Abstract
The vitamin A metabolite retinoic acid (RA) regulates gene transcription by activating the nuclear receptors RAR and PPARβ/δ and their cognate lipid binding proteins CRABP-II, which delivers RA to RAR, and FABP5, which shuttles the hormone to PPARβ/δ. In preadipocytes, RA signals predominantly through CRABP-II and the RAR isotype RARγ to induce the expression of hallmark markers of preadipocytes Pref-1, Sox9, and KLF2. RA thus maintains the preadipocyte phenotype and inhibits adipogenesis. In mature adipocytes, RA activates both of its receptors to upregulate expression of genes that enhance lipid oxidation, energy dissipation, and insulin responses. Consequently, RA potently protects mice from diet-induced obesity and insulin resistance by two distinct mechanisms: by counteracting adipogenesis, thereby moderating the formation of new fat cells, and by promoting energy expenditure, thereby preventing adipocyte hypertrophy.
Keywords: retinoic acid, adipogenesis, nuclear receptors, fatty acid-binding protein, cellular retinoic acid-binding protein, RAR, PPAR
The primary cells of adipose tissue, adipocytes, coordinate energy homeostasis and serve as endocrine cells, giving rise to signaling cytokines that control multiple cellular functions. The adipose tissue begins to develop in late gestation but adipocyte number dramatically expands after birth and continues to increase through puberty.1 Even in adult adipose tissue, about 10% of adipocytes turn over every year2 and adipogenesis can be induced by environmental cues such as consumption of a high-fat diet.3-5 Proper adipogenesis throughout life is thus of critical importance for maintaining health, and malformation or dysfunction of adipocytes underlie the development of various pathologies, including obesity and type 2 diabetes. Adipocytes are generated from mesenchymal stem cells by a two-step process entailing commitment of stem cells to the adipocytes lineage, followed by terminal differentiation of adipocyte progenitors, preadipocytes, into mature fat cells.6 The second step can be triggered by adipogenic signals including insulin, glucocorticoid receptor agonists and agents that elevate cellular cAMP levels.7,8 These signaling molecules modulate the expression of numerous genes, thereby inducing differentiation and allowing adipogenesis to proceed9,10 (reviewed in ref. 11).
Of special note among regulatory factors involved in adipocyte biology is the transcriptionally active metabolite of vitamin A retinoic acid (RA). The biological activities of this hormone originate from its ability to activate several members of the nuclear receptor family of transcription factors: the classical RA receptors RARα, RARβ, and RARγ12 and the peroxisome proliferator activated receptor β/δ (PPARβ/δ).13-17 The partitioning of RA between its receptors is regulated by two intracellular lipid-binding proteins that deliver it from sites of synthesis in the cytosol to cognate receptors in the nucleus, cellular RA binding protein II (CRABP-II) transports RA to RARs and fatty acid binding protein type 5 (FABP5) shuttles it to PPARβ/δ. The spectrum of genes whose expression is regulated by RA and, accordingly, cellular responses to the hormone are thus determined by the relative expression levels of these binding proteins in specific cells; RA controls expression of RAR target genes in cells that display a high CRABP-II/FABP5 ratio, but it regulates PPARβ/δ target genes in cells in which this ratio is low.15,16,18-22 In preadipocytes, RA signals predominantly through the CRABP-II/RAR path. However, adipocyte differentiation is accompanied by downregulation of CRABP-II and RARs and by upregulation of FABP5 and PPARβ/δ, and consequently, the alternative pathway is enabled and RA can activate both of its receptors in mature adipocytes.13,14
Various observations indicate that vitamin A is closely involved in regulation of adipose tissue function. Hence, ablation of retinol dehydrogenase 1 (rdh1) in mice, which results in alterations in vitamin A homeostasis, leads to enhanced size and adiposity.23 Further, fibroblasts with reduced expression of cellular retinol-binding protein I (CRBP-I) undergo adipocyte differentiation more readily than parental cells, and CRBP-I-null mice display increased adiposity.24 It was also reported that CRBP-III plays a role in lipid metabolism.25 Treatment of mice with RA at a pharmacological but non-toxic dose (~3 mg/kg/d) was reported to result in weight loss and improved glucose tolerance despite a larger food intake by treated animals.13 Additional reports showed that RA reduces adiposity in rodents.26-28 However, these latter studies utilized very high RA concentrations (10–100 mg/kg/d), raising the question of whether the observed weight loss may have originated from RA toxicity.
The ability of RA to induce weight loss in animals was traced, at least in part, to activities in mature adipocytes and in muscle, where the hormone signals through both RAR and PPARβ/δ. It has thus been reported that, in cultured adipocytes, RA enhances energy expenditure by inducing the expression of PPARβ/δ target genes that trigger energy dissipation, e.g., uncoupling protein 1 (UCP1), promote fatty acid oxidation, e.g., alcohol dehydrogenase 9 and carnitine palmitoyltransferase, and participate in insulin responses, e.g., GluT4, as well as by upregulating genes that are jointly controlled by RAR and PPARβ/δ and that promote energy dissipation, e.g., UCP3, and lipolysis, e.g., hormone sensitive lipase.13,28,29 In vivo, administration of RA induces the expression of lipid- and sugar-processing PPARβ/δ target genes in adipose tissue and liver, and it recapitulates the reported activity of PPARβ/δ in increasing skeletal muscle mitochondrial content.30 Taken together with the ~0.5 °C higher body temperature of mice treated with RA,13 these observations indicate that induction of weight loss by RA is associated with enhanced energy utilization.
In addition to its activities in mature adipocytes and muscle, RA is also closely involved in regulation of adipogenesis. Interestingly, it has been reported that RA induces commitment of embryonic stem cells to the adipocyte lineage31,32 but potently blocks differentiation of preadipocytes into mature adipose cells.33-35 It has been suggested that induction of adipocyte commitment of stem cells by RA involves glycogen synthase kinase 3 (GSK3)28 and that inhibition of adipocyte differentiation by the hormone involves Smad3.36-38 However, the identity of direct target genes that mediate these activities and the mechanisms by which the effects of RA on adipogenesis are propagated were unknown. Providing insight into some of these questions, our recent observations39 showed that inhibition of adipocyte differentiation by RA is mediated primarily by the RAR subtype RARγ and that, in preadipocytes, RARγ directly controls the expression of several genes that encode known inhibitors of adipogenesis. One of these is the Kruppel-like factor KLF2, a transcription factor that inhibits adipogenesis by suppressing the expression of the adipogenic factors PPARγ, C/EBPα, and SREBP1c.40,41 Interestingly, the data showed that, while suppressing the expression of these genes, KLF2 upregulates the expression of both CRABP-II and RARγ in preadipocyes. Hence, KLF2 participates in a positive feedback loop that amplifies inhibition of adipocyte differentiation by RA. Other RA-regulated genes that block differentiation of preadipocytes into mature fat cells are the preadipocyte marker Pref-1, its activator ADAM17, and its downstream effector, the transcription factor SOX9. Pref-1, a plasma membrane protein exclusively expressed in preadipocytes, is cleaved by ADAM17 to produce an extracellular form that activates ERK signaling, leading to induction of SOX9.42-44 In turn, SOX9 impedes adipogenesis by repressing the expression of C/EBPβ and C/EBPδ.45-48
Mice fed a high fat/high sucrose (HFHS) diet and treated with RA display a lower weight, lower adipose tissue mass, and lower adipocyte size as compared with animals fed a HFHS diet in the absence of administration of RA.13 RA treatment also blunts diet-induced elevation in levels of plasma cholesterol and plasma triglycerides. These effects emanate in part from increased expression of adipose and muscle proteins that enhance lipid oxidation and energy dissipation and that promote insulin signaling. However, the data also showed that adipose tissue of RA-treated mice contains fewer mature, lipid-containing, adipocytes, and displays a higher expression level of the preadipocyte marker Pref-1.39 The data thus establish that RA contributes to maintenance of preadipocytes and inhibits adipocyte differentiation in vivo. CRABP-II+/− mice, in which RA signaling through the CRABP-II/RAR pathway is reduced, were used to further examine whether inhibition of adipogenesis by RA contributes to its ability to protect animals from diet-induced obesity. Expression levels of adipocyte markers in WT and CRABP-II+/− mice were similar. Hence, in agreement with the report that many of the activities of RA in mature adipocytes are mediated by the FABP5/PPARβ/δ path,13 adipocytes of CRABP-II+/− mice retain normal phenotype. In contrast, the levels of expression of Pref-1, SOX9, and KLF2 were markedly lower in adipose tissue of CRABP-II+/− vs. WT mice. These observations further support the identification of these genes as direct targets for the RA-activated CRABP-II/RAR path, and they indicate that the preadipocyte content of adipose tissue of CRABP-II+/− mice is lower than that of WT animals. These findings suggest that these mice are be prone to excess adipogenesis and thus that they may display a propensity for enhanced adiposity. Indeed, CRABP-II+/− mice fed a HFHS diet gained more weight than WT animals although they displayed a lower food consumption. Remarkably, the size of adipocytes in WT and CRABP-II+/− mice was similar, indicating that the increase in the weight of these animals did not result from enhanced adipocyte hypertrophy but directly reflected accelerated generation of mature adipocytes.
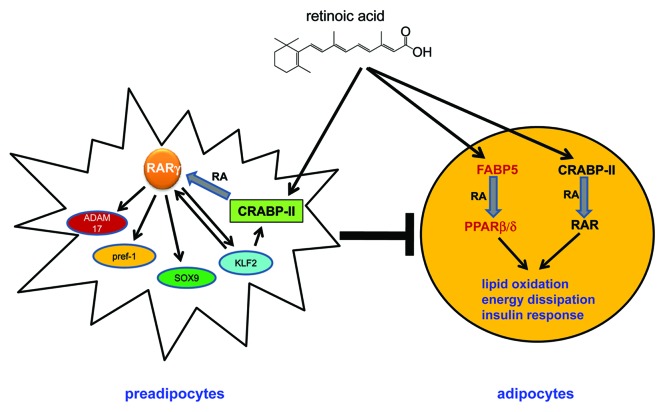
As summarized in the model presented in Figure 1, available information demonstrates that the ability of RA to protect animals from diet-induced obesity and from obesity-related pathologies is exerted by two distinct activities. In preadipocytes, RA activates the CRABP-II/RAR pathway and thereby inhibits adipocyte differentiation, moderating the formation of new fat cells in response to high fat feeding. In mature adipocytes and in muscle, the hormone activates both the CRABP-II/RAR and the FABP5/PPARβ/δ paths to promote lipid oxidation and energy utilization. RA thus suppresses dietary-induced obesity by counteracting both adipogenesis and adipocyte hypertrophy.
Figure 1. Mechanisms by which RA suppresses dietary-induced adiposity and insulin resistance. In preadipocytes, RA activates CRABP-II and RARγ to induce expression of Pref-1, ADAM17, Sox9, and KLF2, all of which contribute to inhibition of adipogenesis. In turn, KLF2 upregulates RARγ and CRABP-II, thereby propagating a positive feedback loop that further potentiates RA-induced inhibition of adipocyte differentiation. In mature adipocytes, RA functions through both RAR and PPARβ/δ to induce the expression of genes that enhance energy expenditure and that promote insulin responses.
Acknowledgments
This work was supported by NIH grant RO1-DK60684.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/23489
References
- 1.Ailhaud G, Grimaldi P, Négrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–33. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- 2.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 3.Lemonnier D. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J Clin Invest. 1972;51:2907–15. doi: 10.1172/JCI107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klyde BJ, Hirsch J. Increased cellular proliferation in adipose tissue of adult rats fed a high-fat diet. J Lipid Res. 1979;20:705–15. [PubMed] [Google Scholar]
- 5.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226–31. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–34. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 8.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–33. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 9.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–34. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 10.Shao D, Lazar MA. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem. 1997;272:21473–8. doi: 10.1074/jbc.272.34.21473. [DOI] [PubMed] [Google Scholar]
- 11.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, et al. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–25. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 13.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–96. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry DC, Soltanian H, Noy N. Repression of cellular retinoic acid-binding protein II during adipocyte differentiation. J Biol Chem. 2010;285:15324–32. doi: 10.1074/jbc.M110.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–33. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schug TT, Berry DC, Toshkov IA, Cheng L, Nikitin AY, Noy N. Overcoming retinoic acid-resistance of mammary carcinomas by diverting retinoic acid from PPARbeta/delta to RAR. Proc Natl Acad Sci U S A. 2008;105:7546–51. doi: 10.1073/pnas.0709981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. 2003;278:41589–92. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 18.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–8. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 19.Budhu AS, Noy N. Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest. Mol Cell Biol. 2002;22:2632–41. doi: 10.1128/MCB.22.8.2632-2641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manor D, Shmidt EN, Budhu A, Flesken-Nikitin A, Zgola M, Page R, et al. Mammary carcinoma suppression by cellular retinoic acid binding protein-II. Cancer Res. 2003;63:4426–33. [PubMed] [Google Scholar]
- 21.Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol Cell. 2005;18:343–53. doi: 10.1016/j.molcel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–27. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Hu P, Krois CR, Kane MA, Napoli JL. Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. FASEB J. 2007;21:2886–96. doi: 10.1096/fj.06-7964com. [DOI] [PubMed] [Google Scholar]
- 24.Zizola CF, Frey SK, Jitngarmkusol S, Kadereit B, Yan N, Vogel S. Cellular retinol-binding protein type I (CRBP-I) regulates adipogenesis. Mol Cell Biol. 2010;30:3412–20. doi: 10.1128/MCB.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zizola CF, Schwartz GJ, Vogel S. Cellular retinol-binding protein type III is a PPARgamma target gene and plays a role in lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E1358–68. doi: 10.1152/ajpendo.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonet ML, Oliver J, Picó C, Felipe F, Ribot J, Cinti S, et al. Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J Endocrinol. 2000;166:511–7. doi: 10.1677/joe.0.1660511. [DOI] [PubMed] [Google Scholar]
- 27.Felipe F, Mercader J, Ribot J, Palou A, Bonet ML. Effects of retinoic acid administration and dietary vitamin A supplementation on leptin expression in mice: lack of correlation with changes of adipose tissue mass and food intake. Biochim Biophys Acta. 2005;1740:258–65. doi: 10.1016/j.bbadis.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Mercader J, Ribot J, Murano I, Felipe F, Cinti S, Bonet ML, et al. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology. 2006;147:5325–32. doi: 10.1210/en.2006-0760. [DOI] [PubMed] [Google Scholar]
- 29.Amengual J, Ribot J, Bonet ML, Palou A. Retinoic acid treatment increases lipid oxidation capacity in skeletal muscle of mice. Obesity (Silver Spring) 2008;16:585–91. doi: 10.1038/oby.2007.104. [DOI] [PubMed] [Google Scholar]
- 30.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, et al. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci. 1997;110:1279–85. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- 32.Monteiro MC, Wdziekonski B, Villageois P, Vernochet C, Iehle C, Billon N, et al. Commitment of mouse embryonic stem cells to the adipocyte lineage requires retinoic acid receptor beta and active GSK3. Stem Cells Dev. 2009;18:457–63. doi: 10.1089/scd.2008.0154. [DOI] [PubMed] [Google Scholar]
- 33.Murray T, Russell TR. Inhibition of adipose conversion in 3T3-L2 cells by retinoic acid. J Supramol Struct. 1980;14:255–66. doi: 10.1002/jss.400140214. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Hiragun A, Mitsui H. Preadipocytes possess cellular retinoid binding proteins and their differentiation is inhibited by retinoids. Biochem Biophys Res Commun. 1980;95:1839–45. doi: 10.1016/S0006-291X(80)80113-6. [DOI] [PubMed] [Google Scholar]
- 35.Kuri-Harcuch W. Differentiation of 3T3-F442A cells into adipocytes is inhibited by retinoic acid. Differentiation. 1982;23:164–9. doi: 10.1111/j.1432-0436.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol. 1997;17:1552–61. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue JC, Schwarz EJ, Chawla A, Lazar MA. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARgamma. Mol Cell Biol. 1996;16:1567–75. doi: 10.1128/mcb.16.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchildon F, St-Louis C, Akter R, Roodman V, Wiper-Bergeron NL. Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J Biol Chem. 2010;285:13274–84. doi: 10.1074/jbc.M109.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61:1112–21. doi: 10.2337/db11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, et al. The Krüppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278:2581–4. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- 41.Wu J, Srinivasan SV, Neumann JC, Lingrel JB. The KLF2 transcription factor does not affect the formation of preadipocytes but inhibits their differentiation into adipocytes. Biochemistry. 2005;44:11098–105. doi: 10.1021/bi050166i. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Kim KA, Kim JH, Sul HS. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J Nutr. 2006;136:2953–6. doi: 10.1093/jn/136.12.2953. [DOI] [PubMed] [Google Scholar]
- 43.Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22:5585–92. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villena JA, Choi CS, Wang Y, Kim S, Hwang YJ, Kim YB, et al. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): a new model of partial lipodystrophy. Diabetes. 2008;57:3258–66. doi: 10.2337/db07-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol. 1997;17:977–88. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Sul HS. Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol Cell Biol. 2006;26:5421–35. doi: 10.1128/MCB.02437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Zhao L, Smas C, Sul HS. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol Cell Biol. 2010;30:3480–92. doi: 10.1128/MCB.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]