Abstract
Dedifferentiated fat cells (DFAT cells) are derived from lipid-containing (mature) adipocytes, which possess the ability to symmetrically or asymmetrically proliferate, replicate, and redifferentiate/transdifferentiate. Robust cell isolation and downstream culture methods are needed to isolate large numbers of DFAT cells from any (one) adipose depot in order to establish population dynamics and regulation of the cells within and across laboratories. In order to establish more consistent/repeatable methodology here we report on two different methods to establish viable DFAT cell cultures: both traditional cell culture flasks and non-traditional (flat) cell culture plates were used for ceiling culture establishment. Adipocytes (maternal cells of the DFAT cells) were easier to remove from flat culture plates than flasks and the flat plates also allowed cloning rings to be utilized for cell/cell population isolation. While additional aspects of usage of flat-bottomed cell culture plates may yet need to be optimized by definition of optimum bio-coating to enhance cell attachment, utilization of flat plate approaches will allow more efficient study of the dedifferentiation process or the DFAT progeny cells. To extend our preliminary observations, dedifferentiation of Wagyu intramuscular fat (IMF)-derived mature adipocytes and redifferentiation ability of DFAT cells utilizing the aforementioned isolation protocols were examined in traditional basal media/differentiation induction media (DMI) containing adipogenic inducement reagents. In the absence of treatment approximately 10% isolated Wagyu IMF-mature adipocytes dedifferentiated spontaneously and 70% DFAT cells displayed protracted adipogenesis 12 d after confluence in vitro. Lipid-free intracellular vesicles in the cytoplasm (vesicles possessing an intact membrane but with no any observable or stainable lipid inside) were observed during redifferentiation. One to 30% DFAT cells redifferentiated into lipid-assimilating adipocytes in the DMI media, with distinct lipid-droplets in the cytoplasm and with no observable lipid-free vesicles inside. Moreover, a high confluence level promoted the redifferentiation efficiency of DFAT cells. Wagyu IMF dedifferentiated DFAT cells exhibited unique adipogenesis modes in vitro, revealing a useful cell model for studying adipogenesis and lipid metabolism.
Keywords: adipocytes, DFAT cells, differentiation, vesicles, adipogenesis, ceiling culture
Introduction
During growth and development of adipose depots, preadipocyte-like cells pass from a proliferation stage to a mitosis quiescent stage resulting in the storage of lipids. This process is called pre-adipocyte conversion/differentiation and is characterized by assimilation of lipid1 and expansion /shrinking in size depending on lipid load.2,3 A general dogma in biology has prevailed that once mitosis ceases, differentiated adipocytes lose any ability to dedifferentiate or proliferate again.4-6 Through the use of ceiling cell cultures, however, cloned mature (lipid-filled) adipocytes have been shown to extrude lipid droplets into the media,7-9 or transfer lipid droplets into daughter cells.10-12 Thus, mature adipocytes appear capable of resuming proliferation in vitro.7-12 Proliferation of mature adipocytes was first reported in human-derived adipocytes,13,14 followed by subsequent reports from other researchers using adipocytes derived from different animal species.7-12,15,16 This (dedifferentiation) process17,18 results in progeny cells with a fibroblast-like appearance,9,16,19-22 which reestablishes the proliferation program,19,22,23 and has been termed proliferative-competent progeny cells or dedifferentiated fat cells (DFAT cells).10,17,18,24-28
Numerous potential stem cells reside within adipose tissue,29,30 but until recently most of these were identified within the mixed stromal vascular (SV) cell fraction.31,32 Demonstration of potential for mature adipocytes to revert back to stem-like cells is a relatively new observation.26,29 Experimental studies will be needed to gain enhanced understanding of the population dynamics and regulation(s) of this (cellular) dedifferentiation process. For example, mature adipocytes were reported to possess the capacity to differentiate into cardiomyocyte-like cells spontaneously in vitro.33 Media/manipulations used to initiate differentiation of mature adipocytes were simple compared with other potential cell sources (such as SV cells).33 An extensive body of literature has been established in this area,7-28,33-46 most of which centers around use of these cells for tissue reconstruction purposes,15,35,37 clinical use,27,33,38,39,47 or in transdifferentiation of DFAT cells to form other types of cells.28,33,35,36,47 Mature adipocytes may be a promising source for cell-based tissue engineering and regeneration.
Isolation methods based on cell culture procedures have evolved with which to harvest populations of mature adipocytes,13,14,20,40,48,49 individual mature adipocytes as clones,11 or maternal precursors of DFAT cells.11,19,37 Principal approaches currently used to isolate mature adipocytes are ceiling culture methods,9,11,19,20,37,40,48-52 which are based on the buoyancy properties of mature lipid- filled adipocytes.52 After preliminary isolation steps, lipid-filled adipocytes float, and subsequently attach, to the ceiling of an inverted cell culture flask.52 Alternatively, and especially due to the multiple wash/removal steps while reserving the mature adipocytes,48 any remnant SV cells fall to the lower surface of the culture flask where they attach and are physically separated from the mature adipocytes.51 Ceiling culture takes from 115 to 7 d11 for mature adipocytes to firmly attach to the cell culture flask. Then the flask is re-inverted and DFAT cells are formed from the lipid-filled adipocytes.9,11,19,37 Eventually, the DFAT cells do not display cytosolic lipids,9,19,37 acquire a spindle-shaped fibroblast-like appearance,9,19,37 and possess strong proliferative ability.19,23 Ceiling cultures are not a universal panacea to produce pure cultures of DFAT cells unless numerous precautions are employed.53 Even with multiple washing steps,48 floating cells available to attach to the ceiling of a cell culture flask may represent a heterogeneous population of both mature adipocytes and SV cells.11,20,40,48,51 For example, during the cell isolation process, a small number of preadipocytes/fibroblasts/stem cells/other cells with undistinguished morphology but similar buoyancy might be co-isolated and attach together with mature adipocytes, influencing observations on DFAT cells.18,20,54
Obtaining pure populations of mature adipocytes is vital for downstream applications. To this end such procedures may involve novel cell culture methods.7,8,11,20,22,41,48 For example, cell culture surgery (after ceiling culture a pasteur pipette or cell scraper is used to remove any non-lipid containing cells by cell morphology) is a common method to remove contaminating cells.11,41 Another method is the use of (early or late) differential platings,11 whereby mature adipocyte from one ceiling culture are re-cultured in sequential ceiling cultures to insure purity from contaminating cells.7,8,11,20,22 These methods are somewhat cumbersome, time-consuming, tedious,11,20,48 and difficult to transfer from one cell isolation to subsequent ones, or from one laboratory to another. The cell culturist must also recognize that there are potential conflicts between obtaining sufficient representatives of tissue-derived mature adipocytes and SV-type cells.11,20 Moreover, not every mature adipocyte seems capable of reforming proliferative-competent cells,19,34 making it somewhat challenging to acquire pure populations of large numbers of DFAT cells.34 We believe that during development of appropriate cell isolation methodology,42 increased numbers of proliferative-competent maternal cells may be obtained through the use of alterations/manipulations of the culture environment to which isolated cells are initially exposed.
In the present study, cell culture flasks were used in traditional ceiling culture methods,7,8,11,19-22,50-52 in which the medium-filled flasks were inverted to allow the attachment of floating populations of mature adipocytes. Cell surgery and differential plating methods described previously were subsequently employed with flasks to ensure the purity of attached cells.11 Narrow flask entrances make use of pipettes/cell scrapers very inefficient making it difficult to insure purity of all cultures. Subsequent additional methods such as differential plating(s) may be employed to insure culture purity.11,20 Even if differential platings were not necessary, use of traditional cell culture flasks does not allow use cloning rings (cylinders) for cell cloning (an established method for isolating colonies derived from a single cell).11 Alternatively, use of traditional, flat-bottomed cell culture plates have been (until this study) discounted, due to an inability to provide a completely sealed environment for (inverted) ceiling culture. Use of glass coverslips or chamber slides,48 are workable with only a limited amount of cells and are thus useful in only a limited number of experimental designs.
Cattle are increasingly used in adipogenesis/lipid metabolism research.55-57 These animals represent useful genetic models,55,58 as cattle have been subject for many years to intensive genetic selection, for enhanced meat quality including the extent of marbling or intramuscular fat.59 Research with such highly selected animals not only refines the animal carcass quality but also provides a specific research platform for human health-related studies.29,52,60 For example, the staining pattern of embryonic stem (ES) cell-specific markers of bovine ES cells is more similar to human ES than mouse ES cells60 suggesting cattle as a more favorable animal model to be used in scientific studies. Adipocyte research with cattle-derived adipocytes may result in dual benefits to both sustainable meat production and human health issues.55 Finally since adipose depots in cattle are large, the potential for increased cell harvests is increased resulting in sufficient adipocytes derived from different adipose depots for comparative studies.57
In the present study we developed an efficient method to acquire homogeneous cultures of mature adipocytes from beef cattle. Our goal is to accurately assess the regulation and physiology of mature adipocytes as they undergo the reversion (dedifferentiation) process to form DFAT cells. To optimize adipocyte isolation, fat tissue was obtained from the subcutaneous adipose depots (SQ) of Angus cattle. We also obtained fat tissue from skeletal muscle of Wagyu cattle. These cattle are attracting more and more attention due to their uniquely disproportional amount of body fat distribution,61 including deposition of a large amount of intramuscular adipose tissue/fat (IMF).62-65 This “marbling” fat56,61,66 enhances the eating quality of meat and sustains the competitive market of highly marbled beef (especially in Japan).61,62,66 Wagyu animals may serve as a novel model for studying fat cell biology, interactions between adipose tissue and muscle tissue,67,68 and signaling pathways regulating adipogenesis in skeletal muscle.69,70
Here we describe improved culture conditions and optimized methods for acquiring purified DFAT cells. We showed that Wagyu-IMF-derived pure mature adipocytes dedifferentiated and redifferentiated under traditional basal/adipogenic induction (DMI) media, suggesting Wagyu-IMF as a useful adipose tissue model to study cell dedifferentiation/adipogenesis and lipid metabolism.
Results
First trial of plate ceiling culture
Two, ten, and 12 lipid-filled adipocytes attached the surface of the three small dishes on d 3, d 4 and d 5 of cell isolation, respectively (Fig. 1A). The ceiling lid (Fig. 1B) and the control flask (Fig. 1C) were re-inverted on d 5, with 10 mature adipocytes attached the flask and zero cells attached to the lid. The lack of success for lids used here may be related to a lack of proper lid pre-use treatment or “TC treated”. However, the results of this dish culture showed that cell culture plates could be employed during ceiling cultures. Although similar numbers of mature adipocytes attached on ceilings of the plate-dishes or flasks, pasteur pipettes were more easily used for marking the lipid-filled adipocytes or removing contaminating cells from the cell culture plates than the flasks (Fig. 1D vs. E). In addition, cloning cylinders could be used with the plates to acquire homogeneous populations more rapidly (Fig. 1F). These observations suggested that plate ceiling culture might be an efficient method to acquire purified DFAT cells.
Figure 1. After re-inverting the plate/flask on d 5 of ceiling culture. (A and B) Re-invert the small dish/lid and put it into new plates. Add small amount of media and observe mature adipocytes under a microscope. (C) Re-invert the flask and add 5 ml media to observe. (D and E) Mark the lipid-containing adipocytes or do cell surgery (remove contaminating cells) under a hood microscope. (F) Apply clone cylinders in the plate ceiling culture.
Second trial of plate ceiling culture
Additional mature adipocytes were isolated compared with control cultures in the second plate/flask ceiling culture by improving isolation procedures to decrease damage/loss of lipid-filled adipocytes. Isolated mature adipocytes floated in media and started to attach the inner surface of cultureware during ceiling culture (Fig. 2A, C, and E). For all combinations of ceiling culture flasks and plates (dishes/lids), several contaminating areas existed together with lipid-filled adipocytes in ceiling cultures. However morphological differences were not observed among the attached mature adipocytes (Fig. 2B, D, and F). About 120 lipid-filled adipocytes were acquired after re-inverting the flask without a prior centrifugation step. Here, more than 20 contaminating cells/clones were found attached together with mature adipocytes, resulting into a mixed cell population (Fig. 2F). Appropriate centrifugation was necessary to ensure the purity of isolated cells. Around 45 lipid-filled adipocytes attached with the 24 h pre-plated flask ceiling culture, together with 4 contaminating cells/clones. This suggested that during differential plating, large numbers of fat cells were lost and contaminating cells still existed in isolated cell cultures. However, the number of attaching mature adipocytes as well as contaminating cells in plates were much less than in control flask (3 mature adipocytes attached the lid ceiling; 7 mature adipocytes and 1 contaminating group attached the dish ceiling). Different sources of cell cultureware may account for the different ceiling attachments in the second trial. Interestingly, the flask with 50 µl fat layer derived from the middle of the centrifuge tube top layer, resulted in 20 lipid-filled adipocytes only, without any differential plating or cell surgery. This result indicated that most contaminating cells might exist in the media underlying the top layer as well as the juncture between the fat layer and the underlying media. The degree of purification and mature adipocyte isolation efficiency appears to be related to source/quality of culture-ware and the exact position of pasteur pipettes during harvesting of mature adipocytes. The present procedure for mature adipocyte isolation and plate/flask ceiling culture (Fig. 3) could be used in further studies.
Figure 2. Comparisons of plate/flask ceiling cultures with equal amount of fat layers. On d 2 of ceiling culture, several adipocytes/lipids/potential contamination slightly attached to the ceiling of the cell culture plate (A)/flask (C), while another flask containing a large number of adipocytes/lipids/potential contamination without centrifugation (E). On d 5 of ceiling culture, all the plates and flasks were re-inverted and observed under a microscope. Several lipid-filled adipocytes stretched membrane and attached to the ceiling surface of the plate (B)/flask (D) with little contaminating cells/clones, while non-centrifugal cell fraction formed numerous lipid-filled adipocytes as well as contaminating cells/clones (black arrows) in the ceiling surface (F). (A, C, E, and F) 200× magnification; (B and D) 100× magnification.
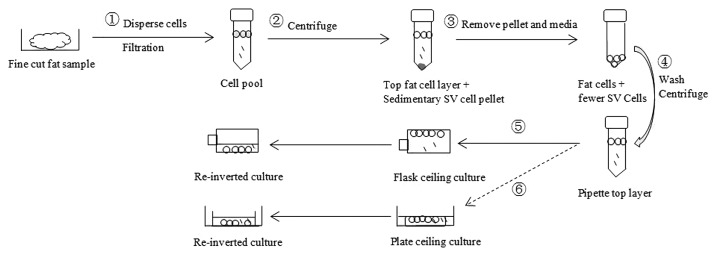
Figure 3. Mature adipocyte isolation and plate/flask ceiling culture. (1) In the hood, collect 5 g of fine cut (about 3 mm pieces) fat fragments into a 50 ml tube containing 20 ml of 0.25% collagenase I (Gibco) solution. Disperse cells gently on a rocker at 37 °C for 1 h, followed by filtering through a 1000 μm mesh into a new 50 ml tube. (2) Centrifuge the cell pool at 186 × g for 10 min. (3) Remove the underlying pellet (SV cells) and media. (4) Wash the fat layer by 20 ml DMEM/F12 + 10% HS, followed by centrifugation at186 x g for 10 min. (5) (flask ceiling culture) Pipette the top layer into a BD Falcon™ T-12.5 cm2 cell culture flask. Fill the flask with DMEM/F12 + 10% HS. The medium-filled flask was tightly closed and inverted for 4–6 d. Then the flask was re-inverted and exposed to a small amount of traditional basal media. (6) (plate ceiling culture) Pipette the top fat layer into a 150 mm × 25 mm cell culture dish containing small amount of media (DMEM/F12 + 10% HS). Then put an inverted 100 mm × 15 mm cell culture dish (ceiling) into the big dish by forceps. Add proper amount of media and chase bubbles out, allowing the solution surface contact with the inner surface of the small dish. Cover the lid of big dish (container) and incubate for 4–6 d for plate ceiling culture. Re-invert the small dish and expose it to small amount of traditional basal media.
Purified ceiling culture and dedifferentiation of Wagyu IMF-mature fat cells
Differential plating was done on d 2 of cell isolation. Five days after ceiling culture, the flask was re-inverted and observed under a microscope. Cell surgery was done to remove any possible cell contamination, leaving pure lipid-filled adipocytes attached. The lipid-filled cells were allowed to dedifferentiate spontaneously, without any induction reagent. Consistent with our previous finding,34 not every attached lipid-filled cell possessed the dedifferentiation/proliferation ability. Most of mature adipocytes died and disappeared several days after cell culturing. Based on close daily observation, 10% of the fat containing cells started to divide and proliferate into daughter cells (this was defined as dedifferentiation efficiency). Once a lipid-filled adipocyte started proliferating, special attention was given to trace its change every day, remove other possible contaminating cells by cell surgery. Eventually, DFAT clones were formed. These cells developed the fibroblast-like appearance, possessed strong proliferative capacity and were used for subsequent experiments.
Adipogenesis of DFAT cells
In the present study, DFAT cells (Fig. 4A) redifferentiated into adipocytes 10 d after applying the FBS-based DMI media. These adipocytes acquired cytoplasmic lipid droplets that were visible by the contrast microscope (Fig. 4B and D) and were confirmed by ORO staining (Fig. 4C and E). Although cells of a single DFAT clone were derived from one mother cell (one lipid-filled mature adipocyte), potential adipogenesis abilities of these cells differed. Few cells in 20% and 50% confluence groups accumulated lipids, cells in the 80% confluence group displayed less than 10% differentiation (Fig. 4E) and DFAT cells in the 100% confluence group had the highest rate of differentiation (around 30%) (Fig. 4C), demonstrating that increased seeding density as well as increased confluence prior to induction by DMI media may promote DFAT cell redifferentiation. Comparing with 100% confluence group cultures exposed to DMI, the control cultures (no application of inducement components) grew into numerous foci (cells grew on top of one another after achieving the maximal cell numbers spread in cell culture plate)41 and lipid devoid intracellular vesicles appeared after 12 d of confluence, which was confirmed by ORO staining (Fig. 4F). In addition, The TEM performed on the vesicles in cells of control cultures illustrated that the vesicles were intact and surrounded by a phospholipid monolayer, indicating the lipid-free vesicles were capable of packaging and holding lipids (Fig. 5). These results support the theory that DFAT cells could differentiate into “immature adipocyte-like” cells,41 with cytoplasmic lipid devoid, but membrane-intact, vesicles.
Figure 4. Adipogenesis of Wagyu-IMF derived DFAT cells. (A) Lipid-laden adipocyte derived DFAT cells possessed fibroblast-like appearance with undetectable lipids. (B) 10 d after DMI inducement, 30% DFAT cells of the 100% confluence group accumulated obvious lipid droplets inside cytoplasm which could be stained by ORO staining (C); while DFAT cells of the 80% confluence group showed less than 10% redifferentiation efficiency (D) which was confirmed by ORO staining (E). (F) 10 d after the basal medium culture, DFAT cells of the 100% confluence group showed numerous ORO stain-free vesicles (black arrows). (A, B, D, and F) 200× magnification; (C and E) 400× magnification.
Figure 5. Transmission electron microscopy of lipid-free vesicles in DFAT cells. Lipid-free vesicles were intact and surrounded by a phospholipid monolayer. [Scale bars: (A) 0.5 µm; (B) 0.2 µm]
Discussion
Significant progress has been made in dedifferentiation studies24 since the first report of a proliferation phenomenon of mature adipocytes in 1975 by Adebonojo.13,14 Confirmatory evidence of dedifferentiation in mature adipocytes came from experiments which showed that lipid-filled cells were dividing by lipid-containing or lipid-expelling methods in vitro in single cell culture systems.8,9,12 The subsequent progeny cells were named DFAT cells,10,17,18,24-28 which were characterized by a fibroblast-like appearance,9,16,19-22 proliferative ability,19,22,23 and multilineage potentials.28,33,35,36,47 In addition, by using immunocytochemistry and flow cytometry techniques, DFAT cells reportedly contained embryonic stem cell markers,71,72 indicating the potential value of DFAT cells in tissue engineering and disease treatments.
Currently published cell isolation methods for acquiring mature adipocytes and DFAT cells have involved cell dispersion, filtration, centrifugation, and flask ceiling culture with (+/−) purification steps, which are initially achieved by differential plating.53 In this method, most of fibroblast-like cells are removed after several early/late differential platings. However, numerous fat cells are lost during this process, and the contaminating cells just decrease but do not disappear. To acquire homologous DFAT cells for research purposes, cell surgery techniques are involved to remove any remaining contaminating cells. However, this method is slow and tedious for monitoring the lipid-laden cells and subsequently removing all contaminating cells under the microscope based on their shapes. In addition, the process is difficult to learn and likely unreliable from inexperienced workers. We employed cell culture plates for plate ceiling culture in this study. This enabled much easier manipulation of cells with pasteur pipettes or cell scrapers compared with using cell culture flasks. Moreover, after observing and marking lipid-filled adipocytes, cloning cylinders could be creatively employed in our protocol which made it easier to acquire homogeneous cell populations.
Mature adipocytes, which typically have a single large lipid droplet, tend to undergo cell lysis during cell isolation.24,48 Gentle manipulation in this process is necessary,48 including cutting, pipetting, and centrifugation. In our experience, slowly pipetting the upper and middle positions of the most top layer (colorless layer) for ceiling culture might help getting a more purified population. Aside from proper manipulation and media, the ceiling surface characteristics of cultureware are one of the key factors for cell attachment.42 Cultureware obtained from different sources may influence the attachment efficiency; in addition bio-coating surfaces might contribute to success of the procedure. Considerations of the proper media and substrata are necessary to maximize mature adipocyte attachment. In present study for testing the plate ceiling culture systems, rules for choosing cultureware were similar diameters with over 10 mm height differences between the ceiling plate and the container plate. The similar diameter would enhance the ceiling culture efficiency. Height differences were necessary to manipulate, decrease the chance of contamination and save the media. Comparing with cell culture dishes, lids may be the first choice in our experiments. The extent of pretreatment of lids needs to be clearly labeled, as lids obtained from some supply companies had not been “TC treated”. Investigators could choose to treat (bio-coat) the lids by themselves and use them in the ceiling culture. In fact, we propose that use of different substratum coatings are the next logical step in flat-bottomed cell cultureware use for this research. Use of collagen, gelatin, fibronectin, Matrigel, laminin, and other bio-coatings would help establish more optimum attachment conditions for mature adipocytes. Improvements in the cell culture conditions can increase the number of dedifferentiating DFAT cells to allow gene expression studies during this process and improve our understanding of basic biological mechanisms. For this study, four 60 mm × 15 mm dishes (ceiling) + one 150 mm × 25 mm dish (container) might be an alternative model to the three 35 mm × 10 mm dishes (ceiling) + one 100 mm × 20 mm dish (container) system with coatings provided by the supplier were used. In all cases, high quality ceiling plates with low height and proper surface area treatment greatly improved the chances of obtaining highly pure cultures of DFAT cells.
To expand our preliminary observations, dedifferentiation and redifferentiation of Wagyu-IMF derived mature adipocytes were tested utilizing a single cell system. For studying adipogenesis, redifferentiation of DFAT cells15,20,22,28,37,45 has been suggested as an alternative to utilizing SV (primary) cells or the 3T3-L1 cell line. DFAT cells enjoy some unique advantages over SV cells/3T3-L1 cells. For example, homogeneous populations of DFAT cells are isolatable (such as DFAT clones); while SV cultures contain various cell types and will be greatly influenced by isolation procedures. We believe that research utilizing proliferative-competent DFAT cells to be an advantage for fully deciphering the physiology of adipocytes, or in transdifferentiation studies to determine ability of the cells to convert to other cell forms. Thus we examined the ability of DFAT cell clones to help ensure the purity of such cells, and determine the redifferentiation efficiency of DFAT clones in this study. However, use of individual (cloned) mature adipocytes (alone) is not sufficient for conduct of gene expression studies. Too few cells are established by the present methods for insuring quality DNA/RNA for amplification. It would be of considerable interest to develop single cell methods for such gene studies, however. As such, with such methods one could determine specific regulation of the dedifferentiation process, which would be extremely eye-opening to the scientific community. As it is, one can use progeny DFAT cells for such gene expression studies. Some of these (DFAT) studies do exist, which show different expression markers are present during re-differentiation of DFAT cells to form lipid-filled adipocytes.22,43,44
Like other cell types that have been thoroughly studied, a proper culture milieu is essential (by providing optimized nutrients and regulators) for progeny cells to show their functions and morphology. With traditional DMI media, 1–30% bovine IMF-derived DFAT cells redifferentiated into adipocytes. Highly confluent DFAT cell cultures resulted in attenuation of proliferation and a switch to differentiate into adipocytes in vitro. Non-confluent cell cultures did not result high numbers of mature cell phenotypes. It should be noted that in all cultures receiving the DMI treatment, lipid-free intracellular vesicles were not observed. However, without specific induction reagents (control vs. cultures), approximately 70% of DFAT cells spontaneously differentiated into “immature adipocyte-like” cells, with cytoplasmic lipid-free but membrane-intact vesicles. This kind of vesicles was reported by our research group previously,41 in which bovine-derived DFAT cells exposed to the HS (horse serum)-based DMI media and displayed protracted adipogenesis. It is possible that bovine-derived DFAT cells possess the adipogenic potential and progress through adipocyte differentiation spontaneously accompanied by lipid-free vesicles, which may be triggered by confluence.
Research with large animals (bovine and pig) for agricultural and biomedical purposes to enhance carcass quality and explore properties of adipocytes related to human health is increasing. In traditional cell cultures, adipogenic inducement for primary SV cultures differs between pig and bovine in the hormone/agent cocktail required for adipocyte differentiation.46 Overall, porcine SV cultures require less induction agents in the media to differentiate compared with bovine SV cultures.46 For example, a DMI media and a TZD (thiazolidinedione) are not necessary for adipocyte differentiation in pig SV cultures46 whereas both (DMI + TZD) are necessary in bovine SV cells. Chen et al.22 previously showed that pig-derived DFAT cells redifferentiated spontaneously from d 6 of confluence, without any inducement reagent. The classic adipogenesis of cattle-derived progeny cells required more induction agents than pig-derived progeny cells to reform the mature adipocyte morphology. Table 1 underscores the differences among differing species (cattle, pig, human, and mouse) regarding adipogenic inducement and effects on DFAT cells, indicating that the redifferentiation ability of DFAT cells varies among species.15,20,22,28,37,41,45
Table 1. Adipogenic inducement of DFAT cells.
| Species | Serum | DMI reagents | Time | Results | References |
|---|---|---|---|---|---|
| Bovine |
10% FBS |
None |
d 12 |
Foci and 70% “immature adipocyte-like” cells |
This study |
| |
10% FBS |
INS, DEX, IBMX, TRO |
d 10 |
Adipocytes with 1–30% differentiation efficiency |
This study |
| |
10% HS |
None |
d 11 |
40% “immature adipocyte-like” cells |
41 |
| |
10% HS |
INS, DEX, IBMX, +/−ROS |
d 17 |
Rare lipids and protracted adipogenesis with vesicles +/− lipids |
41 |
| Porcine |
10% FBS |
None |
d 6–d 16 |
Spontaneous adipogenesis |
22 |
| |
20% FBS |
INS, DEX, IBMX |
d 12 |
Adipocytes |
45 |
| |
10% FBS |
INS, DEX, IBMX |
d 21 |
Adipocytes |
28 |
| Human |
None |
INS, T3, BIO, PAN, TRAN, HYD |
d 21 |
Adipocytes |
20 |
| Murine | 10% FBS | INS, DEX, IBMX | d 8 | Adipocytes | 15 and 37 |
Notes: FBS, fetal bovine serum; HS, horse serum; INS, insulin; DEX, dexamethasone; IBMX, 3-isobutyl-1-methylxanthine; TRO, troglitazone; ROS, rosiglitazone; T3, triiodothyronine; BIO, biotin; PAN, pantothenate; TRAN, transferrin; HYD, hydrocortisone.
Conclusions and Future Directions
New efficient methods (plate ceiling culture) and the improved flask ceiling culture to isolate mature adipocytes and DFAT cells were described. Differential plating, cell surgery and cloning techniques were part of these methods. By practicing patience and caution during isolation procedures, collecting the correct layer of the centrifugate with a pipette, the usage of proper cultureware and simple clean-up methods, purified mature adipocytes and DFAT cells may be acquired efficiently. Moreover, as Wagyu cattle are good donors for studying IMF adipocyte physiology, the subsequent DFAT cells may, indeed, be a unique model for the lipid metabolism and adipogenesis studies. However, the cellular physiology of this adipogenesis (including the lipid-free vesicles) remains unclear; further investigations especially biochemical analyses in gene and protein expressions are necessary in order to improve/validate this new model. The dedifferentiation of mature fat cells and redifferentiation of the progeny cells detailed in this work would enable further study of the novel model for elucidating adipocyte physiology, adipogenesis, and lipid metabolism. Once lipid-filled adipocytes dedifferentiate into proliferative-competent progeny cells, the subsequent DFAT clones can be a valuable resource as stem cells to generate into other cell types with the potential to be an attractive target for clinical applications in regenerative medicine.
Adipogenesis is a complex phenomenon involving a plethora of regulatory network of genes and key adipogenic transcription factors including CAAT/enhancer-binding proteins (C/EBPs), peroxisome proliferator-activated receptor γ (PPARγ), Krüppel-like factors (KLFs), and sterol regulatory element-binding protein (SREBP).73 Besides these, microRNAs (miRNA) are known to regulate multiple targets as well as several other transcriptional regulators in modulating adipogenic gene expression. Recent studies have suggested an important role of miRNA178 and several other miRNAs in adipogenesis in bovine back-fat tissues.74,75 In future, genome-wide mRNaseq and miRNA profiling combined with proteome mapping strategies, of homogenous populations of mature fat cells, dedifferentiated DFAT cells and redifferentiated lipid-assimilating adipocytes, will elucidate the full complement of key transcriptional cascades that orchestrate adipogenesis.
This comprehensive understanding of the intricacies of adipogenesis using the DFAT cells as a model can be exploited for efficient targeting and manipulation of fat depots in livestock species.66 Furthermore, it may result in adoption of strategies for selective breeding and enhanced animal productivity through declined financial costs and reduced environmental impact of the livestock industry. New molecular insights into fat development by using a combination of cellular, biochemical, and functional genomic approaches, may also prove useful in revealing therapeutic strategies for treatment of obesity and its associated diseases.
Materials and Methods
Animal and tissue samples
Samples of subcutaneous fat (Angus cross steers, n = 2) and sternomandibularis (skeletal) muscle (Wagyu steers, n = 2) were harvested separately at the Washington State University (WSU) abattoir, placed in warm phosphate-buffered saline (PBS) and immediately transported to the cell culture laboratory. The WSU Animal Care and Use Committee approved the use of animals in this research. Further, this work adhered to standards for animal use imposed by both the United States Department of Agriculture (USDA) and the Public Health Service (PHS). PBS and Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (DMEM/F12; Gibco) media used in this study were supplemented with 100 IU/ml penicillin (Gibco), 100 µg/ml streptomycin (Gibco), 2.5 ng/ml Fungizone B (Gibco) and 50 µg/ml gentamicin (Gibco). In addition, horse serum (HS; Gibco) and fetal bovine serum (FBS; Gibco) were used in the study. The present procedure for isolating mature adipocytes and cells possessing similar buoyancy was based on earlier methods described by Fernyhough et al.11
Mature adipocyte isolation and first trial of plate ceiling culture
Subcutaneous fat samples from an Angus steer were washed with PBS several times before being placed into an appropriate culture hood. About 15 g fat tissue was collected from trimmed samples into a 100 mm dish. Five grams of fine cut fat (about 3 mm) fragments were transferred into each fresh sterile 50 ml centrifuge tube (n = 3). To this, pre-warmed collagenase type I (Gibco) was added. The tissue-collagenase mixture was incubated in a continuously shaking 37 °C water bath for 1 h. Following collagenase digestion, contents of the tubes were filtered through a 1000 μm sterile plastic mesh into fresh sterile 50 ml centrifuge tubes. Centrifugation at 186 × g for 10 min was performed to separate the collagenase digested tissue into three layers; the supernatant (top layer) containing adipocytes; the infranatant (middle layer) containing the collagenase; and the pellet at the bottom formed from the stromal vascular fraction. The top layers were subsequently transferred into 3 fresh sterile 50 ml centrifuge tubes each containing 20 ml DMEM/F12 + 10% HS. Centrifugation at 186 × g for 10 min was performed, followed by two repeated washing steps. After filtration, centrifugation and washing, the floating top layers containing adipocytes were collected and used in the first trial plate ceiling culture (Fig. 6). Two combinations [three 35 mm × 10 mm dishes (ceiling; Falcon) + one 100 mm × 20 mm dish (container; Nunc), and one 100 mm × 10 mm lid (ceiling; Nunc) + one 150 mm × 20 mm dish (container; Nunc)] were chosen to initiate plate ceiling culture; one T-12.5 cm2 flask (Falcon) ceiling culture served as control (Fig. 6). An inverted phase contrast microscope with 4× and 10× objectives was available in the hood to mark lipid containing cells/ remove contaminating cells. Adipocytes were transferred into the previously mentioned cell culture plates and flask. Culture medium was then carefully added. The inverted small dishes (Fig. 6A) and the lid (Fig. 6B) were placed into the bigger dishes, while the medium-filled flask (Fig. 6C) was tightly closed and inverted. On d 3, 4, and 5 of cell isolation the three small dishes were re-inverted and small amount of basal media (DMEM/F12 + 10% FBS) was added to check the mature adipocyte attaching efficiency. The big lid and the flask were re-inverted on d 5. Mature adipocyte attaching efficiency was measured in three methods (dish ceiling culture, lid ceiling culture and flask ceiling culture).

Figure 6. First attempt at plate ceiling culture. (A) Pipette top layer of the 1st tube into a Nunclon® 100 mm × 20 mm cell culture dish (container). Then put three inverted BD Falcon™ 35 mm × 10 mm dishes into the big dish by forceps. Add proper amount of media (DMEM/F12 + 10% HS) and chase bubbles out, allowing its surface contact with the inner surface of the small dishes (the bottom of the small dishes then became the “ceiling”). (B) Pipette top layer of the 2nd tube into a Nunclon® 150 mm × 20 mm cell culture dish. Then put an inverted Nunclon® 100 mm × 10 mm lid into the big dish by forceps. Add proper amount of media (DMEM/F12 + 10% HS) and chase bubbles out, allowing its surface contact with the inner surface of the lid. (C) Pipette top layer of the 3rd tube into a BD Falcon™ T-12.5 cm2 cell culture flask. Fill the flask with DMEM/F12 + 10% HS. The medium-filled flask was tightly closed and inverted (the bottom of the flask then became the “ceiling”).
Mature adipocyte isolation and use of cell culture plates
The results of first trial proved the advantages of plate ceiling culture compared with traditional methods and the 35 mm × 10 mm dishes with a container might be a good operational system to acquire mature adipocytes. However, the size of the 35 mm dish was small and only several cloning cylinders were available in the dish. Although the 100 mm lids were bigger and easier to handle than the small dishes, no cells attached to these lids most likely due to lack of tissue culture (TC) treatment. Therefore, we expanded the choice range of cell cultureware and the special sized 100 mm × 15 mm cell culture dishes (Celltreat) and 100 mm × 10 mm “TC treated” lids (Celltreat) were used in the subsequent experiment, with the Corning® 150 mm × 25 mm cell culture dishes as the containers. In addition, due to the small amount of attached mature adipocytes in the previous ceiling culture, we improved the procedure to get more mature adipocytes.
About 20 g subcutaneous fat samples of one Angus steer were used. The fat samples were cut into small (about 3 mm) fragments and 5 g of these fragments were transferred into each 50 ml tube (n = 4). Pre-warmed collagenase type I was added to the tubes followed by incubation in a continuously gentle shaking 37 °C water bath for 1 h. After the collagenase digestion, the tissue\collagenase mixture was filtered through a 1000 μm plastic mesh and collected into new tubes. Two hundred microliters of the upper floating layer from one tube was carefully pipetted into a cell culture flask after 5 min following filtration. The flask was completely filled with culture media, tightly closed and inverted for 5 d to check the isolation efficiency without centrifugation.
The collected filtrates from the 3 other tubes were centrifuged at 186 × g for 10 min. The underlying media and pellets were pipetted out instead of transferring the top layer to decrease lipid-containing mature adipocyte loss and damage. The remaining adipocyte containing layers were carefully washed by adding 20 ml DMEM/F12 + 10% HS along the wall of the tubes and then centrifuged at 186 × g for 10 min. Following the washing process, 50 μl from the middle of the top (fat) layer was pipetted and used in flask ceiling culture to check the purity in this area. Then equal amounts (200 μl) of the top (fat) layer were pipetted into 2 plates; one 100 mm × 15 mm cell culture dish (Celltreat) and one 100 mm × 10 mm “TC treated” lid (Celltreat) as the ceiling plate, respectively and placed each into 150 mm × 25 mm cell culture dishes (container; Corning). One BD Falcon™ T-12.5 cm2 flask ceiling culture with 200 μl of the top (fat) layer was used as control, with one differential plating (pipette mature adipocytes from one ceiling culture into another to get rid of some contaminating cells) on d 2 of cell isolation process.
Dedifferentiation of Wagyu IMF- mature adipocytes by purified ceiling culture
Sternomandibularis muscle samples (Wagyu steer, n = 2) were collected and washed with PBS several times before taking into the hood. Then fat tissue was collected from trimmed muscle samples into a 100 mm dish. The present adipocyte isolation procedure is shown in Figure 3, 1–5. Differential platings,11 cell surgery,11 and close observations were included in the culture process to insure high purity of DFAT cells. In addition, based on single cell systems, one DFAT clone (derived from a single lipid-filled mature adipocyte) of each donor was kept and used in subsequent experiment. Media was changed every 2 d after re-inverting the cell cultures.
Adipogenesis of Wagyu IMF-DFAT cells
When Wagyu IMF mature adipocyte-derived clones reached 70% confluence, the cells were subcultured in propagation plates and finally cultured into individual wells of three 12-well plates. Two clones derived from the two Wagyu cattle were used in the subsequent test. Each DFAT cell clone was divided into five groups [four DMI treatment groups (1.25 × 104, 2.5 × 104, 4 × 104, and 5 × 104 cells per well) and one control group (5 × 104 cells per well)], with 3 replicates (wells) for each group. Two days after the cells reached 20%, 50%, 80%, and 100% confluence respectively, the traditional DMI cocktail [DMEM/F12 + 10% FBS supplemented with insulin (1 µg/ml), dexamethasone (0.1 µg/ml), methyl-isobutylxanthine (27.8 µg/ml), and troglitazone (10 µM/l)] was applied to each treatment group and kept for 3 d.76 On d 4, culture media was changed by fresh DMI media and incubated for 3 additional days. On d 7, the DMI media was replaced by DMEM/F12 + 10% FBS supplemented with insulin (1 µg/ml) only and cultured for 2 additional days, with the same media being changed every 2 d.77-79 Total 10 d were needed for redifferentiation of DFAT cells. The control group was cultured with DMEM/F12 + 10% FBS (basal media) only and the media was changed every 2 or 3 d. All cells were observed and stained on d 10 of applying DMI cocktail media.
Morphological observation and ORO staining
All cells were cultured in a 37 °C incubator with 95% air and 5% CO2. Morphological changes were observed daily by a phase contrast microscope. At d 10 of adipogenic treatment, cells were stained for lipids with ORO (oil red-O) as method described by Kinkel et al.80 Photographs of cells were taken by a Sony RGB digital camera (3/4 in chip) married to a Nikon Diaphot phase contrast microscope (Nikon) and analyzed by Image Pro Plus® analysis software. In addition, a transmission electron microscope (TEM; JEM 1200 EX; JOEL) was used to detect if the intracellular ultrastructure was present for lipid storage. The method for fixing the cells for TEM was described previously by Fernyhough et al.41 and present images were taken with a Mega View III digital camera.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/24589
References
- 1.Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 2008;9:338–52. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 2.Jo J, Shreif Z, Periwal V. Quantitative dynamics of adipose cells. Adipocyte. 2012;1:80–8. doi: 10.4161/adip.19705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flatt JP. Use and storage of carbohydrate and fat. Am J Clin Nutr. 1995;61(Suppl):952S–9S. doi: 10.1093/ajcn/61.4.952S. [DOI] [PubMed] [Google Scholar]
- 4.Rigamonti A, Brennand K, Lau F, Cowan CA. Rapid cellular turnover in adipose tissue. PLoS One. 2011;6:e17637. doi: 10.1371/journal.pone.0017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn L, Woodhouse KA. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4:228–35. doi: 10.4161/org.4.4.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crescenzi M, Soddu S, Tatò F. Mitotic cycle reactivation in terminally differentiated cells by adenovirus infection. J Cell Physiol. 1995;162:26–35. doi: 10.1002/jcp.1041620105. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Guridi M, Fernyhough M, Jiang Z, Guan L, Hausman G, et al. Initial differences in lipid processing leading to pig- and beef- derived mature adipocyte dedifferentiation. Basic Appl Myol. 2009;19:243–6. [Google Scholar]
- 8.Chen J, Guridi M, Fernyhough ME, Jiang Z, Guan L, Hausman GJ, et al. Clonal mature adipocyte production of proliferative-competent daughter cells requires lipid export prior to cell division. Int J Stem Cells. 2009;2:76–9. doi: 10.15283/ijsc.2009.2.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei S, Duarte MS, Du M, Jiang Z, Paulino PVR, Chen J, et al. Like pigs, and unlike other breeds of cattle examined, mature Angus-derived adipocytes may extrude lipid prior to proliferation in vitro. Adipocyte. 2012;1:237–41. doi: 10.4161/adip.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodson MV, Fernyhough ME, Vierck JL, Hausman GJ. Adipocytes may not be a terminally differentiated cell type: implications for animal production. Anim Sci. 2005;80:239–40. [Google Scholar]
- 11.Fernyhough ME, Vierck JL, Hausman GJ, Mir PS, Okine EK, Dodson MV. Primary adipocyte culture: adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnology. 2004;46:163–72. doi: 10.1007/s10616-005-2602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernyhough ME, Bucci LR, Hausman GJ, Antonio J, Vierck JL, Dodson MV. Gaining a solid grip on adipogenesis. Tissue Cell. 2005;37:335–8. doi: 10.1016/j.tice.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Adebonojo FO. Monolayer cultures of disaggregated human adipocytes. In Vitro. 1975;11:50–4. doi: 10.1007/BF02615322. [DOI] [PubMed] [Google Scholar]
- 14.Adebonojo FO. Studies on human adipose cells in culture: relation of cell size and multiplication to donor age. Yale J Biol Med. 1975;48:9–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008;332:435–46. doi: 10.1007/s00441-008-0593-9. [DOI] [PubMed] [Google Scholar]
- 16.Vierck JL, McNamara JP, Dodson MV. Proliferation and differentiation of progeny of ovine unilocular fat cells (adipofibroblasts) In Vitro Cell Dev Biol Anim. 1996;32:564–72. doi: 10.1007/BF02722983. [DOI] [PubMed] [Google Scholar]
- 17.Fernyhough ME, Helterline DL, Vierck JL, Hausman GJ, Hill RA, Dodson MV. Dedifferentiation of mature adipocytes to form adipofibroblasts: more than just a possibility. Adipocytes. 2005;1:17–24. [Google Scholar]
- 18.Fernyhough ME, Vierck JL, Dodson MV. Assessing a non-traditional view of adipogenesis: adipocyte dedifferentiation--mountains or molehills? Cells Tissues Organs. 2006;182:226–8. doi: 10.1159/000093970. [DOI] [PubMed] [Google Scholar]
- 19.Wei S, Duarte MS, Du M, Paulino PV, Jiang Z, Albrecht E, et al. Bovine mature adipocytes readily return to a proliferative state. Tissue Cell. 2012;44:385–90. doi: 10.1016/j.tice.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Tholpady SS, Aojanepong C, Llull R, Jeong JH, Mason AC, Futrell JW, et al. The cellular plasticity of human adipocytes. Ann Plast Surg. 2005;54:651–6. doi: 10.1097/01.sap.0000158065.12174.40. [DOI] [PubMed] [Google Scholar]
- 21.Vierck JL, McNamara JP, Dodson MV. Two alternative procedures for isolating adipofibroblasts from sheep skeletal muscle. Methods Cell Sci. 1996;18:309–14. doi: 10.1007/BF00127908. [DOI] [Google Scholar]
- 22.Chen J, Dodson MV, Jiang Z. Cellular and molecular comparison of redifferentiation of intramuscular- and visceral-adipocyte derived progeny cells. Int J Biol Sci. 2010;6:80–8. doi: 10.7150/ijbs.6.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen JF, Sugawara A, Yamashita J, Ogura H, Sato S. Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues. Int J Oral Sci. 2011;3:117–24. doi: 10.4248/IJOS11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei S, Duarte MS, Zan L, Du M, Jiang Z, Guan L, et al. Cellular and molecular implications of mature adipocyte dedifferentiation. J Genomics. 2012;1:5–12. doi: 10.7150/jgen.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodson MV, Fernyhough ME. Mature adipocytes: are there still novel things that we can learn from them? Tissue Cell. 2008;40:307–8. doi: 10.1016/j.tice.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Fernyhough ME, Hausman GJ, Guan LL, Okine E, Moore SS, Dodson MV. Mature adipocytes may be a source of stem cells for tissue engineering. Biochem Biophys Res Commun. 2008;368:455–7. doi: 10.1016/j.bbrc.2008.01.113. [DOI] [PubMed] [Google Scholar]
- 27.Ohta Y, Takenaga M, Tokura Y, Hamaguchi A, Matsumoto T, Kano K, et al. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877–86. doi: 10.3727/096368908786576516. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–22. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 29.Dodson MV, Wei S, Duarte M, Du M, Jiang Z, Hausman GJ, et al. Cell supermarket: Adipose tissue as a source of stem cells. J Genomics. 2012;1:39–44. doi: 10.7150/jgen.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett. 2011;16:236–57. doi: 10.2478/s11658-011-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hausman GJ, Dodson MV. Stromal vascular cells and adipogenesis: cells within adipose depots regulate adipogenesis. J Genomics. 2012;1:56–66. doi: 10.7150/jgen.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565–75. doi: 10.1016/j.yjmcc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Dodson MV, Hausman GJ, Guan L, Du M, Jiang Z. Potential impact of mature adipocyte dedifferentiation in terms of cell numbers. Int J Stem Cells. 2011;4:76–8. doi: 10.15283/ijsc.2011.4.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct. 2008;33:211–22. doi: 10.1247/csf.08038. [DOI] [PubMed] [Google Scholar]
- 36.Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780–5. doi: 10.1016/j.bbrc.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 37.Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–74. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 38.Obinata D, Matsumoto T, Ikado Y, Sakuma T, Kano K, Fukuda N, et al. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model. Int J Urol. 2011;18:827–34. doi: 10.1111/j.1442-2042.2011.02865.x. [DOI] [PubMed] [Google Scholar]
- 39.Kishimoto N, Momota Y, Hashimoto Y, Ando K, Omasa T, Kotani J. Dedifferentiated fat cells differentiate into osteoblasts in titanium fiber mesh. Cytotechnology. 2013;65:15–22. doi: 10.1007/s10616-012-9456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigematsu M, Watanabe H, Sugihara H. Proliferation and differentiation of unilocular fat cells in the bone marrow. Cell Struct Funct. 1999;24:89–100. doi: 10.1247/csf.24.89. [DOI] [PubMed] [Google Scholar]
- 41.Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. Cells Tissues Organs. 2008;188:359–72. doi: 10.1159/000134007. [DOI] [PubMed] [Google Scholar]
- 42.Dodson MV, Mathison BA, Mathison BD. Effects of medium and substratum on ovine satellite cell attachment, proliferation and differentiation in vitro. Cell Differ Dev. 1990;29:59–66. doi: 10.1016/0922-3371(90)90024-Q. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi M, Guan LL, Zhang B, Dodson MV, Okine E, Moore SS. Adipogenesis of bovine perimuscular preadipocytes. Biochem Biophys Res Commun. 2008;366:54–9. doi: 10.1016/j.bbrc.2007.11.110. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi M, Guan LL, Zhang B, Dodson MV, Okine E, Moore SS. Gene expression patterns of bovine perimuscular preadipocytes during adipogenesis. Biochem Biophys Res Commun. 2008;366:346–51. doi: 10.1016/j.bbrc.2007.11.111. [DOI] [PubMed] [Google Scholar]
- 45.Nobusue H, Kano K. Establishment and characteristics of porcine preadipocyte cell lines derived from mature adipocytes. J Cell Biochem. 2010;109:542–52. doi: 10.1002/jcb.22431. [DOI] [PubMed] [Google Scholar]
- 46.Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood) 2010;235:1185–93. doi: 10.1258/ebm.2010.010063. [DOI] [PubMed] [Google Scholar]
- 47.Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, et al. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355–65. doi: 10.1016/j.juro.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 48.Zhang HH, Kumar S, Barnett AH, Eggo MC. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol. 2000;164:119–28. doi: 10.1677/joe.0.1640119. [DOI] [PubMed] [Google Scholar]
- 49.Funatsumaru S. Cellular structure and function of rat fat cells in the primary culture. Cell Struct Funct. 1995;20:23–32. doi: 10.1247/csf.20.23. [DOI] [PubMed] [Google Scholar]
- 50.Sugihara H, Funatsumaru S, Yonemitsu N, Miyabara S, Toda S, Hikichi Y. A simple culture method of fat cells from mature fat tissue fragments. J Lipid Res. 1989;30:1987–95. [PubMed] [Google Scholar]
- 51.Sugihara H, Yonemitsu N, Miyabara S, Toda S. Proliferation of unilocular fat cells in the primary culture. J Lipid Res. 1987;28:1038–45. [PubMed] [Google Scholar]
- 52.Sugihara H, Yonemitsu N, Miyabara S, Yun K. Primary cultures of unilocular fat cells: characteristics of growth in vitro and changes in differentiation properties. Differentiation. 1986;31:42–9. doi: 10.1111/j.1432-0436.1986.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 53.Wei S, Bergen WG, Hausman GJ, Zan L, Dodson MV. Cell culture purity issues and DFAT cells. Biochem Biophys Res Commun. 2013;433:273–5. doi: 10.1016/j.bbrc.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–46. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, et al. Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int J Biol Sci. 2010;6:691–9. doi: 10.7150/ijbs.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dodson MV, Jiang Z, Chen J, Hausman GJ, Guan L, Novakofski J, et al. Allied industry approaches to alter intramuscular fat content and composition in beef animals. J Food Sci. 2010;75:R1–8. doi: 10.1111/j.1750-3841.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- 57.Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, et al. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 2009;87:1218–46. doi: 10.2527/jas.2008-1427. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Z, Michal JJ, Chen J, Daniels TF, Kunej T, Garcia MD, et al. Discovery of novel genetic networks associated with 19 economically important traits in beef cattle. Int J Biol Sci. 2009;5:528–42. doi: 10.7150/ijbs.5.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehnert SA, Reverter A, Byrne KA, Wang Y, Nattrass GS, Hudson NJ, et al. Gene expression studies of developing bovine longissimus muscle from two different beef cattle breeds. BMC Dev Biol. 2007;7:95. doi: 10.1186/1471-213X-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Duan E, Sung LY, Jeong BS, Yang X, Tian XC. Generation and characterization of pluripotent stem cells from cloned bovine embryos. Biol Reprod. 2005;73:149–55. doi: 10.1095/biolreprod.104.037150. [DOI] [PubMed] [Google Scholar]
- 61.Du M, Dodson MV. Advanced techniques to enhance marbling in meat. In: Joo S, ed. Control of Meat Quality. Kerala: Research Signpost, 2011:105-15. [Google Scholar]
- 62.Zembayashi M, Nabeta H, Mototsuji T. Effects of breeds and nutritional plans on intramuscular lipid deposition of fatting of steers. Jpn J Zootech Sci. 1988;59:39–48. [Google Scholar]
- 63.Zhang L, Michal JJ, O’Fallon JV, Pan Z, Gaskins CT, Reeves JJ, et al. Quantitative genomics of 30 complex phenotypes in Wagyu x Angus F₁ progeny. Int J Biol Sci. 2012;8:838–58. doi: 10.7150/ijbs.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albrecht E, Gotoh T, Ebara F, Xu JX, Viergutz T, Nürnberg G, et al. Cellular conditions for intramuscular fat deposition in Japanese Black and Holstein steers. Meat Sci. 2011;89:13–20. doi: 10.1016/j.meatsci.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Gotoh T, Albrecht E, Teuscher F, Kawabata K, Sakashita K, Iwamoto H, et al. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Sci. 2009;82:300–8. doi: 10.1016/j.meatsci.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 66.Basu U, Guan LL, Taniguchi M, Zhao Y, Dodson MV. Application of 'omics” technologies on improvement of meat quality. In: Haugen S, Meijer S, eds. Nutritional Biochemistry: Genomics, Metabolomics and Food Supply. New York: Nova Science, 2009:165-94. [Google Scholar]
- 67.Du M, Tong J, Zhao J, Underwood KR, Zhu M, Ford SP, et al. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. 2010;88(Suppl):E51–60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- 68.Wang YH, Byrne KA, Reverter A, Harper GS, Taniguchi M, McWilliam SM, et al. Transcriptional profiling of skeletal muscle tissue from two breeds of cattle. Mamm Genome. 2005;16:201–10. doi: 10.1007/s00335-004-2419-8. [DOI] [PubMed] [Google Scholar]
- 69.Du M, Yin J, Zhu MJ. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci. 2010;86:103–9. doi: 10.1016/j.meatsci.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Kokta TA, Dodson MV, Gertler A, Hill RA. Intercellular signaling between adipose tissue and muscle tissue. Domest Anim Endocrinol. 2004;27:303–31. doi: 10.1016/j.domaniend.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Poloni A, Maurizi G, Leoni P, Serrani F, Mancini S, Frontini A, et al. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells. 2012;30:965–74. doi: 10.1002/stem.1067. [DOI] [PubMed] [Google Scholar]
- 72.Gao Q, Zhao L, Song Z, Yang G. Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol Biol Rep. 2012;39:5791–804. doi: 10.1007/s11033-011-1371-4. [DOI] [PubMed] [Google Scholar]
- 73.Basu U, Romao JM, Guan LL. Adipogenic transcriptome profiling using high throughput technologies. J Genomics. 2012;1:22–8. doi: 10.7150/jgen.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin W, Dodson MV, Moore SS, Basarab JA, Guan LL. Characterization of microRNA expression in bovine adipose tissues: a potential regulatory mechanism of subcutaneous adipose tissue development. BMC Mol Biol. 2010;11:29. doi: 10.1186/1471-2199-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romao JM, Jin W, Dodson MV, Hausman GJ, Moore SS, Guan LL. MicroRNA regulation in mammalian adipogenesis. Exp Biol Med (Maywood) 2011;236:997–1004. doi: 10.1258/ebm.2011.011101. [DOI] [PubMed] [Google Scholar]
- 76.Klemm DJ, Leitner JW, Watson P, Nesterova A, Reusch JE, Goalstone ML, et al. Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J Biol Chem. 2001;276:28430–5. doi: 10.1074/jbc.M103382200. [DOI] [PubMed] [Google Scholar]
- 77.Kim JE, Chen J. regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53:2748–56. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 78.Yada E, Yamanouchi K, Nishihara M. Adipogenic potential of satellite cells from distinct skeletal muscle origins in the rat. J Vet Med Sci. 2006;68:479–86. doi: 10.1292/jvms.68.479. [DOI] [PubMed] [Google Scholar]
- 79.Yamanouchi K, Hosoyama T, Murakami Y, Nishihara M. Myogenic and adipogenic properties of goat skeletal muscle stem cells. J Reprod Dev. 2007;53:51–8. doi: 10.1262/jrd.18094. [DOI] [PubMed] [Google Scholar]
- 80.Kinkel AD, Fernyhough ME, Helterline DL, Vierck JL, Oberg KS, Vance TJ, et al. Oil red-O stains non-adipogenic cells: a precautionary note. Cytotechnology. 2004;46:49–56. doi: 10.1007/s10616-004-3903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]






