Abstract
Background
Deep brain stimulation (DBS) of the subcallosal cingulate (SCC) is reported to be a safe and effective new treatment for treatment-resistant depression (TRD). However, the optimal electrical stimulation parameters are unknown and generally selected by trial and error. This pilot study investigated the relationship between stimulus parameters and clinical effects in SCC-DBS treatment for TRD.
Methods
Four patients with TRD underwent SCC-DBS surgery. In a double-blind stimulus optimization phase, frequency and pulse widths were randomly altered weekly, and corresponding changes in mood and depression were evaluated using a visual analogue scale (VAS) and the 17-item Hamilton Rating Scale for Depression (HAM-D-17). In the open-label postoptimization phase, depressive symptoms were evaluated biweekly for 6 months to determine long-term clinical outcomes.
Results
Longer pulse widths (270–450 μs) were associated with reductions in HAM-D-17 scores in 3 patients and maximal happy mood VAS responses in all 4 patients. Only 1 patient showed acute clinical or mood effects from changing the stimulation frequency. After 6 months of open-label therapy, 2 patients responded and 1 patient partially responded.
Limitations
Limitations include small sample size, weekly changes in stimulus parameters, and fixed-order and carry-forward effects.
Conclusion
Longer pulse width stimulation may have a role in stimulus optimization for SCC-DBS in TRD. Longer pulse durations produce larger apparent current spread, suggesting that we do not yet know the optimal target or stimulus parameters for this therapy. Investigations using different stimulus parameters are required before embarking on large-scale randomized sham-controlled trials.
Introduction
Deep brain stimulation (DBS) has recently emerged as a viable option for treatment-resistant depression (TRD). Among several neuroanatomical targets (subcallosal cingulate, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle and habenula),1 DBS of the subcallosal cingulate (SCC) has been the most investigated. Four open-label studies of SCC-DBS involving a total of 66 patients with TRD (major depressive disorder and bipolar depression) showed efficacy and safety.2–7 Although these results are encouraging, about 40%–50% of patients did not respond and 70%–80% did not achieve clinical remission with SCC-DBS.3,4,7
Adjusting stimulus parameters in patients with poor or suboptimal response may improve outcomes in Parkinson disease and TRD.5,8 However, the selection of optimal stimulation parameters (frequency, pulse width, amplitude) can be time-consuming, even when studying movement disorders for which there are immediate objective motor outcomes to measure.9 At present, there is no evidence-based approach for the selection of optimal stimulus parameters for TRD. The optimization of stimulation settings for individual patients is guided by the experience and preference of the clinician and by adapting DBS parameters used for movement disorders.3
To establish an evidence-based standardized algorithm for optimal DBS for TRD, we need double-blind, controlled studies examining the effect of each electrical stimulation parameter on clinical symptoms. In the present study, we examined the clinical and mood responses of patients with TRD to weekly changes in frequency and pulse width stimulation in a double-blind fashion for the first 3 months after DBS implantation. We then examined the clinical efficacy and safety of these stimulus parameters over the following 6 months.
Methods
This pilot study consisted of 4 phases: preoperative screening and baseline evaluation, DBS surgery, double-blind postoperative stimulus optimization, and open-label postoptimization stimulation. The study was conducted at Foothills Hospital in Calgary, Alta., and approved by the University of Calgary research ethics board.
Preoperative screening and evaluation
Two study psychiatrists (R.R. and G.M.) independently screened patients referred by local psychiatrists to determine their eligibility to participate in the study. Criteria for inclusion were presence of major depressive disorder, as determined using the Structured Clinical Interview for DSM-IV;10 severe depression with a minimum score of 20 (out of 52) on the 17-item Hamilton Depression Rating Scale (HAM-D-17);11 treatment resistance, as determined by failure to respond to 4 different classes of antidepressants (including augmentation or combination strategies with lithium, atypical antipsychotics, anticonvulsants and antidepressants), evidence-based psychotherapy or electroconvulsive treatment despite adequate dosage, duration and compliance with treatment;12–14 willingness to comply with long-term follow-up; and age between 20 and 60 years. Exclusion criteria were other Axis I psychiatric disorders, including schizophrenia, bipolar disorder or obsessive compulsive disorder; dementia; psychotic symptoms; history of substance abuse in the 6 months preceding the study; and active suicidal ideation. In addition, cerebrovascular risk factors, previous stroke, head injury, pregnancy or medical and general contraindications for DBS surgery were also exclusion criteria.
Enrolled patients were among the most treatment-resistant. Both patients and family members were fully informed about the aims, benefits and risks of the study. After providing consent, participants underwent magnetic resonance imaging (MRI), neuropsychological tests and clinical assessment using the HAM-D-17, Montgomery–Åsberg Depression Rating Scale (MADRS),15 Hamilton Anxiety Rating Scale (HAM-A)16 and Clinical Global Impression (CGI) scales.17 These clinical assessments were repeated 1 week before surgery, and the averaged scores of 2 time evaluations before surgery were considered baseline scores.
Surgery
The surgical implantation procedure was performed as described by others.18 Briefly, under local anesthesia, the SCC was targeted, as suggested by Hamani and colleagues,19 using stereotactic frame-based MRI, surgical planning software (Atamai) and microelectrode recording. Quadripolar DBS electrodes (lead model 3387; Medtronic) were implanted bilaterally spanning the grey–white–grey matter of the SCC gyrus, such that at least 1–2 active poles of the DBS lead (labelled 0,1,2,3 for the left brain and 4,5,6,7 for the right brain) were in the subgenual target. Three days later, the DBS electrodes were connected to the implantable pulse generator (Kinetra; Medtronic) placed subcutaneously in the left infraclavicular region under general anesthesia. We used MRI (Appendix 1, Fig. S1, available at cma.ca/jpn) and high-resolution computed tomography postoperatively to evaluate the contact location within the SCC and rule out any intracranial hemorrhage. Patients were discharged 1–2 days after the implantation of the pulse generator with the stimulator turned off.
Double-blind stimulus optimization
The optimization of electrical stimulation parameters took place in the first 3 months after the implantation of the DBS system. Monopolar stimulation was applied and pulse width (60–450 μs), frequency (2–185 Hz) and amplitude (0–10.5 V) were adjusted. Two weeks of sham stimulation were included in this double-blind randomized stimulus optimization phase.
At week 1, when the stitches were removed, each electrode was tested for immediate effects on mood using the Positive and Negative Affective Scale (PANAS)20 and the visual analogue scale (VAS). The VAS involved a 100 mm horizontal line with a schematic neutral face at one end corresponding to a score of 0 and a schematic mood state at the other end corresponding to a score of 10. The VAS was used to assess the following moods: sadness, happiness, anger, fear, disgust, anxiety and alertness. For this initial testing, we used monopolar stimulation with a frequency of 135 Hz and a pulse width of 60 μs.7 The amplitude was progressively increased, allowing time for patients to identify an effect. We recorded the amplitude at which positive effects on mood or adverse effects, such as dizziness, paraesthesia and disorientation, were identified. The optimal electrode contact was selected as the one with the lowest amplitude required to elicit a positive mood effect and the highest threshold for an adverse effect. If there were no positive or adverse effects, then the 1 or 2 poles of the DBS lead that were in the described SCC white matter target19 (Appendix 1, Fig. S1) were selected for stimulation. The pulse generator was programmed at this initial setting, and patients were sent home.
During weeks 2–7, different frequencies of stimulation (0, 5, 20, 50, 130 and 185 Hz) were tested in a randomized manner, changing the frequency weekly and keeping pulse width (90 μs) and amplitude (5 V) constant, and assessing clinical and mood responses using the PANAS, VAS and the HAM-D-17. Patients and psychiatrists were blinded to the frequencies tested. During weeks 8–11, the pulse widths were altered while keeping the frequency constant at 130 Hz. Various pulse widths were tested (0, 90, 150, 270, 450 μs) in a randomized and double-blind fashion. For pulse widths up to 150 μs, the voltage was set at 5 V, but at higher pulse widths, the voltage was reduced to 3 V to limit charge density (30 μC/cm).3,21 Clinical and mood responses were assessed using the PANAS, VAS and the HAM-D-17. At week 12, the optimal stimulation parameters for each patient were selected based on the specific frequency or pulse width that was associated with 50% reduction in HAM-D-17 score from the pretreatment baseline and maximal mood response in either the VAS or PANAS.
Open-label postoptimization stimulation
For a further 6 months after this double-blind period, all patients received open-label continuous stimulation using the stimulus parameters that were considered optimal at the end of the optimization phase. No changes were allowed in stimulus parameters or electrode contacts, even if there was no improvement in depression. However, downward titration of pulse width or amplitude occurred if the patient experienced any side effects (e.g., dizziness, paraesthesia, worsening anxiety, restlessness, insomnia) that may have been related to stimulation. We evaluated clinical efficacy every 2 weeks using the HAM-D-17,11 MADRS,15 HAM-A16 and the CGI scales.17 The same comprehensive battery of neuropsychological tests to assess general cognitive, intellectual and emotional functions as well as 4 domains of frontal lobe function that we performed before surgery was repeated at 9 months. We monitored patients for potential adverse events that had been reported in previous SCG-DBS studies.4,5
Results
The demographic and clinical characteristics of each patient, including current and past treatments for drepression are shown in Table 1. All 4 patients completed both the double-blind stimulus optimization and postoptimization phases of the study. Postoperative imaging confirmed adequate placement of electrodes with either 1 or 2 contacts in the correct location (Appendix 1, Fig. S1). No reproducible acute effects of stimulation were observed during intraoperative testing of individual DBS contacts. No patient had surgery- or device-related adverse events. Owing to the small sample size, data are presented in descriptive format only.
Table 1.
Clinical and demographic characteristics of patients undergoing subcallosal cingulate deep brain stimulation for treatment-resistant depression
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age, yr | 49 | 56 | 46 | 50 |
| Sex | Female | Female | Male | Female |
| Marital status | Married | Divorced | Single | Married |
| Family history of depression | Yes | Yes | Yes | Yes |
| Age at onset of MDD, yr | 23 | 20 | 13 | 13 |
| Current episode duration, mo | 32 | 120 | 72 | 84 |
| Lifetime no. of depressive episodes | 2 | 5 | 6 | 1 |
| Prior psychiatric hospital admission | 1 | 3 | 2 | 2 |
| Prior suicide attempt | No | No | No | No |
| Secondary Axis I diagnosis | Agoraphobia with panic disorder | Chronic fatigue syndrome | Hypochondriasis | Anorexia nervosa as a teenager |
| Prior psychotherapy | Yes | Yes | Yes | Yes |
| Previous medications | Fluoxetine, citalopram, bupropion, lamotrigine, venlafaxine, plus augmentation with lithium, quetiapine | Phenelzine, doxepin, fluoxetine, fluoxamine, sertraline, paroxetine, moclobemide, venlafaxine, amitriptyline, plus augmentation with lithium, trazodone, buspirone, tryptophan | Amitriptyline, desipramine, imipramine, nortriptyline, phenelzine, fluoxetine tranylcypromine, fluoxamine, paroxetine, sertraline, venlafaxine, bupropion, moclobemide, lamotrigine, with thyroxine augmentation, St. John’s wort | Bupropion, fluoxetine, phenelzine, moclobemide dualoxetine, escitalopram, mirtazepine, methylphenidate, plus augmentation with lithium, atypical antipsychotics, dexamphetamine |
| Medications (dosage) during SCC-DBS | Citalopram (60 mg/d), clonazepam (0.5 mg qhs), lithium carbonate (600 mg/qhs), quetiapine (150 mg/qhs), multivitamins | Dualoxetine (60 mg/d), zopiclone (22.5 mg/qhs), gabapentin (1200 mg/d), clonazepam (1.5 mg/d), synthyroid (0.15 mg/d) | No antidepressants, vitamin D 4000 IU + calcium, sporadic testosterone im | Dextroamphetamine (10 mg/d), risperidone (0.75 mg/qhs), mirtazepine (11.25 mg/qhs), aripiprazole (6 mg/qhs) |
| Prior ECT | Transient positive response | No response | No response with severe memory deficits | Positive response with severe memory deficits |
| Prior rTMS | No prior treatment | No prior treatment | No response | Responded but relapsed |
| Stages of treatment resistance* | Stage IV | Stage IV | Stage IV | Stage IV |
ECT = electroconvulsive therapy; im = intramuscular; MDD = major depressive disorder; qhs = every evening or bedtime; rTMS = repetitive transcranial magnetic stimulation; SCC-DBS = deep brain stimulation of the subcallosal cingulate.
As per Thase and Rush.12
Stimulus optimization phase
During the first 6 postoperative weeks, we altered the frequency of stimulation, keeping pulse width (90 μs) and voltage (5V) constant. Patient 2 showed a 50% reduction in HAM-D-17 scores as well as a maximal increase in positive affect and decrease in negative affect at DBS frequencies of 20 Hz and 130 Hz. However, this patient did not respond to the same settings later during the pulse width optimization phase, possibly owing to carry forward effects or changes in life events. Patient 4 showed a maximal increase in positive affect at 50 Hz without change in depression scores. The other 2 patients did not respond to changes in frequency (Table 2).
Table 2.
Results of blinded pulse width and frequency alterations in the stimulus optimization phase in patients undergoing subcallosal cingulate deep brain stimulation for treatment-resistant depression
| Pulse width | Frequency | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Scale | Baseline | Off | 90/5 | 150/5 | 270/3 | 450/2 | Off | 5 Hz | 20 Hz | 50 Hz | 130 Hz | 185 Hz |
| Patient 1 | ||||||||||||
| HAM-D-17 | 33 | 26 | 25 | 21 | 17* | 28 | 34 | 25 | 27 | 24 | 29 | 29 |
| VAS-H | 0 | 0 | 1.5 | 0 | 2.5↑ | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| PANAS-P | 14 | 15 | 13 | 21 | 16 | 15 | 11 | 11 | 16 | 15 | 10 | 12 |
| PANAS-N | 41 | 36 | 27↓ | 28 | 27↓ | 36 | 25 | 37 | 27 | 38 | 45 | 28 |
| Patient 2 | ||||||||||||
| HAM-D-17 | 30 | 17 | 18 | 22 | 24 | 13* | 26 | 17 | 13* | 20 | 12* | 27 |
| VAS-H | 1 | 3 | 4 | 0 | 1 | 6↑ | 0 | 1 | 1 | 1 | 4.5↑ | 0 |
| PANAS-P | 15 | 31 | 32↑ | 25 | 15 | 32↑ | 14 | 17 | 15 | 16 | 19 | 14 |
| PANAS-N | 34 | 18 | 21 | 28 | 29 | 16↓ | 14 | 17 | 20 | 16 | 16↓ | 16 |
| Patient 3 | ||||||||||||
| HAM-D-17 | 33 | 26 | 23 | 27 | 22 | 16* | 28 | 28 | 28 | 29 | 31 | 29 |
| VAS-H | 0.3 | 0.2 | 0.4 | 0.4 | 0.5 | 6↑ | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| PANAS-P | 17 | 16 | 20 | 12 | 25 | 37↑ | 14 | 17 | 15 | 16 | 19 | 14 |
| PANAS-N | 16 | 13 | 14 | 13 | 20 | 14 | 14 | 17 | 20 | 16 | 16 | 18 |
| Patient 4 | ||||||||||||
| HAM-D-17 | 27 | 25 | 22 | 24 | 24 | 24 | 23 | 25 | 25 | 21 | 21 | 24 |
| VAS-H | 0.8 | 0.8 | 1 | 1.7 | 1.2 | 4.5↑ | 3 | 1.2 | 1.2 | 3.5↑ | 2.7 | 2.4 |
| PANAS-P | 17 | 15 | 15 | 16 | 14 | 16 | 19 | 16 | 14 | 14 | 17 | 16 |
| PANAS-N | 30 | 28 | 27 | 23↓ | 30 | 25 | 26 | 28 | 29 | 25 | 27 | 30 |
↓ = maximal decrease; ↑ = maximal increase; HAM-D-17 = 17-item Hamilton Rating Scale for Depression;11 PANAS = Positive and Negative Affective Scale, P for positive, N for negative;20 VAS-H = visual analogue scale for happy mood.
Signifies a 50% reduction in HAM-D-17 scores. Two patients inadvertently used flat affect (emotionally blunt) to score the sad feelings on the VAS sadness scale; therefore, those results are not reported. Frequency was kept constant at 130 Hz, and voltage was 2–4 V during pulse width changes. Pulse width and voltage were kept constant at 90 μs and 5 V during frequency changes. Patients 1, 2 and 3 showed 50% reduction in HAM-D-17 scores from the baseline following long pulse width (210 and 450 μs) stimulation. Only patient 2 showed clinical response with 20 and 130 Hz stimulation.
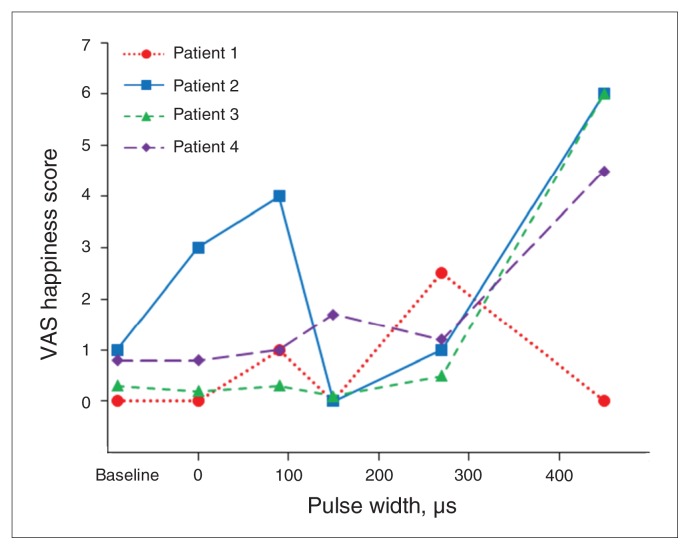
During the next 5 weeks, we altered pulse width while frequency (130 Hz) was kept constant. All 4 patients showed maximal response in happy mood (VAS-H) at longer pulse widths (270 or 450 μs; Fig. 1) and 3 patients (1–3) showed a 50% reduction in HAM-D-17 scores at these longer pulse widths (Table 2). Patient 1 experienced confusion and drowsiness within 2 days after her pulse width was increased to 450 μs, therefore we decreased voltage from 2 V to 1 V to mitigate these adverse effects. Sham stimulation was applied twice during this optimization phase, and depressive symptoms remained reduced in some patients (Table 2).
Fig. 1.
Visual analogue scale (VAS) of patient-reported happiness as a function of pulse width applied. Frequency was kept constant (130 Hz), and changes were made weekly to pulse width. Stimulus intensity was applied at 3–5 V but was reduced at higher pulse durations to keep the charge density within the maximum allowable limit (30 μC/cm2). A pulse width of 0 indicates that the stimulation was turned off. Each patient is shown as an individual line/symbol/colour.
Poststimulus optimization phase
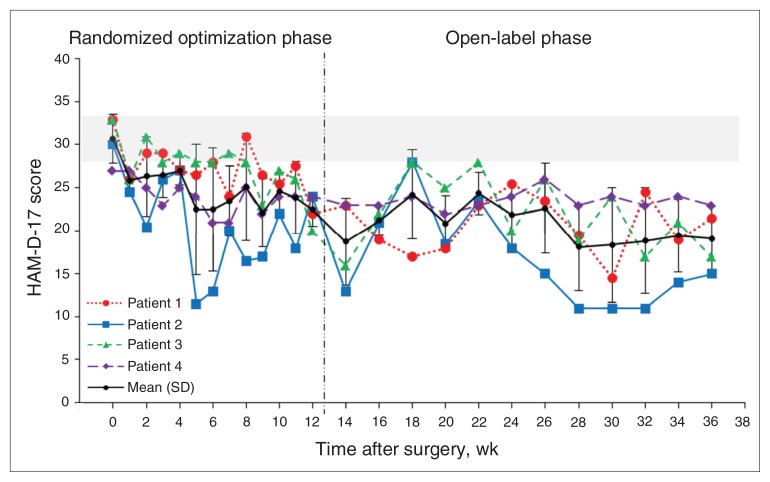
Table 3 details the electrode contacts and stimulation parameters used chronically. Patients 2 and 3 reached the clinical response criterion (50% reduction in HAM-D-17 scores from pre-DBS baseline), and patient 1 achieved a partial response of 35% reduction in HAM-D-17 scores from baseline (Fig. 2). Patient 4 (nonresponder) received standard stimulation settings as previously published (90 μs, 130 Hz, 5 V),4 because she did not respond acutely to any setting tested during optimization. Table 4 provides secondary outcomes, including MADRS and HAM-A scores.
Table 3.
Optimal and chronically used stimulation parameters in patients undergoing subcallosal cingulate deep brain stimulation for treatment-resistant depression
| Electrode; stimulation | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Type of stimulation | Monopolar | Monopolar | Monopolar | Monopolar |
| Electrode contacts*† | C+ 2− C+ 5− |
C+ 3− C+ 4− |
C+ 0− C+ 4−/5− |
C+ 2−/3− C+ 4−/5− |
| Optimal stimulation parameters determined by 12-week optimization phase (blinded) | 2 V, 270 μs, 130 Hz | 2 V, 450 μs, 130 Hz | 2 V, 450 μs, 130 Hz | Not determined |
| Stimulation setting used in postoptimization phase (open label) | 3 V, 150 μs, 130 Hz | 3 V, 210 μs, 130 Hz | 2 V, 450 μs, 130 Hz | 5 V, 90 μs, 130 Hz |
0–3 for left brain and 4–7 for right brain, where 3 and 7 are most dorsal, and 0 and 4 are most ventral poles of deep-brain stimulation lead.
C+ neurostimulator case as anode and − electrode contact as cathode.
Fig. 2.
Mean and individual scores on the 17-item Hamilton Rating Sale for Depression (HAM-D-17)11 over time in all 4 patients. The horizontal grey bar indicates the baseline HAM-D-17 scores with standard deviation (SD). Each patient is shown as an individual line/symbol/colour, and the black indicates mean HAM-D-17 scores and SD for the entire group.
Table 4.
Postoptimization phase test scores of patients undergoing subcallosal cingulate deep brain stimulation for treatment-resistant depression
| Scale | Pre-DBS | Week 14 | Week 16 | Week 18 | Week 20 | Week 22 | Week 24 | Week26 | Week 28 | Week 30 | Week 32 | Week 34 | Week 36 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAM-D-17 | |||||||||||||
| Patient 1 | 33 | 19 | 17 | 18 | 23 | 26 | 24 | 20 | 15 | 25 | 19 | 22 | 23 |
| Patient 2 | 30 | 13 | 21 | 28 | 19 | 24 | 18 | 15 | 11 | 11 | 11 | 14 | 15 |
| Patient 3 | 33 | 22 | 28 | 25 | 28 | 20 | 26 | 19 | 24 | 17 | 21 | 17 | 16 |
| Patient 4 | 27 | 23 | 24 | 22 | 23 | 24 | 26 | 23 | 24 | 23 | 24 | 25 | 25 |
| MADRS | |||||||||||||
| Patient 1 | 42 | 27 | 24 | 30 | 32 | 34 | 31 | 24 | 24 | 25 | 29 | 27 | 24 |
| Patient 2 | 35 | 16 | 32 | 32 | 22 | 25 | 20 | 19 | 14 | 13 | 11 | 22 | 17 |
| Patient 3 | 40 | 25 | 33 | 29 | 32 | 20 | 26 | 24 | 29 | 21 | 26 | 19 | 17 |
| Patient 4 | 34 | 36 | 38 | 34 | 38 | 32 | 40 | 36 | 39 | 38 | 39 | 38 | 39 |
| HAM-A | |||||||||||||
| Patient 1 | 39 | 23 | 25 | 24 | 25 | 31 | 29 | 23 | 27 | 28 | 29 | 26 | 26 |
| Patient 2 | 33 | 17 | 25 | 32 | 27 | 28 | 26 | 21 | 15 | 14 | 17 | 24 | 17 |
| Patient 3 | 28 | 25 | 27 | 24 | 26 | 22 | 22 | 20 | 25 | 21 | 22 | 18 | 19 |
| Patient 4 | 27 | 20 | 21 | 22 | 22 | 22 | 22 | 23 | 23 | 22 | 24 | 23 | 22 |
Adverse events included anxiety manifested as dizzy spells and fainting in patient 1, which resolved without stimulus adjustment and were attributed to her preoperative panic attacks. Anxiety in patient 2 resolved by reducing DBS pulse width from 450 μs to 210 μs. Nocturnal insomnia that began 2 months after the initiation of 450 μs, 130Hz, 2 V stimulation in patient 3 was improved by turning the stimulation off at night.
In neuropsychological testing, 2 DBS responders showed small improvements on speed in timed tasks, consistent with improvement in depression. Patient 1 did not complete post-DBS testing owing to poor motivation, and patient 4 showed improved performances in spatial working memory, selective attention and phonemic fluency.
Discussion
To our knowledge, this is the first study to systematically examine the effects of electrical parameters (pulse width and frequency) on clinical response to SCC-DBS in patients with TRD in a double-blind manner. Stimulation using longer pulse widths (270 or 450 μs) was related to short-term clinical improvement and positive mood response in 3 of 4 patients. Two of these patients showed clinical improvement at 6 months with similar long pulse length stimulation. There was no consistent association between DBS frequency and mood or clinical response. Two patients (50%) showed clinical response at the end of 6 months of the open-label optimal stimulation phase.
Despite the limitations of our study design (listed in the next section), our results are consistent with those of a recent report in which 8 patients also responded to longer pulse width stimulation (180–270 μs, 135 Hz, 3.5–5 V).6 However, 3 other SCC-DBS studies used shorter pulse width stimulation in the range of 60–90 μs, frequencies of 110–140 Hz and amplitudes of 2.5–9 V.4,5,7 Taken together, these data suggest that shorter pulse widths with higher amplitude stimulation (up to 9 V) and longer pulse widths with lower amplitude stimulation may produce comparable benefit, as might be expected from the physics of current injection into nervous tissue.22 Individual variations in response may depend on differences in individual anatomy, fibre pathways, clinical factors or electrode placement. In the absence of predictors of response to DBS, testing both low and high pulse widths could help optimize the response for individual patients. For example, when clinical efficacy is lacking at a low pulse width, increasing the pulse width may provide clinical benefit.
The mechanism underlying the association between long pulse width and clinical efficacy/mood response in SCC-DBS remains unclear. One possibility relates to the spread of current to other pathways projecting to or from ventromedial and orbitofrontal cortical areas that are modulated by SCC-DBS in responders.2 Longer pulse duration could influence pathways farther from the electrodes.22,23 The increased happiness that was experienced by all of our patients using longer pulse widths suggests activation of the SCC–nucleus accumbens network.24
Long pulse width stimulation has disadvantages. It narrows the therapeutic window between beneficial and adverse effects, as demonstrated by 2 of our patients not tolerating a pulse width of 450 μs. In these patients, the long pulse width stimulation induced insomnia, anxiety, confusion and drowsiness, which could be related to downstream fibre tracts connecting the SCC with the amygdala, thalamus/hypothalamus and brainstem.25 Other concerns with long pulse width stimulation are decreased battery life of the pulse generator and risk of tissue damage due to higher electrical charge density. Rechargeable batteries are now readily available to eliminate the need for frequent battery replacements. To prevent tissue damage, longer pulse width was paired with a decrease in voltage to keep charge density below the allowable maximum limit.21
Our results of a clinical response rate of 50% after 6 months of optimal stimulation are similar to those reported in previous open-label studies. Deep brain stimulation failure may have been with our selection of stimulation parameters, based on 1 week of trial stimulation in the optimization phase. We did not allow changes in DBS parameters, even if there was inadequate clinical response, whereas other groups modified parameters frequently to obtain clinical responses in the 50%–60% range.3–5,7 When we increased pulse width to 330 μs (3 V, 130 Hz) in the nonresponder (patient 4) at the end of the 9-month study, her HAM-D-17 score dropped by 30% within 1 month. This highlights the error in our study design, which was planned before recent publications suggesting a cumulative effect of stimulation over time for patients with TRD3,5 and other conditions.26–28
Limitations
There are several limitations to our pilot study, including small sample size, and carry-forward and fixed-order effects during the optimization phase, because of the within-subject design. Owing to fixed-order design, the administration of pulse width changes at weeks 8–12 following the frequency changes at weeks 2–7 may have confounded our results. Recent studies have suggested a carry-forward cumulative effect of stimulation over time.3,5 The successive improvement observed in mean HAM-D scores from weeks 9 to 12 (Fig. 2) regardless of pulse width changes may be due to cumulative effects and/or effective stimulation. The changes in pulse width (90, 150, 270 and 450 μs) and frequencies (long and short) were administered in random order, though the results of pulse width and frequency are shown in ascending order for clarity (Table 2, Fig. 1). That means some patients received long pulse width stimulation in earlier sessions, whereas others received it in later sessions; the same applies to frequency changes. Hence, it is unlikely that the observed temporal association of long pulse width stimulation with 50% change in HAM-D-17 score from baseline and 2- to 6-fold increases in happy scores can be exclusively attributed to carry-forward effects.
We designed this pilot study for feasibility and before recent studies suggested time as an important factor in benefit. Therefore, we used weekly adjustments in frequency and pulse width based on programming used for movement disorders. Weekly changes were likely too short a period to evaluate the relationship between stimulation parameters and clinical response in depression. The effect of negative or positive life events within a 1-week period may have confounded the clinical responses. Such frequent changes might have also caused carry-forward effects, which may explain, in part, the ongoing reduction in depressive symptoms during sham stimulation.5 Other possibilities include too short a period of null stimulation (1 wk) or placebo effect.29 The clinical efficacy assessment in postoptimization phase was an open-label design, making it impossible to tease out the placebo effects of stimulation.
Other issues of importance to future studies of SCC-DBS include electrode placement, which cannot be easily standardized for this target. Contrary to deep brain targets for movement disorders, where placement is based on standard anatomic landmarks, cortical gyral anatomy is variable, and therefore targets must be individualized. This was why we used asymmetric electrode contacts in some patients to optimize the cathode(s) in the white matter of the subgenual cingulate gyrus. This highlights the importance of learning what the true target is for this therapy, such as which white matter tract emanating from the SCC is the relevant one for TRD.
Conclusion
Our preliminary results suggest that there is a role for SCC-DBS in patients with TRD and that more rigorous evaluation of different electrical parameters are required before embarking on large sham-controlled trials. Future studies should consider a parallel 2-arm randomized controlled design without crossover; however, such trials are often difficult and have failed for other conditions.30
Acknowledgements
This study was supported by a grant from the Hotchkiss Brain Institute (HBI) Clinical Research Unit, Faculty of Medicine, University of Calgary, and Calgary Health Region, Calgary, Alberta, Canada, to R. Ramasubbu and Z.H.T. Kiss. Z.H.T. Kiss is a Clinical Scholar of the Alberta Heritage Foundation for Medical Research. S. Chavda was the Denyse Lajoie Lake Fellow of the HBI. We thank Dr. G. MacQueen for screening patients, Dr. M. Eliasziw for assistance in study design, Dr. Y. Starreveld for stereotactic planning, and Haifeng Zhu for statistical assistance. Preliminary results were presented as a poster at the annual conference of Society of Biological Psychiatry at New Orleans, May 2010.
Footnotes
Competing interests: As above for R. Ramasubbu, who also declares having received an investigator-initiated grant from AstraZeneca and from Lundbeck for the WORKER study, speaker’s honoraria from AstraZeneca and payment for developing educational presentations from the Canadian Network for Mood and Anxiety Treatments. S. Chavda declares having won competitive fellowships from HBI as above and from the University of Calgary (International Resident Fellowship award). As above for Z.H.T. Kiss, who also declares receiving grant support through her institution from Medtronic Australia and St. Jude Australia, as well as travel support from Medtronic Inc. None declared for A. Haffenden.
Contributors: R. Ramasubbu and Z.H.T. Kiss designed the study, analyzed the data and wrote the article. R. Ramasubbu, S. Anderson, A. Haffenden and S. Chavda acquired the data. All authors reviewed the article and approved its publication.
References
- 1.Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–10. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 4.Lozano AM, Giacobbe P, Hamani C, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116:315–22. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- 5.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puigdemont D, Perez-Egea R, Portella MJ, et al. Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. Int J Neuropsychopharmacol. 2011 Jul.:1–13. doi: 10.1017/S1461145711001088. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Moro E, Poon YY, Lozano AM, et al. Subthalamic nucleus stimulation: improvements in outcome with reprogramming. Arch Neurol. 2006;63:1266–72. doi: 10.1001/archneur.63.9.1266. [DOI] [PubMed] [Google Scholar]
- 9.Hunka K, Suchowersky O, Wood S, et al. Nursing time to program and assess deep brain stimulators in movement disorder patients. J Neurosci Nurs. 2005;37:204–10. doi: 10.1097/01376517-200508000-00006. [DOI] [PubMed] [Google Scholar]
- 10.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 11.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(Suppl 13):23–9. [PubMed] [Google Scholar]
- 13.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 14.Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–28. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 17.Kadouri A, Corruble E, Falissard B. The improved Clinical Global Impression Scale (iCGI): development and validation in depression. BMC Psychiatry. 2007;7:7. doi: 10.1186/1471-244X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter EO, Davis KD, Hamani C, et al. Cingulotomy for psychiatric disease: microelectrode guidance, a callosal reference system for documenting lesion location, and clinical results. Neurosurgery. 2004;54:622–8. doi: 10.1227/01.neu.0000108644.42992.95. , discussion 28–30. [DOI] [PubMed] [Google Scholar]
- 19.Hamani C, Mayberg H, Snyder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111:1209–15. doi: 10.3171/2008.10.JNS08763. [DOI] [PubMed] [Google Scholar]
- 20.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 21.Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol. 2004;115:2431–41. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 23.Wu YR, Ashby P, Tasker RR, et al. Does stimulation of the GPi control dyskinesia by activating inhibitory axons? Mov Disord. 2001;16:208–16. doi: 10.1002/mds.1046. [DOI] [PubMed] [Google Scholar]
- 24.Johansen-Berg H, Gutman DA, Behrens TE, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–83. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamani C, Mayberg H, Stone S, et al. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–8. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 26.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 27.Fridley J, Thomas JG, Navarro JC, et al. Brain stimulation for the treatment of epilepsy. Neurosurg Focus. 2012;32:E13. doi: 10.3171/2012.1.FOCUS11334. [DOI] [PubMed] [Google Scholar]
- 28.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty DD, Carpenter LL, Bhati MT, et al. A randomized sham-controlled trial of DBS of the VC/VS for treatment-resistant depression [abstract]. Society of Biological Psychiatry 67th annual scientific convention; 2012 May 3–5; Philadelphia, Pa. [Google Scholar]
- 30.Wiebe S, Kiss Z, Ahmed N, et al. Medical vs electrical therapy for mesial temporal lobe epilepsy: a multicenter randomized trial [abstract 2.271] American Epilepsy Society Abstracts. 2012. [accessed 2013 Mar. 19]. Available: www.aesnet.org/go/publications/aes-abstracts/abstract-search/mode/display/st/jette/sy/all/sb/All/id/16444.