Abstract
Background
Glutamate N-methyl-d-aspartate (NMDA) receptor antagonists exert fast-acting antidepressant effects, providing a promising way to develop a new classification of antidepressant that targets the glutamatergic system. In the present study, we examined the potential antidepressant action of 7-chlorokynurenic acid (7-CTKA), a glycine recognition site NMDA receptor antagonist, in a series of behavioural models of depression and determined the molecular mechanisms that underlie the behavioural actions of 7-CTKA.
Methods
We administered the forced swim test, novelty-suppressed feeding test, learned helplessness paradigm and chronic mild stress (CMS) paradigm in male rats to evaluate the possible rapid antidepressant-like actions of 7-CTKA. In addition, we assessed phospho-glycogen synthase kinase-3β (p-GSK3β) level, mammalian target of rapamycin (mTOR) function, and postsynaptic protein expression in the medial prefrontal cortex (mPFC) and hippocampus.
Results
Acute 7-CTKA administration produced rapid antidepressant-like actions in several behavioural tests. It increased p-GSK3β, enhanced mTOR function and increased postsynaptic protein levels in the mPFC. Activation of GSK3β by LY294002 completely blocked the antidepressant-like effects of 7-CTKA. Moreover, 7-CTKA did not produce rewarding properties or abuse potential.
Limitations
It is possible that 7-CTKA modulates glutamatergic transmission, thereby causing enduring alterations of GSK3β and mTOR signalling, although we did not provide direct evidence to support this possibility. Thus, the therapeutic involvement of synaptic adaptions engaged by 7-CTKA requires further study.
Conclusion
Our findings demonstrate that acute 7-CTKA administration produced rapid antidepressant-like effects, indicating that the behavioural response to 7-CTKA is mediated by GSK3β and mTOR signalling function in the mPFC.
Introduction
Depression is one of the most devastating mental illnesses, with a lifetime prevalence of about 16%.1 The rate of suicidal ideation is as high as 58% among depressed patients, and suicidal behaviour increases the mortality associated with depression, leading to a major burden on society.2 Conventional antidepressants require several weeks to achieve therapeutic responses. This treatment delay is a major limitation to current depression therapies. Therefore, developing faster-acting and more effective antidepressants is important, especially for depressed patients at risk for suicide.
Human postmortem studies reported that glutamate levels in the frontal cortex are increased in patients with depression, suggesting that abnormal glutamatergic neurotransmission may play a role in the pathophysiology of depression and the action mechanism of antidepressants.3–5 Ketamine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, has been widely reported to have rapid antidepressant effects in both preclinical and clinical studies.6,7 A single dose of ketamine exerted fast antidepressant effects within a few hours in patients with treatment-resistant major depression.8–10 Based on the addictive and psychotomimetic potential of ketamine, however, developing fast-acting antidepressants that target the NMDA receptor system without causing psychotropic side effects is necessary. Unfortunately, the adverse effects of ketamine, particularly psychotomimetic consequences and cognitive impairment, limit its clinical utility.11 Increasing evidence suggests that several NMDA receptor antagonists are effective in the treatment of depression. Memantine, a noncompetitive NMDA receptor antagonist, has produced antidepressant effects in the forced swim test in rats,12 and CP-101606, an NR2B subunit-selective NMDA receptor antagonist, has been shown to elicit an antidepressant response in depressed patients.13 These findings suggest that NMDA receptor antagonists could have therapeutic potential for the treatment of major depression.
7-Chlorokynurenic acid (7-CTKA), a glycine recognition site NMDA receptor antagonist, has been shown to have neuroprotective and antinociceptive effects.14 Whether 7-CTKA is effective for the treatment of depression has not yet been clarified. Glycogen synthase kinase 3β (GSK3β) is a serine/threonine kinase, and this signalling molecule modulates the effects of many psychiatric therapeutic agents.15,16 Numerous studies have suggested the involvement of GSK3β in NMDA receptor-dependent long-term potentiation (LTP) and long-term depression (LTD). Specifically, activation of GSK3β is required for LTD, whereas inhibition of GSK3β activity induces LTP.17,18 The NMDA receptor antagonist memantine has been reported to inhibit GSK3β activation,19 suggesting that 7-CTKA may similarly inhibit GSK3β. The mammalian target of rapamycin (mTOR) regulates cell growth and survival and is implicated in activity-dependent synaptic plasticity by controlling protein synthesis. A recent study found that an NMDA receptor antagonist rapidly activated mTOR, causing an increase in the function of spine synapses.6 In the present study, we sought to determine whether 7-CTKA has antidepressant-like effects in animal models of depression and whether GSK3β and mTOR signalling in the medial prefrontal cortex (mPFC) are involved in the behavioural mechanism of action of 7-CTKA.
Methods
Animals
Male Sprague–Dawley rats that weighed 200–220 g upon arrival were individually housed under a constant temperature (23°C ± 2°C) and a 12-hour light:dark cycle with free access to food and water. All of the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Peking University Animal Use Committee (LA2012/21) approved all procedures. All of the behavioural tests and drug administrations were performed during the dark phase of the light:dark cycle.
Drugs
We purchased 7-CTKA hydrochloride from Enzo Life Sciences International Inc. and dissolved it in saline containing 10% dimethylsulfoxide (DMSO). We administered 7-CTKA intraperitoneally at doses of 0.05, 0.1 and 1 mg/kg. We chose these doses based on our previous study20 and on another report that demonstrated effective antagonism of the NMDA receptor.21 We purchased LY294002 (10 nM/0.5 μl) from Sigma and dissolved it in saline containing 50% DMSO, as described in previous reports.6,22 Rapamycin (50 μg/0.5 μl) was also purchased from Sigma and dissolved in saline containing 5% DMSO, as described in a previous report.23 We purchased D-serine (50 μg/0.5 μL) from Beijing Redwood Fine Chemical Co. Ltd. and dissolved it in saline containing 2% DMSO, as described in a previous report.24 All drugs and vehicles were bilaterally infused into the mPFC at a volume of 0.5 μl per side. Nissl staining showed that the vehicle (5%–50% DMSO) used in the microinjection did not cause cell death after a single infusion. We obtained venlafaxine from Chengdu Daxi’nan Pharmaceutical Co. Ltd. and ketamine from Jiangsu Hengrui Medicine Co. Ltd.
Behavioural tests
Forced swim test
We placed the rats in a plastic cylinder 25 cm in diameter and 65 cm high filled to a depth of 30 cm with 23ºC–25ºC water for 15 minutes. The rats were tested 24 hours later. Immobility was defined as the minimum movement required to passively keep the animal’s head above the water without other motions. Results are expressed as the amount of time (in seconds) that the animals spent immobile during the 5-minute test.25
Open field test
We administered the open field test to measure locomotor activity, as previously described.26 Briefly, the apparatus consisted of a 75 cm × 75 cm × 40 cm square arena divided into 25 equal squares (15 cm × 15 cm). A single rat was placed in the centre of the apparatus, and we counted the number of crossings (i.e., entries into adjacent squares) for 5 minutes.
Novelty-suppressed feeding test
We adapted the novelty-suppressed feeding test from previous studies,27,28 making minor modifications. The rats were deprived of food in their home cages for 24 hours before the test. On the test day, rats were individually placed in an open field arena (75 cm × 75 cm × 40 cm) with several pellets of food placed in the centre. The animal was placed in a corner of the arena and allowed to explore for 8 minutes. We recorded the latency (in seconds) to approach the food and begin eating. Subsequent home cage food consumption in 5 minutes was the quantitative control measure for appetite.
Learned helplessness
We used the learned helplessness paradigm, as described previously.29 The shuttle box apparatus consisted of 2 equal compartments divided by a central barrier (Jiliang Software Technology). During training on day 1, we administered an inescapable footshock on 1 side of the apparatus with the guillotine door closed (100 footshocks, 0.85 mA intensity, 15 s average duration, 60 s average intershock interval). Control rats were placed in the same chambers but received no foot-shock. We used failures and the latency to escape in an active avoidance test to detect learned helplessness. In the test session on day 2, 30 escapable footshock trials (0.65 mA intensity, 35 s maximum duration, 90 s average intertrial interval) were presented with the guillotine door open. During the shock period, the rats needed to cross to the other side of the shuttle box twice to terminate the shock. If rats failed to escape within 35 seconds, the shock was automatically terminated. The numbers of escape failures and latency to escape were recorded by computer.
Chronic mild stress protocol and sucrose preference test
We adapted the chronic mild stress protocol from our earlier study.30 Briefly, rats were subjected to different mild stressors for 28 days: day 1 (cold immobilization for 1 h at 4°C, tilted cages [45°] for 24 h), day 2 (immobilization for 1 h, crowding for 24 h), day 3 (forced cold swim for 5 min, soiled bedding for 24 h), day 4 (immobilization for 1 h, vibration for 1 h), day 5 (tilted cages [45°] for 24 h, cold immobilization for 1 h at 4°C), day 6 (forced cold swim for 5 min at 4°C, crowding for 24 h) and day 7 (vibration for 1 h, soiled bedding for 24 h). This schedule was repeated 3 more times. Control rats were handled daily without any stress in the housing room.
We measured sucrose preference as previously described.31 The rats were trained to adapt to a 1% sucrose solution (w/v) for 48 hours at the beginning of the experiment, during which 2 bottles of 1% sucrose solution were placed in each cage. After adaptation, rats were deprived of water and food for 24 hours and then submitted to the sucrose preference test, in which they were housed in individual cages for 4 hours and had free access to 2 bottles containing 1% sucrose or tap water. We counterbalanced the bottles across the left and right sides of the cages throughout the experiment. The position of the 2 bottles varied every 2 hours during the test. At the end of 4 hours, we measured sucrose and water consumption (in millilitres) and calculated sucrose preference (%) as the ratio of sucrose consumption to sucrose plus water consumption.
Conditioned place preference
We administered the conditioned place preference (CPP) test based on our previous work.32,33 The apparatus consisted of 3-chamber polyvinyl chloride (PVC) boxes with 2 large side chambers (27.9 cm × 21.0 cm × 20.9 cm) separated by a smaller chamber (12.1 cm × 21.0 cm × 20.9 cm) with a smooth PVC floor. The floor textures are different (bar or grid) in the 2 chambers to provide distinct contexts paired with drug (7-CTKA, ketamine) or saline injections. Before the CPP test, the rats were placed in the centre compartment of the CPP apparatus with the doors removed for 15 minutes to measure baseline place preference. Subsequently, rats were conditioned for 8 consecutive days with alternating intraperitoneal injections of drug (1 mg/kg 7-CTKA or 10 mg/kg ketamine) or saline (1 mL/kg) in corresponding compartments using an unbiased, counterbalanced protocol. A separate set of rats received only saline during the 8-day conditioning period. After each injection, rats were confined to the drug- or saline-conditioned chamber for 45 minutes before being returned to their home cages. We assessed the expression of 7-CTKA-and ketamine-induced CPP 1 day after the last training session on day 9 under conditions identical to those described in the preconditioning test. The CPP score was defined as the time (in seconds) spent in the drug-paired chamber minus the time spent in the saline-paired chamber.
Further methodological details, including those for tissue sample preparation,34 Western blot assays, intracerebral cannula implantation and intracranial injections,35,36 together with detailed experimental design of the 6 main experiments performed37–39 are included in Appendix 1 (available at cma.ca/jpn).
Statistical analysis
The data are expressed as mean (and standard error of the mean [SEM]). We analyzed the data using 1- or 2-way analysis of variance (ANOVA) followed by the Tukey post hoc test. We considered results to be significant at p < 0.05.
Results
Acute 7-CTKA administration produced rapid antidepressant-like effects
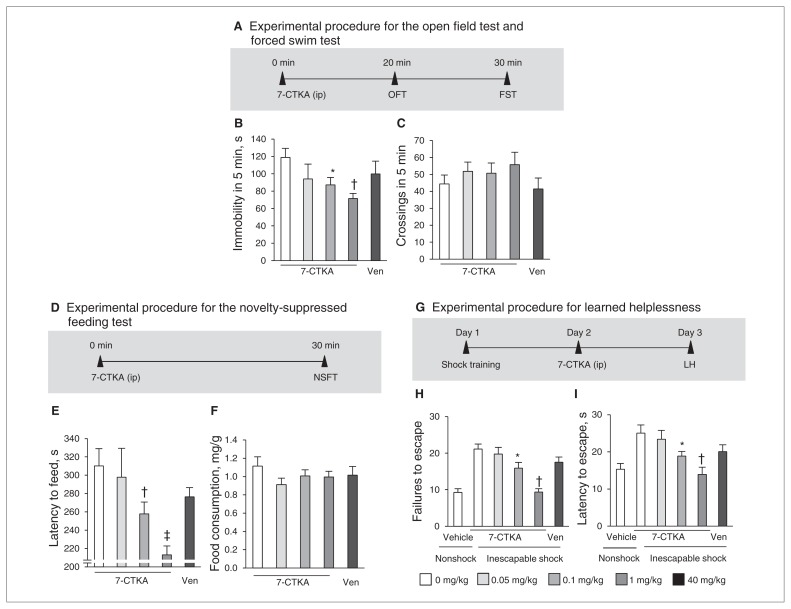
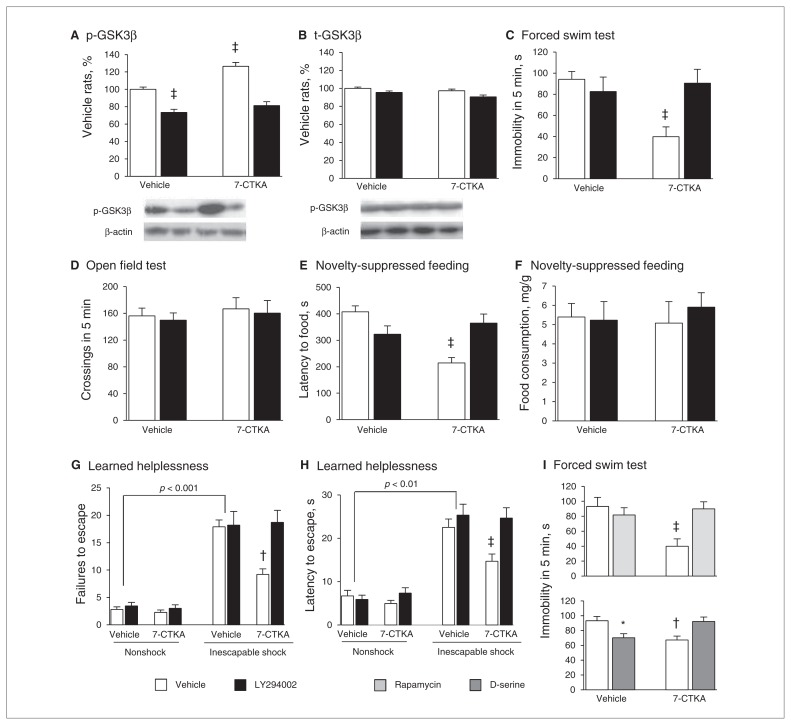
In the forced swim test (Fig. 1A), acute 7-CTKA (0.1 and 1 mg/kg) significantly reduced immobility (Fig. 1B) but had no effects on locomotor activity (Fig. 1C). In the novelty-suppressed feeding test (Fig. 1D), acute 7-CTKA significantly decreased the latency to feed (Fig. 1E), whereas 7-CTKA did not affect food consumption in the home cage (Fig. 1F). In the learned helplessness paradigm (Fig. 1G), inescapable foot-shock significantly increased failures compared with non-shock rats (Fig. 1H) and increased the latency to escape (Fig. 1I). In contrast, rats treated with 7-CTKA exhibited a significant decrease in escape failures (F4,39 = 9.86, p < 0.001) and decreased latency to escape (F4,39 = 4.83, p = 0.002). These behavioural results suggest that a single dose of 7-CTKA produces a rapid antidepressant-like response. However, a single dose of a traditional antidepressant, venlafaxine, was not effective in any of these 3 tests (Fig. 1). Previous evidence has shown that 3 injections of venlafaxine were needed to produce an antidepressant-like action by decreasing the immobility in the forced swim test.40,41
Fig. 1.
Acute intraperitoneal administration of 7-CTKA dose-dependently produced antidepressant-like effects. (A) Experimental procedure for the open-field test (OFT) and forced swim test (FST). (B) Immobility time measured in the FST (n = 8). (C) Locomotor activity measured in the OFT (n = 8). (D) Experimental procedure for the novely-suppressed feeding test (NSFT). (E, F) Latency to feed and food consumption in the NSFT (n = 9). (G) Experimental procedure for learned helplessness (LH). (H, I) Escape failures and latency in the LH paradigm (n = 8). The data are expressed as mean and standard error of the mean. *p < 0.05, †p < 0.01, ‡p < 0.001, compared with 0 mg/kg of 7-CTKA. Ven = venlafaxine.
Acute 7-CTKA administration rapidly reversed depressive-like behaviour induced by chronic mild stress
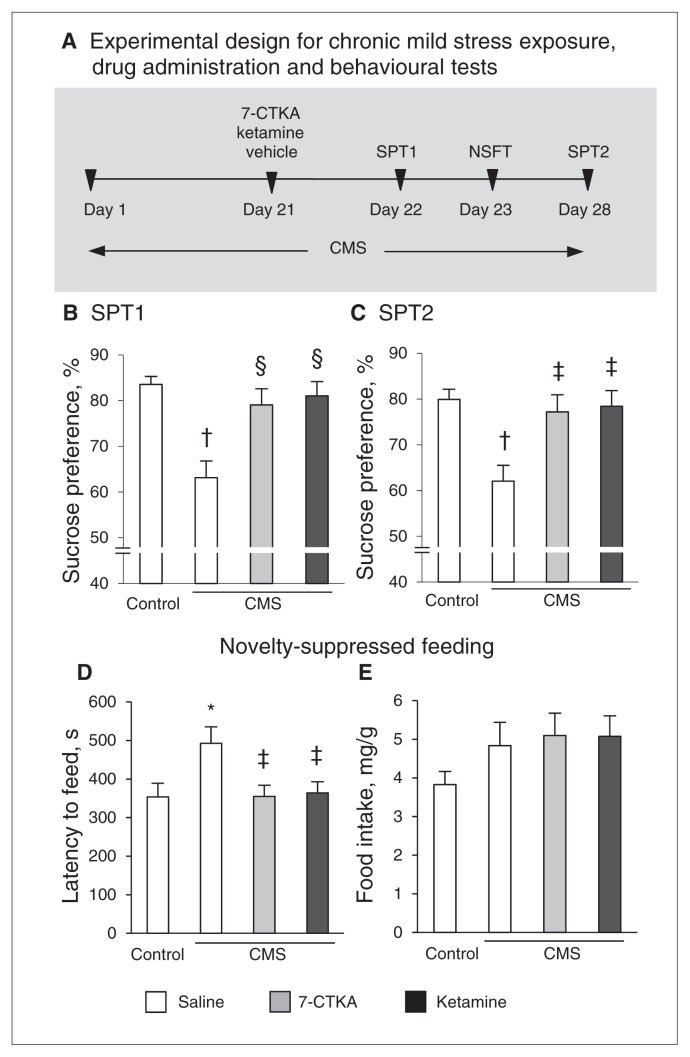
We further assessed the fast antidepressant-like effect of 7-CTKA in the chronic mild stress (CMS) paradigm (Fig. 2A), one of the most valid models of depression that requires continuous administration of traditional antidepressants to produce a therapeutic response. The rats subjected to CMS exhibited a key symptom of depression, anhedonia, reflected by a decrease in sucrose preference (F1,13 = 25.58, p < 0.001). A single injection of 7-CTKA significantly increased sucrose preference (F1,13 = 9.89, p = 0.008), consistent with the effect of ketamine (Fig. 2B). The rapid antidepressant-like effects of 7-CTKA lasted for 7 days, indicated by the sucrose preference test results on day 28 (Fig. 2C). The CMS-treated rats exhibited an increased latency to feed in a novel environment, and this behavioural deficit was reversed by a single injection of 7-CTKA (F1,13 = 7.06, p = 0.021), with no changes in home cage food intake (Fig. 2D and E). These results suggest that the effectiveness of 7-CTKA in decreasing depression-related behaviours is similar to that of the fast-acting antidepressant ketamine.
Fig. 2.
Acute intraperitoneal administration of 7-CTKA produced rapid antidepressant effects in the chronic mild stress (CMS) procedure. (A) Schematic of the experimental design for CMS exposure, drug administration and the behavioural tests. The rats were exposed to CMS for 28 days and received 7-CTKA (1 mg/kg) or ketamine (10 mg/kg) on day 21. The sucrose preference test (SPT) was conducted on (B) day 22 and again on (C) day 28 to test the maintenance of the antidepressant effect of 7-CTKA. (D, E) The novelty-suppressed feeding test (NSFT) was performed 2 days after drug treatment on day 23. The data are expressed as mean and standard error of the mean (n = 7). *p < 0.05, †p < 0.001, compared with control group; ‡p < 0.05, §p < 0.01, compared with vehicle-treated CMS rats.
Acute 7-CTKA treatment increased p-GSK3β level and enhanced mTOR function and synaptic protein levels in rats subjected to CMS
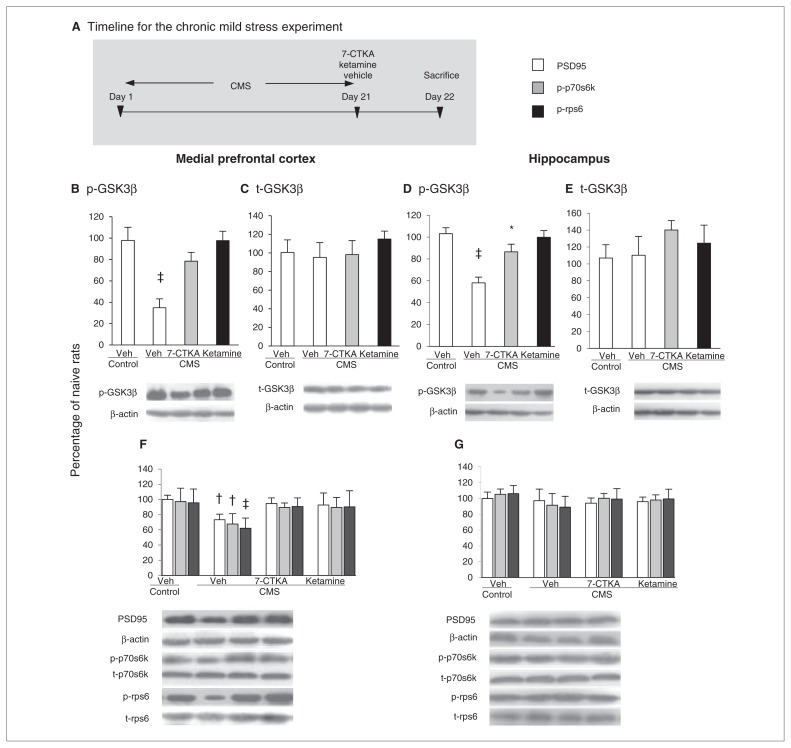
To further examine the mechanisms that underlie the rapid antidepressant activity of 7-CTKA, we exposed 3 groups of rats (n = 6 per group) to CMS for 21 days, and we intraperitoneally administered vehicle, 7-CTKA or ketamine acutely on day 21. One day after the drug treatment, we measured GSK3β, postsynaptic density 95 (PSD95) protein, p70s6k and rps6 in the mPFC and hippocampus using Western blot (Fig. 3A). Exposure to CMS significantly decreased p-GSK3β in the mPFC (p < 0.001; Fig. 3B) without altering t-GSK3β levels (Fig. 3C). The results showed that the decrease in p-GSK3β in the mPFC was reversed by acute 7-CTKA administration. We observed a similar effect in the ketamine-treated group; 7-CTKA did not alter hippocampal p-GSK3β level induced by CMS (Fig. 3D and E), suggesting that the mPFC is a key target region involved in the behavioural effects of 7-CTKA.
Fig. 3.
Acute intraperitoneal administration of 7-CTKA rapidly increased p-GSK3β level, reversed the deficits in the mammalian target of rapamycin (mTOR) function and blocked the loss of postsynaptic protein caused by chronic mild stress (CMS). (A) Experimental procedure. Chronic mild stress decreased p-GSK3β level in the medial prefrontal cortex (mPFC) and had no effects on t-GSK3β. Systemic 7-CTKA administration rapidly increased the expression of p-GSK3β. The percentages of (B) p-GSK3β and (C) t-GSK3β in the mPFC are shown. Chronic mild stress decreased p-GSK3β protein levels in the hippocampus and did not change t-GSK3β. Systemic 7-CTKA treatment had no effects on the expression of p-GSK3β. The percentages of (D) p-GSK3β and (E) t-GSK3β in the hippocampus are shown. Representative Western blot images are shown on the bottom. (F) The levels of PSD95, p-p70s6k and p-rps6 in the mPFC were decreased by CMS, whereas acute 7-CTKA or ketamine administration increased the expression of these proteins. (G) The levels of PSD95, p70s6k and rps6 in the hippocampus were not altered by CMS, 7-CTKA or ketamine. The data are expressed as mean and standard error of the mean (n = 6). *p < 0.05, †p < 0.01, ‡p < 0.001, compared with corresponding control group. GSK3β = glycogen synthase kinase-3β; p = phosphorylated; p70s6k = p70s6 kinase; PSD95 = postsynaptic density protein 95; rps6 = ribosomal protein s6; t = total.
Next we examined whether acute 7-CTKA treatment pre-vented the changes in the mTOR signalling pathway. Chronic mild stress significantly decreased p-p70s6k and p-rps6 in the mPFC (Fig. 3F) but not in the hippocampus (Fig. 3G). We found that 7-CTKA blocked the deficits of PSD95 induced by chronic stress (Fig. 3F), suggesting that induction of synaptogenesis might underlie the antidepressant action of 7-CTKA.
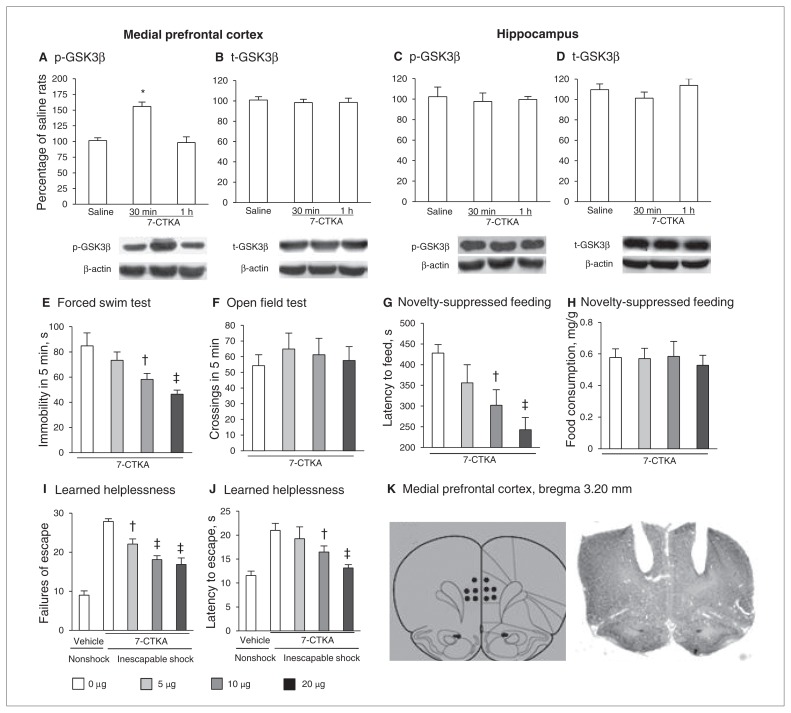
We also found that systemic 7-CTKA treatment selectively increased p-GSK3β in the mPFC (Fig. 4A and B) but not in the hippocampus in nonstressed rats (Fig. 4C and D), suggesting that acute 7-CTKA administration increased the p-GSK3β level specifically in the mPFC and that this inhibition was necessary for the rapid antidepressant action of 7-CTKA. Furthermore, microinfusion of 7-CTKA into the mPFC exerted significant antidepressant-like effects in the forced swim test (Fig. 4E and F), novelty-suppressed feeding test (Fig. 4G and H) and learned helplessness paradigm (Fig. 4I and J), confirming that the mPFC is a selective region for the antidepressant effects of 7-CTKA. The location of 7-CTKA infusions into the mPFC is shown in Figure 4K.
Fig. 4.
The p-GSK3β level in the medial prefrontal cortex (mPFC) is involved in the antidepressant action of 7-CTKA. Systemic 7-CTKA administration increased p-GSK3β in the mPFC but not in the hippocampus. The percentages of (A) p-GSK3β and (B) t-GSK3β in the mPFC are shown. The p-GSK3β level was increased by 7-CTKA (30 min after intraperitoneal administration). The percentages of (C) p-GSK3β and (D) t-GSK3β in the hippocampus are shown. The data are expressed as a percentage of the values obtained for the rats treated with saline. *p < 0.001, different from other experimental groups (n = 6). Intra-mPFC administration of 7-CTKA produced dose-dependent antidepressant-like effects. (E) Immobility time measured in the forced swim test. (F) Locomotor activity measured in the open field test. (G, H) Latency to feed and food consumption in the novelty-suppressed feeding test. (I, J) Failures and latency to escape in the learned helplessness paradigm. (K) Schematic representations of the injection sites and photomicrographs of representative cannula placements in the mPFC by Nissl staining are shown. The data are expressed as mean and standard error of the mean (n = 8). †p < 0.05, ‡p < 0.01, compared with 0 mg/kg 7-CTKA. p = phosphorylated; t = total.
Activation of GSK3β by LY294002 reversed the antidepressant-like effect of 7-CTKA
Microinjection of the GSK3β activator LY294002 into the mPFC decreased the level of p-GSK3β (p < 0.001). A single injection of 7-CTKA increased p-GSK3β (p < 0.001; Fig. 5A and B). In the absence of pretreatment with LY294002, systemic 7-CTKA administration decreased immobility in the forced swim test (Fig. 5C), with no effects on locomotor activity (Fig. 5D).
Fig. 5.
Activation of GSK3β blocked the antidepressant effect of 7-CTKA. Infusion of the GSK3β activator LY294002 (10 nM/side) into the medial prefrontal cortex (mPFC) decreased the p-GSK3β protein level. The percentages of (A) p-GSK3β and (B) t-GSK3β in the mPFC are shown (n = 6). Representative Western blot images are shown on the bottom. Infusion of the GSK3β activator LY294002 (10 nM/side) into the mPFC blocked the antidepressant effect of 7-CTKA (1 mg/kg) in the (C) forced swim test (FST; n = 8–11) and (D) open field test (n = 8–11). A 2-way analysis of variance (ANOVA) with the between-subjects factors 7-CTKA (0 and 1 mg) and LY294002 (0 and 10 nM) revealed significant effects of 7-CTKA (F1,36 = 8.07, p < 0.01) and LY294002 (F1,36 = 5.69, p < 0.05) on immobility and a 7-CTKA × LY294002 interaction (F1,36 = 14.47, p < 0.01). These results demonstrate that pretreatment with LY294002 completely abolished the antidepressant actions of 7-CTKA in the FST. (E, F) LY294002 (10 nM/side) blocked the antidepressant effect of 7-CTKA in the novelty-suppressed feeding test (n = 8–11). A 2-way ANOVA with the between-subjects factors 7-CTKA (0 and 1 mg) and LY294002 (0 and 10 nM) revealed significant effects of 7-CTKA (F1,36 = 7.44, p < 0.05) and LY294002 (F1,36 = 6.39, p < 0.01) on the latency to feed and a 7-CTKA × LY294002 interaction (F1,36 = 17.92, p < 0.001). (G, H) The antidepressant effect of 7-CTKA was blocked by LY294002 in the learned helplessness paradigm (n = 8–10). A 3-way ANOVA with the between-subjects factors treatment (nonshock, shock), 7-CTKA (0 and 1 mg) and LY294002 (0 and 10 nM) revealed significant effects of treatment (F1,67 = 163.55, p < 0.001), 7-CTKA (F1,67 = 5.02, p < 0.05) and LY294002 (F1,67 = 7.43, p < 0.01) on the number of escape failures and a treatment × 7-CTKA × LY294002 interaction (F1,67 = 4.91, p < 0.05). A 3-way ANOVA also revealed significant effects of treatment (F1,67 = 151.25, p < 0.001), 7-CTKA (F1,67 = 3.04, p < 0.05) and LY294002 (F1,67 = 7.99, p < 0.01) on the latency to escape and a treatment × 7-CTKA × LY294002 interaction (F1,67 = 5.60, p < 0.05). (I) Pretreatment of rapamycin (50 μg) and D-serine (50 μg) administered in the mPFC blocked the antidepressant effect of 7-CTKA (1 mg/kg) in the FST (n = 8). The data are expressed as mean and standard error of the mean. *p < 0.05, †p < 0.01, ‡p < 0.001, compared with vehicle-treated rats.
In the novelty-suppressed feeding test, acute 7-CTKA administration significantly decreased the latency to feed (p < 0.001; Fig. 5E), whereas the reduction of the latency to feed in 7-CTKA-treated rats was reversed by LY294002 pretreatment (p = 0.002; Fig. 5E and F). These results indicate that activation of GSK3β effectively reversed the behavioural effect of 7-CTKA in the novelty-suppressed feeding test.
In the learned helplessness paradigm, inescapable shock significantly increased the number of failures (p < 0.001; Fig. 5G) and latency to escape (p = 0.001; Fig. 5H) compared with the nonshock condition. Acute 7-CTKA administration completely reversed the behavioural deficits with regard to failures and latency to escape induced by inescapable shock (Fig. 5G and H). However, the reversal effects of 7-CTKA on failures and the latency to escape was blocked by intra-mPFC administration of LY294002 (Fig. 5G and H).
Furthermore, pretreatment with the mTOR inhibitor rapamycin into the mPFC (50 μg per side) reversed the antidepressant effect of 7-CTKA in the forced swim test (p < 0.001; Fig. 5I) without altering normal locomotion (data not shown). We also found that intra-mPFC administration of D-serine, a glycine site NMDA receptor agonist, produced a significant antidepressant-like action by decreasing the immobility in the forced swim test (p = 0.011; Fig. 5J), whereas the combination of D-serine with 7-CTKA did not change the immobility time (Fig. 5J).
Systemic 7-CTKA administration produced no rewarding effects in the CPP test
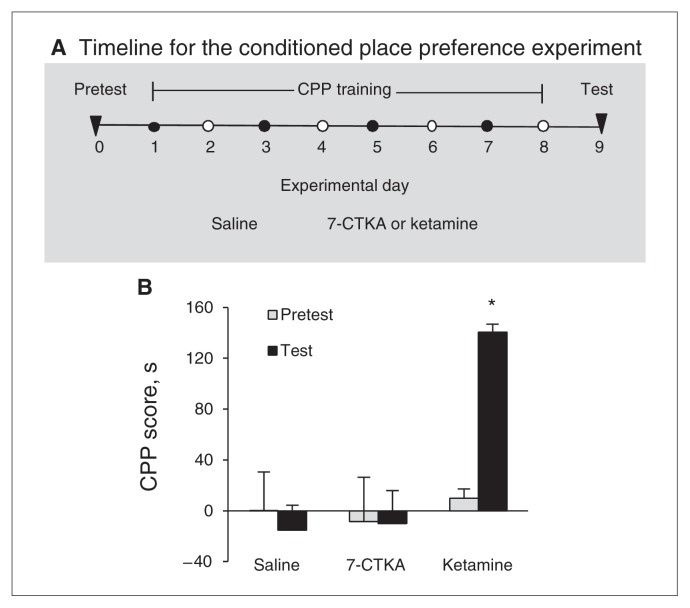
In this experiment, we determined whether systemic 7-CTKA (1 mg/kg, intraperitoneal) has abuse potential when administered at an effective antidepressant dose (Fig. 6A). The analysis of the behavioural data (i.e., the expression of CPP) included drug (saline, 7-CTKA and ketamine) as the between-subjects factor and test condition (pretest baseline preference and test preference) as a within-subjects factor. This analysis of CPP scores after 8 days of conditioning revealed a significant drug × test condition interaction for ketamine (F1,27 = 15.12, p = 0.001) but not 7-CTKA (F1,25 = 0.058, p = 0.81; Fig. 6B). We observed the expression of CPP in rats injected with ketamine but not those injected with 7-CTKA, suggesting that 7-CTKA at an effective antidepressant dose (1 mg/kg) did not have rewarding property.
Fig. 6.
Systemic 7-CTKA treatment produced no rewarding effects in the conditioned place preference (CPP) test. (A) Timeline of the experiment. (B) Conditioned place preference scores for rats during baseline (pretest) preference and during a test for the expression of CPP 1 day after training. The results showed that systemic intraperitoneal administration of 7-CTKA (1 mg/kg) did not alter the expression of CPP. The data are expressed as mean and standard error of the mean (n = 6–7). *p < 0.05, compared with the other experimental groups.
Discussion
The present study showed that a single injection of the glycine binding site NMDA receptor antagonist 7-CTKA produced rapid antidepressant-like effects and also reversed the behavioural deficits induced by CMS. Notably, 7-CTKA at an effective antidepressant dose produced no rewarding effects, reflected by the CPP scores, suggesting that 7-CTKA has no addictive potential and indicating the potential benefits of further exploring 7-CTKA rather than ketamine as a promising antidepressant agent.
The important roles of the GSK3β in depressive behaviour and antidepressant treatment have been previously demonstrated. Chronic administration of traditional antidepressants, including fluoxetine and venlafaxine, significantly increased phospho-Ser9-GSK3β protein in the hippocampus.42 Increasing evidence suggests that the PFC plays a key role in depression.43–46 Previous studies have also reported that ketamine rapidly inhibited GSK3 activation and that GSK3 inhibition is required for the rapid antidepressant effects of ketamine.47 The present results also showed that acute 7-CTKA administration increased p-Ser9-GSK3β in the mPFC, suggesting that the antidepressant response of 7-CTKA is likely mediated by the increase of p-GSK3β in the mPFC. The requirement for GSK3β inhibition for the antidepressant effects of 7-CTKA was further confirmed by the results that acute 7-CTKA reversed GSK3β activation induced by CMS (Fig. 3). We also found that the antidepressant effects of 7-CTKA were blocked by the GSK3β activator LY294002, indicating that the anti-depressant effect of 7-CTKA is GSK3β-dependent. Further studies are required to determine the mechanism by which 7-CTKA increases p-GSK3β and subsequently affects behaviour phenotype.
A growing body of evidence suggests that dysfunction of the NMDA receptor system is critical in depression.48 D-serine, a glycine site NMDA receptor agonist, has been shown to produce antidepressant-like effects in rodents.49 Consistently, we found that intra-mPFC infusion of D-serine produced antidepressant effects. However, pretreatment with D-serine blocked the behavioural response of 7-CTKA. It appears contradictory that both the NMDA receptor agonist and antagonist produced similar antidepressant effects. This may be explained by the regulatory effects of D-serine on glutamatergic neurotransmission and synaptic plasticity by influencing neuronal–glial communication. This regulation might mediate the behavioural effects of D-serine.50 Ketamine also increased glutamatergic neurotransmission in the PFC at non-NMDA glutamate receptors.51 Therefore, 7-CTKA might have similar effects on glutamatergic neurotransmission, which could be a potential mechanism underlying the antidepressant effects of 7-CTKA. Additional glycine site NMDA receptor antagonists will be required to determine the antidepressant efficacy of glycine site antagonism in the treatment of depression.
Synaptogenesis is accompanied by upregulation of post-synaptic proteins, including PSD-95,6 a key synaptic protein that colocalizes with NMDA receptors at synapses in primary neurons and is important for the structure and function of brain cells.52 Decreased PSD95 may result in the dysfunction of NMDA receptors with consequently reduced LTP associated with NMDA receptors.53 Induction of PSD-95 is also consistent with increased synapse formation and function. Consistent with previous reports, our results showed that CMS markedly reduced PSD95 in the mPFC (Fig. 3), showing that PSD95 loss could underlie the behavioural deficits caused by CMS. In contrast, the decrease of PSD95 was rapidly reversed by 7-CTKA, suggesting that synaptic function underlies the antidepressant response of 7-CTKA. In addition, brain-derived neurotrophic factor (BDNF) also participates in the synaptogenesis and neurogenesis.54 Several NMDA receptor antagonists, including ketamine, memantine and MK-801, increased BDNF, which is necessary for their antidepressant responses.55,56 Based on these findings and our observation that 7-CTKA enhanced mTOR function and increased postsynaptic protein levels, we hypothesize that 7-CTKA produces anti-depressant response through multiple pathways just like other NMDA receptor antagonists.
The mTOR signalling pathways have been implicated in the increase in synaptic plasticity and new spine formation. Ketamine activated mTOR by increasing phosphorylated p70s6k and rps6 in the mPFC, indicating that the behavioural effects of ketamine require mTOR activation. Therefore, we hypothesized that stimulation of the mTOR may be involved in the antidepressant-like effects of 7-CTKA. Our findings that pre-treatment with the mTOR inhibitor rapamycin reversed the antidepressant action of 7-CTKA confirmed this possibility. Chronic mild stress significantly decreased phosphorylated p70s6k and rps6 in the mPFC, whereas a single dose of 7-CTKA prevented the deficits in mTOR function induced by CMS. These findings suggest that inhibition of mTOR may be involved in the impairment of synaptic function induced by CMS and that stimulation of mTOR in the mPFC may increase synaptic plasticity and mediate the antidepressant effects of 7-CTKA. However, our findings are not consistent with previous reports that phosphorylated p70s6k and rps6 levels were not changed by chronic stress.7 This may have 2 explanations. First, the exact brain tissues used in these studies were different. Whole cytosolic proteins in the mPFC were selected in the present study, whereas synaptoneurosomes were used in the earlier study. The differences in the neural circuits associated with glutamate-mediated neurotransmission between the cytosol and synaptoneurosome may lead to various behavioural and neurophysiological phenotypes in rats exposed to chronic stress. Further studies are needed to address the mechanisms by which chronic stress induces distinct effects on mTOR signalling cascades in the subcellular components of the mPFC. Second, although CMS protocols were used in these investigations, the stressors were not identical. In the previous study,7 food and water deprivation was one of the stressors used in the 21-day procedure to produce depression-related behaviour, whereas the stressors used in present study did not include food and water deprivation. Dietary restriction has been shown to significantly increase synaptic expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors that underlie changes in synaptic strength.57,58 Therefore, food and water deprivation in the previous study may have been beneficial for rodents to cope with the adaptation of synaptic function without altering phosphorylated p70s6k and rps6 and ultimately normalized the mTOR signalling. Altogether, these results support the hypothesis that 7-CTKA has fast-acting anti-depressant effects by activating the mTOR.
Limitations
Numerous lines of evidence have shown that glutamate neurotransmission dysfunction may be a core feature of psychiatric disorders. Accordingly, both traditional antidepressants and rapid-antidepressant ketamine reduce glutamate release and synaptic transmission and are likely contributing to their therapeutic action. Thus, a limitation of our study is that the effects of 7-CTKA on glutamate release were not directly investigated. Future studies are warranted, particularly to investigate the sites of regulation of the glutamate synapse by 7-CTKA, including presynaptic release of glutamate and postsynaptic NMDA receptors. Additionally, previous investigations have shown that the rapid antidepressant effect of ketamine involved the changes of dendritic spines; thus, whether 7-CTKA has an effect on the modulation of dendritic remodelling and morphological changes also requires further investigation.
Conclusion
The present data showed that acute 7-CTKA administration produced rapid antidepressant-like effects and had no addictive potential. Our results suggest that inhibition of the GSK3β pathway and activation of mTOR in the mPFC are involved in the rapid behavioural response of 7-CTKA. The advantages of quick actions and no abuse potential suggest that specific agents that target the glycine site of NMDA receptors may be promising for the treatment of depression.
Acknowledgements
This work was supported in part by the National Basic Research Program of China (no. 2009CB522004) and National Natural Science Foundation of China (no. 30800362, no. 81071079 and no. 81201038). We thank Prof. Hao-Wei Shen for his helpful comments on this manuscript.
Footnotes
Competing interests: As above. Otherwise, none declared.
Contributors: W.-L. Zhu and L. Lu designed the study. W.-L. Zhu, S.-J. Wang and M.-M. Liu carried out the experiment. S.-J. Wang and H.-S. Shi acquired the data, which M.-M. Liu, R.-X. Zhang, J.-F. Liu and Z.-B. Ding analyzed. W.-L. Zhu wrote the article, which all other authors reviewed. All authors approved publication.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Ruengorn C, Sanichwankul K, Niwatananun W, et al. Factors related to suicide attempts among individuals with major depressive disorder. Int J Gen Med. 2012;5:323–30. doi: 10.2147/IJGM.S30874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–6. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 4.McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26:368–75. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- 5.McEwen AM, Burgess DT, Hanstock CC, et al. Increased glutamate levels in the medial prefrontal cortex in patients with postpartum depression. Neuropsychopharmacology. 2012;37:2428–35. doi: 10.1038/npp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 9.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 10.Liebrenz M, Borgeat A, Leisinger R, et al. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137:234–6. doi: 10.4414/smw.2007.11852. [DOI] [PubMed] [Google Scholar]
- 11.aan het Rot M, Zarate CA, Jr, Charney DS, et al. Ketamine for depression: Where do we go from here? Biol Psychiatry. 2012;72:537–47. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Réus GZ, Stringari RB, Kirsch TR, et al. Neurochemical and behavioural effects of acute and chronic memantine administration in rats: Further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res Bull. 2010;81:585–9. doi: 10.1016/j.brainresbull.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Preskorn SH, Baker B, Kolluri S, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–7. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Graham S, Moroni F, et al. A study of the dose dependency of a glycine receptor antagonist in focal ischemia. J Pharmacol Exp Ther. 1993;267:937–41. [PubMed] [Google Scholar]
- 15.Mao Y, Ge X, Frank CL, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–31. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva R, Mesquita AR, Bessa J, et al. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience. 2008;152:656–69. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu JM, Zhang X, Rodriguiz RM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A. 2008;105:1333–8. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peineau S, Taghibiglou C, Bradley C, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–17. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 19.De Sarno P, Bijur GN, Zmijewska AA, et al. In vivo regulation of GSK3 phosphorylation by cholinergic and NMDA receptors. Neurobiol Aging. 2006;27:413–22. doi: 10.1016/j.neurobiolaging.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou SJ, Xue LF, Wang XY, et al. NMDA receptor glycine modulatory site in the ventral tegmental area regulates the acquisition, retrieval, and reconsolidation of cocaine reward memory. Psychopharmacology (Berl) 2012;221:79–89. doi: 10.1007/s00213-011-2551-6. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Espejo E, Ramiro-Fuentes S, Portavella M, et al. Role for D-serine within the ventral tegmental area in the development of cocaine’s sensitization. Neuropsychopharmacology. 2008;33:995–1003. doi: 10.1038/sj.npp.1301495. [DOI] [PubMed] [Google Scholar]
- 22.Sui L, Wang J, Li BM. Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem. 2008;15:762–76. doi: 10.1101/lm.1067808. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Luo YX, He YY, et al. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–41. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorenza NG, Rosa J, Izquierdo I, et al. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232:210–6. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Porsolt RD, Anton G, Blavet N, et al. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 26.Lin YH, Liu AH, Xu Y, et al. Effect of chronic unpredictable mild stress on brain-pancreas relative protein in rat brain and pancreas. Behav Brain Res. 2005;165:63–71. doi: 10.1016/j.bbr.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Greene J, Banasr M, Lee B, et al. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–68. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, et al. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 29.Hajszan T, Dow A, Warner-Schmidt JL, et al. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu WL, Shi HS, Wang SJ, et al. Increased Cdk5/p35 activity in the dentate gyrus mediates depressive-like behaviour in rats. Int J Neuropsychopharmacol. 2012;15:795–809. doi: 10.1017/S1461145711000915. [DOI] [PubMed] [Google Scholar]
- 31.Willner P, Towell A, Sampson D, et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 32.Wang XY, Zhao M, Ghitza UE, et al. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–10. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue YX, Luo YX, Wu P, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–5. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu L, Hope BT, Dempsey J, et al. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–9. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 35.Aguiar DC, Terzian AL, Guimaraes FS, et al. Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology (Berl) 2009;205:217–25. doi: 10.1007/s00213-009-1532-5. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–49. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 37.Budni J, Lobato KR, Binfare RW, et al. Involvement of PI3K, GSK-3beta and PPARgamma in the antidepressant-like effect of folic acid in the forced swimming test in mice. J Psychopharmacol. 2012;26:714–23. doi: 10.1177/0269881111424456. [DOI] [PubMed] [Google Scholar]
- 38.Ling Poon S, Lau MT, Hammond GL, et al. Gonadotropin-releasing hormone-II increases membrane type I metalloproteinase production via beta-catenin signaling in ovarian cancer cells. Endocrinology. 2011;152:764–72. doi: 10.1210/en.2010-0942. [DOI] [PubMed] [Google Scholar]
- 39.Friedrichs N, Trautmann M, Endl E, et al. Phosphatidylinositol-3′-kinase/AKT signaling is essential in synovial sarcoma. Int J Cancer. 2011;129:1564–75. doi: 10.1002/ijc.25829. [DOI] [PubMed] [Google Scholar]
- 40.Rénéric JP, Lucki I. Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology (Berl) 1998;136:190–7. doi: 10.1007/s002130050555. [DOI] [PubMed] [Google Scholar]
- 41.Estrada-Camarena E, Rivera NM, Berlanga C, et al. Reduction in the latency of action of antidepressants by 17 beta-estradiol in the forced swimming test. Psychopharmacology (Berl) 2008;201:351–60. doi: 10.1007/s00213-008-1291-8. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto H, Voleti B, Banasr M, et al. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry. 2010;68:521–7. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covington HE, III, Lobo MK, Maze I, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–90. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koenigs M, Huey ED, Calamia M, et al. Distinct regions of pre-frontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341–8. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 47.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–70. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malkesman O, Austin DR, Tragon T, et al. Acute d-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol. 2012;15:1135–48. doi: 10.1017/S1461145711001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Billard JM. D-serine signalling as a prominent determinant of neuronal-glial dialogue in the healthy and diseased brain. J Cell Mol Med. 2008;12:1872–84. doi: 10.1111/j.1582-4934.2008.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kornau HC, Schenker LT, Kennedy MB, et al. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–40. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 53.Sultana R, Banks WA, Butterfield DA. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J Neurosci Res. 2010;88:469–77. doi: 10.1002/jnr.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondo M, Takei Y, Hirokawa N. Motor protein KIF1A is essential for hippocampal synaptogenesis and learning enhancement in an enriched environment. Neuron. 2012;73:743–57. doi: 10.1016/j.neuron.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Jantas D, Szymanska M, Budziszewska B, et al. An involvement of BDNF and PI3-K/Akt in the anti-apoptotic effect of memantine on staurosporine-evoked cell death in primary cortical neurons. Apoptosis. 2009;14:900–12. doi: 10.1007/s10495-009-0370-6. [DOI] [PubMed] [Google Scholar]
- 56.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng XX, Ziff EB, Carr KD. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011;65:1024–31. doi: 10.1002/syn.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–86. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]