fondos de Investigación Sanitaria (PI100747), Centro de Investigación Biomedica en Red Fisiopatología de Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III, and Fundación de Endocrinología y Nutrición.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/24075
References
- 1.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99:931–40. doi: 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]
- 2.Kanter R, Caballero B. Global gender disparities in obesity: a review. Adv Nutr. 2012;3:491–8. doi: 10.3945/an.112.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiyama MG, Agellon LB. Sex differences in lipid metabolism and metabolic disease risk. Biochem Cell Biol. 2012;90:124–41. doi: 10.1139/o11-067. [DOI] [PubMed] [Google Scholar]
- 4.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer A, Kauter K, Brown L. Gender differences in metabolic syndrome: a key research issue? Endocr Metab Immune Disord Drug Targets. 2011;11:182–8. doi: 10.2174/187153011796429808. [DOI] [PubMed] [Google Scholar]
- 6.Lovejoy JC, Sainsbury A, Stock Conference 2008 Working Group Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–67. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 7.He Q, Horlick M, Thornton J, Wang J, Pierson RN, Jr., Heshka S, et al. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. J Clin Endocrinol Metab. 2002;87:2164–70. doi: 10.1210/jc.87.5.2164. [DOI] [PubMed] [Google Scholar]
- 8.Taylor RW, Grant AM, Williams SM, Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring) 2010;18:1410–6. doi: 10.1038/oby.2009.399. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–39. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 10.Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–2. [PubMed] [Google Scholar]
- 11.Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. Gender differences in plasma leptin concentrations. Nat Med. 1996;2:949–50. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- 12.Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58:803–12. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–93. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman-Gruen D, Barrett-Connor E. Sex differences in measures of body fat and body fat distribution in the elderly. Am J Epidemiol. 1996;143:898–906. doi: 10.1093/oxfordjournals.aje.a008833. [DOI] [PubMed] [Google Scholar]
- 15.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–8. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86:353–9. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/er.21.6.697. [DOI] [PubMed] [Google Scholar]
- 19.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–20. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–59. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 21.Green ED, Maffei M, Braden VV, Proenca R, DeSilva U, Zhang Y, et al. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res. 1995;5:5–12. doi: 10.1101/gr.5.1.5. [DOI] [PubMed] [Google Scholar]
- 22.Argente J, Barrios V, Chowen JA, Sinha MK, Considine RV. Leptin plasma levels in healthy Spanish children and adolescents, children with obesity, and adolescents with anorexia nervosa and bulimia nervosa. J Pediatr. 1997;131:833–8. doi: 10.1016/S0022-3476(97)70029-5. [DOI] [PubMed] [Google Scholar]
- 23.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 24.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 25.Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, et al. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59:1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- 26.Landt M, Gingerich RL, Havel PJ, Mueller WM, Schoner B, Hale JE, et al. Radioimmunoassay of rat leptin: sexual dimorphism reversed from humans. Clin Chem. 1998;44:565–70. [PubMed] [Google Scholar]
- 27.Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 2009;6:17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellström L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. 2000;247:457–62. doi: 10.1046/j.1365-2796.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–300. doi: 10.1210/jc.82.4.1293. [DOI] [PubMed] [Google Scholar]
- 30.Montague CT, Prins JB, Sanders L, Digby JE, O’Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–7. doi: 10.2337/diabetes.46.3.342. [DOI] [PubMed] [Google Scholar]
- 31.Masuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, Shigemoto M, et al. Human obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes. 1995;44:855–8. doi: 10.2337/diabetes.44.7.855. [DOI] [PubMed] [Google Scholar]
- 32.Devaskar SU, Ollesch C, Rajakumar RA, Rajakumar PA. Developmental changes in ob gene expression and circulating leptin peptide concentrations. Biochem Biophys Res Commun. 1997;238:44–7. doi: 10.1006/bbrc.1997.7237. [DOI] [PubMed] [Google Scholar]
- 33.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–74. doi: 10.1210/en.140.4.1567. [DOI] [PubMed] [Google Scholar]
- 34.Karastergiou K, Fried SK, Xie H, Lee M-J, Divoux A, Rosencrantz MA, et al. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab. 2013;98:362–71. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nookaew I, Svensson P-A, Jacobson P, Jernås M, Taube M, Larsson I, et al. Adipose Tissue Resting Energy Expenditure and Expression of Genes Involved in Mitochondrial Function Are Higher in Women than in Men. J Clin Endocrinol Metab. 2013;98:E370–8. doi: 10.1210/jc.2012-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viguerie N, Montastier E, Maoret J-J, Roussel B, Combes M, Valle C, et al. Determinants of human adipose tissue gene expression: impact of diet, sex, metabolic status, and cis genetic regulation. PLoS Genet. 2012;8:e1002959. doi: 10.1371/journal.pgen.1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gómez-Abellán P, Madrid JA, Luján JA, Frutos MD, González R, Martínez-Augustín O, et al. Sexual dimorphism in clock genes expression in human adipose tissue. Obes Surg. 2012;22:105–12. doi: 10.1007/s11695-011-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schäffler A, Schölmerich J, Buechler C. The role of ‘adipotropins’ and the clinical importance of a potential hypothalamic-pituitary-adipose axis. Nat Clin Pract Endocrinol Metab. 2006;2:374–83. doi: 10.1038/ncpendmet0197. [DOI] [PubMed] [Google Scholar]
- 39.Flint DJ, Binart N, Boumard S, Kopchick JJ, Kelly P. Developmental aspects of adipose tissue in GH receptor and prolactin receptor gene disrupted mice: site-specific effects upon proliferation, differentiation and hormone sensitivity. J Endocrinol. 2006;191:101–11. doi: 10.1677/joe.1.06939. [DOI] [PubMed] [Google Scholar]
- 40.del Rincon J-P, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, et al. Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes. 2007;56:1638–46. doi: 10.2337/db06-0299. [DOI] [PubMed] [Google Scholar]
- 41.Erman A, Veilleux A, Tchernof A, Goodyer CG. Human growth hormone receptor (GHR) expression in obesity: I. GHR mRNA expression in omental and subcutaneous adipose tissues of obese women. Int J Obes (Lond) 2011;35:1511–9. doi: 10.1038/ijo.2011.23. [DOI] [PubMed] [Google Scholar]
- 42.Bell A, Gagnon A, Dods P, Papineau D, Tiberi M, Sorisky A. TSH signaling and cell survival in 3T3-L1 preadipocytes. Am J Physiol Cell Physiol. 2002;283:C1056–64. doi: 10.1152/ajpcell.00058.2002. [DOI] [PubMed] [Google Scholar]
- 43.Menendez C, Baldelli R, Camiña JP, Escudero B, Peino R, Dieguez C, et al. TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol. 2003;176:7–12. doi: 10.1677/joe.0.1760007. [DOI] [PubMed] [Google Scholar]
- 44.Antunes H, Santos C, Carvalho S. Serum leptin levels in overweight children and adolescents. Br J Nutr. 2009;101:1262–6. doi: 10.1017/S0007114508055682. [DOI] [PubMed] [Google Scholar]
- 45.Tong YA, Zhao HF, Labrie F, Pelletier G. Ontogeny of prolactin mRNA in the rat pituitary gland as evaluated by in situ hybridization. Mol Cell Endocrinol. 1989;67:11–6. doi: 10.1016/0303-7207(89)90225-6. [DOI] [PubMed] [Google Scholar]
- 46.Chowen JA, García-Segura LM, González-Parra S, Argente J. Sex steroid effects on the development and functioning of the growth hormone axis. Cell Mol Neurobiol. 1996;16:297–310. doi: 10.1007/BF02088097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meinhardt UJ, Ho KKY. Modulation of growth hormone action by sex steroids. Clin Endocrinol (Oxf) 2006;65:413–22. doi: 10.1111/j.1365-2265.2006.02676.x. [DOI] [PubMed] [Google Scholar]
- 48.Ramis JM, Salinas R, García-Sanz JM, Moreiro J, Proenza AM, Lladó I. Depot- and gender-related differences in the lipolytic pathway of adipose tissue from severely obese patients. Cell Physiol Biochem. 2006;17:173–80. doi: 10.1159/000092079. [DOI] [PubMed] [Google Scholar]
- 49.Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci. 2012;32:15913–21. doi: 10.1523/JNEUROSCI.2591-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology. 1990;52:581–8. doi: 10.1159/000125647. [DOI] [PubMed] [Google Scholar]
- 51.Brussaard HE, Gevers Leuven JA, Frölich M, Kluft C, Krans HM. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia. 1997;40:843–9. doi: 10.1007/s001250050758. [DOI] [PubMed] [Google Scholar]
- 52.Lee CC, Kasa-Vubu JZ, Supiano MA. Differential effects of raloxifene and estrogen on insulin sensitivity in postmenopausal women. J Am Geriatr Soc. 2003;51:683–8. doi: 10.1034/j.1600-0579.2003.00214.x. [DOI] [PubMed] [Google Scholar]
- 53.Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res. 2001;89:823–30. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 54.Sun WH, Keller ET, Stebler BS, Ershler WB. Estrogen inhibits phorbol ester-induced I kappa B alpha transcription and protein degradation. Biochem Biophys Res Commun. 1998;244:691–5. doi: 10.1006/bbrc.1998.8324. [DOI] [PubMed] [Google Scholar]
- 55.Ribas V, Nguyen MTA, Henstridge DC, Nguyen A-K, Beaven SW, Watt MJ, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–19. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorres BK, Bomhoff GL, Gupte AA, Geiger PC. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J Appl Physiol. 2011;110:1046–53. doi: 10.1152/japplphysiol.00541.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J-Q, Cammarata PR, Baines CP, Yager JD. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta. 2009;1793:1540–70. doi: 10.1016/j.bbamcr.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 59.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung J, Nguyen A-K, Henstridge DC, Holmes AG, Chan MHS, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–44. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasseur F, Meyre D, Froguel P. Adiponectin, type 2 diabetes and the metabolic syndrome: lessons from human genetic studies. Expert Rev Mol Med. 2006;8:1–12. doi: 10.1017/S1462399406000147. [DOI] [PubMed] [Google Scholar]
- 62.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-Y M, Rudling M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–5. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 63.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–61. doi: 10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 65.Gorres BK, Bomhoff GL, Morris JK, Geiger PC. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J Physiol. 2011;589:2041–54. doi: 10.1113/jphysiol.2010.199018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rüegg J, Cai W, Karimi M, Kiss NB, Swedenborg E, Larsson C, et al. Epigenetic regulation of glucose transporter 4 by estrogen receptor β. Mol Endocrinol. 2011;25:2017–28. doi: 10.1210/me.2011-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Chen X, Sairam MR. Novel genes of visceral adiposity: identification of mouse and human mesenteric estrogen-dependent adipose (MEDA)-4 gene and its adipogenic function. Endocrinology. 2012;153:2665–76. doi: 10.1210/en.2011-2008. [DOI] [PubMed] [Google Scholar]
- 68.Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153:3100–10. doi: 10.1210/en.2011-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu I-C, Lin H-Y, Liu N-C, Wang R-S, Sparks JD, Yeh S, et al. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149:2361–8. doi: 10.1210/en.2007-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192:585–94. doi: 10.1677/joe.1.07070. [DOI] [PubMed] [Google Scholar]
- 71.Stelmanska E, Kmiec Z, Swierczynski J. The gender- and fat depot-specific regulation of leptin, resistin and adiponectin genes expression by progesterone in rat. J Steroid Biochem Mol Biol. 2012;132:160–7. doi: 10.1016/j.jsbmb.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Estrany ME, Proenza AM, Lladó I, Gianotti M. Isocaloric intake of a high-fat diet modifies adiposity and lipid handling in a sex dependent manner in rats. Lipids Health Dis. 2011;10:52. doi: 10.1186/1476-511X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estrany ME, Proenza AM, Gianotti M, Lladó I. High-fat diet feeding induces sex-dependent changes in inflammatory and insulin sensitivity profiles of rat adipose tissue. Cell Biochem Funct. 2013;31:504–10. doi: 10.1002/cbf.2927. [DOI] [PubMed] [Google Scholar]
- 74.Garg N, Thakur S, McMahan CA, Adamo ML. High fat diet induced insulin resistance and glucose intolerance are gender-specific in IGF-1R heterozygous mice. Biochem Biophys Res Commun. 2011;413:476–80. doi: 10.1016/j.bbrc.2011.08.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 2010;34:989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nickelson KJ, Stromsdorfer KL, Pickering RT, Liu T-W, Ortinau LC, Keating AF, et al. A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice. Exp Diabetes Res. 2012;2012:859395. doi: 10.1155/2012/859395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El Akoum S, Lamontagne V, Cloutier I, Tanguay J-F. Nature of fatty acids in high fat diets differentially delineates obesity-linked metabolic syndrome components in male and female C57BL/6J mice. Diabetol Metab Syndr. 2011;3:34. doi: 10.1186/1758-5996-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pettersson US, Waldén TB, Carlsson P-O, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7:e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pennington KA, Harper JL, Sigafoos AN, Beffa LM, Carleton SM, Phillips CL, et al. Effect of food restriction and leptin supplementation on fetal programming in mice. Endocrinology. 2012;153:4556–67. doi: 10.1210/en.2012-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nadal-Casellas A, Proenza AM, Lladó I, Gianotti M. Sex-dependent differences in rat hepatic lipid accumulation and insulin sensitivity in response to diet-induced obesity. Biochem Cell Biol. 2012;90:164–72. doi: 10.1139/o11-069. [DOI] [PubMed] [Google Scholar]
- 81.Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond) 2012;36:262–72. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- 82.Lê K-A, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes. 2011;60:2802–9. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amengual-Cladera E, Lladó I, Gianotti M, Proenza AM. Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolism. 2012;61:1108–17. doi: 10.1016/j.metabol.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Amengual-Cladera E, Lladó I, Proenza AM, Gianotti M. High-fat diet feeding induces a depot-dependent response on the pro-inflammatory state and mitochondrial function of gonadal white adipose tissue. Br J Nutr. 2013;109:413–24. doi: 10.1017/S0007114512001171. [DOI] [PubMed] [Google Scholar]
- 85.Yasmeen R, Reichert B, Deiuliis J, Yang F, Lynch A, Meyers J, et al. Autocrine function of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes. 2013;62:124–36. doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palanivel R, Fullerton MD, Galic S, Honeyman J, Hewitt KA, Jorgensen SB, et al. Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance. Diabetologia. 2012;55:3083–93. doi: 10.1007/s00125-012-2665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cano P, Jiménez-Ortega V, Larrad A, Reyes Toso CF, Cardinali DP, Esquifino AI. Effect of a high-fat diet on 24-h pattern of circulating levels of prolactin, luteinizing hormone, testosterone, corticosterone, thyroid-stimulating hormone and glucose, and pineal melatonin content, in rats. Endocrine. 2008;33:118–25. doi: 10.1007/s12020-008-9066-x. [DOI] [PubMed] [Google Scholar]
- 88.Cifuentes M, Morano AB, Chowdhury HA, Shapses SA. Energy restriction reduces fractional calcium absorption in mature obese and lean rats. J Nutr. 2002;132:2660–6. doi: 10.1093/jn/132.9.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shinoda M, Latour MG, Lavoie J-M. Effects of physical training on body composition and organ weights in ovariectomized and hyperestrogenic rats. Int J Obes Relat Metab Disord. 2002;26:335–43. doi: 10.1038/sj.ijo.0801900. [DOI] [PubMed] [Google Scholar]
- 90.Forney JP, Milewich L, Chen GT, Garlock JL, Schwarz BE, Edman CD, et al. Aromatization of androstenedione to estrone by human adipose tissue in vitro. Correlation with adipose tissue mass, age, and endometrial neoplasia. J Clin Endocrinol Metab. 1981;53:192–9. doi: 10.1210/jcem-53-1-192. [DOI] [PubMed] [Google Scholar]
- 91.Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–7. doi: 10.1210/jcem-60-1-174. [DOI] [PubMed] [Google Scholar]


