Abstract
Background
Evidence that tic behaviour in individuals with Tourette syndrome reflects difficulties inhibiting prepotent motor actions is mixed. Response conflict tasks produce sensitive measures of response interference from prepotent motor impulses and the proficiency of inhibiting these impulses as an act of cognitive control. We tested the hypothesis that individuals with Tourette syndrome show a deficit in inhibiting prepotent motor actions.
Methods
Healthy controls and older adolescents/adults with persistent Tourette syndrome without a history of obsessive–compulsive disorder or attention-deficit/hyperactivity disorder and presenting with stable mood functioning (i.e., no history of well-treated anxiety or depression) participated in this study. They performed a Simon task that induced conflict between prepotent actions and goal-directed actions. A novel theoretical framework distinguished group differences in acting impulsively (i.e., fast motor errors) from the proficiency of inhibiting interference by prepotent actions (i.e., slope of interference reduction).
Results
We included 27 controls and 28 individuals with Tourette syndrome in our study. Both groups showed similar susceptibility to making fast, impulsive motor errors (Tourette syndrome 26% v. control 23%; p = 0.10). The slope (m) reduction of the interference effect was significantly less pronounced among participants with Tourette syndrome than controls (Tourette syndrome: m = −0.07 v. control: m = −0.23; p = 0.022), consistent with deficient inhibitory control over prepotent actions in Tourette syndrome.
Limitations
This study does not address directly the role of psychiatric comorbidities and medication effects on inhibitory control over impulsive actions in individuals with Tourette syndrome.
Conclusion
The results offer empirical evidence for deficient inhibitory control over prepotent motor actions in individuals with persistent Tourette syndrome with minimal to absent psychiatric comorbidities. These findings also suggest that the frontal–basal ganglia circuits involved in suppressing unwanted motor actions may underlie deficient inhibitory control abilities in individuals with Tourette syndrome.
Introduction
Individuals with Tourette syndrome often describe premonitory urges that precede tic behaviour. This has motivated imaging studies to focus on somatosensory and sensorimotor processes as neural correlates of such involuntary urges.1–4 However, tic behaviour in individuals with Tourette syndrome may reflect a dynamic interplay between involuntary urges to act and deficient reactive cognitive control efforts to suppress such action urges. In this study, we examine the hypothesis that individuals with Tourette syndrome have a deficit in top–down inhibitory control over prepotent motor actions.5
Notions about the nature and the relative contributions of bottom–up somatosensory/sensorimotor urges and top–down control processes to tic behaviour are complex and debated.1 Some theories assert that top–down control processes are intact, with individuals with Tourette syndrome issuing tic behaviour voluntarily to reduce the tension produced by involuntary premonitory urges.6 Alternatively, it has been argued that the ability to control or inhibit motor behaviour voluntarily is impaired among individuals with Tourette syndrome and that this, in turn, contributes to tic movements interfering with goal-directed behaviour.7 The facts that many individuals with Tourette syndrome are capable of suppressing tic behaviour, at least transiently, and that tic urges and movements are diminished during performance of complex motor tasks requiring high levels of cognitive control (e.g., playing music or sports) add to the complexity of the role of top–down cognitive control in tic behaviour.8 In the present study, we contribute to this line of investigation by assessing the effects of Tourette syndrome on the expression and suppression of prepotent motor actions.
Conflict tasks produce sensitive measures of the proficiency of action control.9 The goal of these tasks is to issue a speeded manual response to a task-relevant feature of a stimulus display. Concurrent with this deliberate response selection process is an involuntary but prepotent response tendency that is activated rapidly by an irrelevant, but salient, stimulus feature. When the prepotent and goal-driven processing routes converge to the same response, reaction time (RT) speeds and response accuracy increase. Conversely, RT slows and response accuracy decreases when the activation of the prepotent but incorrect response urge interferes with the deliberate selection of the correct goal-driven response. In some instances of conflict, the response system is captured sufficiently to produce an overt response error. The magnitude of interference effects in conflict tasks has been used widely to study individual and group differences in cognitive control over interfering prepotent responses.10
A more elaborate conceptual framework for studying pre-potent action control in interference tasks is provided by the dual-process activation suppression (DPAS) model.11 This model uses distributional analyses to dissociate 2 temporally and functionally distinct processes. The first is the strength of the initial prepotent response urge in conflict trials, henceforth referred to as response capture. The second is the proficiency of inhibitory control engaged subsequently to suppress this urge. This methodology has elucidated and dissociated deviancies in the strength of prepotent response capture and of top–down inhibitory control in clinical populations, including attention-deficit/hyperactivity disorder (ADHD)12 and Parkinson disease,13 and in studies of the effects of targeted interventions on these processes, such as stimulation of the subthalamic nucleus.14
An emerging literature indicates that inhibitory control networks involving frontal–basal ganglia circuits are engaged during conflict trials to prevent capture and interference.15–17 Imaging studies of Tourette syndrome implicate structural and functional changes to these neural circuits, supporting the plausibility that inhibitory cognitive control processes might be impaired.6,18 The few studies on performance of patients with Tourette syndrome on response conflict tasks have produced mixed findings, showing either the absence or presence of exacerbated interference effects in patients compared with healthy controls.19–22 In the present investigation, we used the Simon conflict task and the theoretical framework of the DPAS model to distinguish the effect of Tourette syndrome on the strength of involuntary capture by prepotent motor urges from the proficiency of inhibiting these action urges. Given evidence for dysfunctional frontal–basal ganglia circuitry in individuals with Tourette syndrome, we predicted they would show a reduction in the proficiency of top–down inhibitory control to suppress the response conflict produced by prepotent motor urges. We also tested whether Tourette syndrome is characterized by increased susceptibility to involuntary capture by prepotent response urges.
Methods
Participants
We recruited individuals with a diagnosis of Tourette syndrome and healthy controls through a specialized movement disorder clinic and community advertisement, respectively. A neurologist specializing in movement disorders (D.C.) confirmed the clinical diagnosis of Tourette syndrome, including tic onset before age 18, and presence of motor and vocal tics. Groups were matched for age, education and sex. Individuals with a diagnosis of ADHD or obsessive–compulsive disorder (OCD) were excluded from participation, but those with a diagnosis of mood disorder (depression or anxiety) were included if, at the time of testing, they reported stable and well-controlled mood symptoms on the basis of questionnaire and interview data. Thus, the Tourette syndrome group represented patients with vocal and motor tics, but without potentially confounding psychiatric comorbidities. All participants provided informed consent before participating in the study, which was fully compliant with standards of ethical conduct in human research, as regulated by the University of Virginia and Vanderbilt University institutional human investigation committees.
Screening measures
All participants completed the American National Adult Reading Test23 to estimate verbal intelligence and questionnaires to assess depression (Beck Depression Inventory II),25 anxiety (Beck Anxiety Inventory [BAI]),24 OCD (Yale–Brown Obsessive Compulsive Scale)26 and ADHD (Conners’ Adult ADHD Rating Scales-Short Version).27 In addition, participants with Tourette syndrome were rated on the Yale Global Tic Severity Scale (YGTSS).28
Simon task and procedures
The Simon task produces sensitive measures of prepotent response activation and suppression.9 In the version used in our study, participants issued speeded manual reactions (thumb presses using hand-held grips) based on the colour of circles that appeared sequentially, but randomly, to the left or right of a central fixation point on a computer screen. Goal responses were based on a predetermined mapping between the colour of a presented circle and a response hand (e.g., green circle, right-thumb press; blue circle, left-thumb press), which was counterbalanced across participants. Competing with this deliberate, goal-driven selection process is a spontaneous impulse to respond with the hand that is in the direction corresponding to the spatial location of the circle (i.e., a circle appearing in the left visual field involuntarily triggers an impulse to respond with the left hand, irrespective of colour). When the action impulse triggered by the stimulus location corresponds to the action signalled by the stimulus colour, the dual engagement of the same action speeds RTs and increases accuracy. Thus, for these corresponding trials, the side of fixation on which the circle appeared corresponded with the side of the response signalled by the colour of the stimulus (e.g., a green circle calling for a right-hand response appeared on the right side of fixation).
Conversely, RT slows and accuracy decreases when the action impulse triggered by the circle’s location and the action signalled by its colour are noncorresponding. Thus, for these noncorresponding trials, the circle appeared on the side of fixation opposite to the side of the response signalled by the circle’s colour (e.g., a coloured circle signalling a left-hand response appeared in the right visual field). In this case, involuntary activation of the incorrect action impulse interferes with selection of the goal-directed response and, in some instances, captures the response system sufficiently to produce a fast impulsive error. Slowing of correct responses in this conflict situation is typically attributed to the extra time required to inhibit the interfering action impulse. The detrimental influence of location-driven response activation on the mean RTs and accuracy rates of noncorresponding trials relative to the facilitative influence on corresponding trials is called the Simon effect. This effect has been used with considerable success to study individual and group differences in cognitive control (i.e., inhibition) over interfering action impulses.11
Participants completed a block of 68 practice trials followed by 6 blocks of 68 experimental trials. Short rest breaks of 1–2 minutes were provided between blocks. Within each block of trials, corresponding and noncorresponding trial types were presented randomly, but equiprobably. In total, participants completed 204 corresponding and 204 noncorresponding experimental trials. Stimulus duration was response-terminated, and a variable interstimulus interval ranged from 1750 to 2250 ms in steps of 100 ms. Additional task details regarding stimulus and response features have been described in detail previously.13
Statistical analysis
Extreme RT values, either excessively fast (so-called anticipatory errors; < 150 ms) or slow (> 3 standard deviations [SDs]), were removed from the analysis using a combination of statistical procedures (e.g., value > 3 SDs above the mean) followed by visual inspection to ensure that extreme outliers were excluded.13 On average, these procedures led to the exclusion of fewer than 1% of trials per participant. Mean RT and square-rooted accuracy data were submitted to separate overall analyses (repeated-measures analysis of variance [ANOVA]; Huynh–Feldt adjustments for violations of sphericity) to determine group differences in average Simon effect (i.e., mean RT for noncorresponding trials minus mean RT for corresponding trials). The ANOVAs included the within-subject factor of correspondence (corresponding, non-corresponding) and the between-subjects factor of group (Tourette syndrome, control).
In addition, the strength of response capture by incorrect action impulses was inferred from the proportion of fast errors revealed in conditional accuracy functions (CAFs) that plot accuracy rates as a function of the entire RT distribution for each level of correspondence. Accuracy rates for the fastest RT bin of the CAFs have been demonstrated to be the most sensitive measure of response capture, with stronger capture reflected by a higher percentage of fast errors.15 The proficiency of suppression was inferred from Δ plots, which plot the Simon effect (i.e., mean RT for noncorresponding trials minus mean RT for corresponding trials) as a function of RT. The slope between the Δ values of the 2 slowest RT bins was the primary dependent measure because this value has been demonstrated to be the most sensitive measure of the proficiency of inhibitory control over action impulses.10,11 More proficient inhibition is reflected by steeper reduction of interference (i.e., a larger negative-going final Δ slope). All values derived from the CAFs and the Δ plots were then submitted to separate repeated-measures ANOVAs to examine group differences on the entire functions. We then followed these analyses up with ANOVAs focusing on accuracy rates from the fastest bin of RTs in the CAFs and the slope between the slowest 2 bins of the Δ plot to more precisely measure response capture and suppression of action impulses, respectively. Our detailed methods for computing and analyzing CAFs and Δ plots derived from the Simon task can also be found elsewhere.15,19 Pearson correlations were computed to test associations between questionnaire ratings and performance variables.
Results
Participants
We enrolled 55 participants: 28 with Tourette syndrome and 27 controls. Participant demographic and clinical characteristics are summarized in Table 1. Of the 28 participants with Tourette syndrome, 11 were taking medications to treat tic symptoms: atypical antipsychotics (n = 4), tetrabenazine (n = 2), clonidine (n = 4) and haloperidol (n = 1). Eleven participants with Tourette syndrome were taking either a serotonergic reuptake inhibitor (n = 7) or tetracyclic antidepressant (n = 4) at the time of testing to treat past difficulties with mild depression and/or anxiety; all reported good control over these symptoms at present. All participants had normal or corrected-to-normal vision and denied colour blindness. All but 2 participants (1 from each group) were right-handed.
Table 1.
Demographic and clinical characteristics of the Tourette syndrome and healthy control groups
| Group; mean (SD)* | ||
|---|---|---|
|
|
||
| Characteristic | Healthy control, n = 27 | Tourette syndrome, n = 28 |
| Age, yr | 26.1 (11.9) | 26.6 (13.5) |
| Median [range] | 21 [16–62] | 22 [16–66] |
| Education, yr | 14.7 (2.9) | 13.8 (3.3) |
| Estimated verbal IQ23 | 121.7 (10.7) | 122.6 (14.5) |
| Sex, male:female | 21:6 | 24:4 |
| Beck Anxiety Inventory score24 | 5.6 (5.4) | 11.3 (7.3)† |
| Beck Depression Inventory score25 | 3.1 (2.6) | 5.8 (5.7)‡ |
| Yale–Brown Obsessive Compulsive Scale score26 | 3.1 (4.2) | 9.05 (6.7)§ |
| Conners’ Adult ADHD Index score27 | 8.1 (4.9) | 11.5 (6.3)‡ |
| Yale Global Tic Severity Scale score28 | — | 30.7 (12.9) |
| Motor tic severity, current | — | 12.1 (4.0) |
| Vocal tic severity, current | — | 5.9 (4.9) |
ADHD = attention-deficit/hyperactivity disorder; SD = standard deviation.
Unless otherwise indicated.
p < 0.01.
p < 0.05.
p < 0.001.
Clinical measures
Mean scores from the self-report clinical screening measures for each group are presented in Table 1. Compared with the control group, the Tourette syndrome group had higher anxiety ratings (F1,53 = 10.99, p = 0.002), depression (F1,53 = 5.31, p = 0.025), ADHD (F1,53 = 5.98, p = 0.018) and OCD symptoms (F1,53 = 15.35, p < 0.001). Notably, the Tourette syndrome mean scores on these measures fell in the subclinical to very mild ranges.
Influence of Tourette syndrome on the expression and suppression of prepotent responses
Mean RT and accuracy
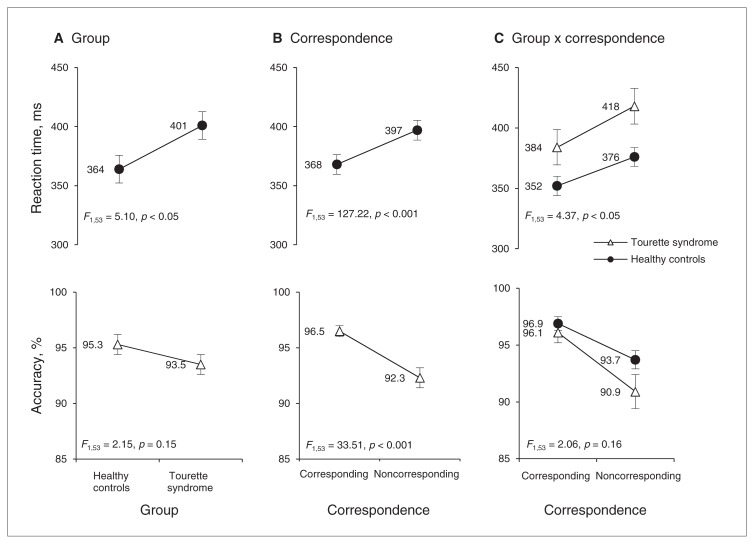
The overall mean RTs and accuracy rates of the Tourette syndrome and control groups are depicted in Figure 1A. The Tourette syndrome group was 38 ms slower to react than the control group, but equally accurate (RT, F1,53 = 5.10, p = 0.028; accuracy, F1,53 = 2.15, p = 0.15). As illustrated in Figure 1B, RTs were slower and accuracy rates were lower for noncorresponding than for corresponding trials (i.e., the Simon effect; RT, F1,53 = 127.22, p < 0.001; accuracy, F1,53 = 33.51, p < 0.001). The cost of noncorrespondence on RT was greater among participants with Tourette syndrome (35 ms) than controls (24 ms; group × correspondence: RT, F1,53 = 4.37, p = 0.041; Fig. 1C). In contrast, the cost on accuracy was similar across the groups (Tourette syndrome 5.2%; control 3.2%; group × correspondence: accuracy, F1,53 = 2.06, p = 0.16).
Fig. 1.
Mean reaction times (RT) and accuracy rates (% correct) as a function of A) group (Tourette syndrome, healthy controls), B) correspondence (corresponding, noncorresponding) and C) the interaction between group and correspondence. All participants show a slowing of RT and reduction in accuracy for noncorresponding compared with corresponding trials, confirming that incorrect motor impulses interfered with selection of correct responses and sometimes captured the response system sufficiently to produce errors. The Tourette syndrome group showed greater mean interference effects on RT; however, unlike the distributional analytic methods described in the captions of Figs. 2 and 3, mean effects cannot distinguish the strength of the incorrect prepotent motor action from the proficiency of inhibiting this action. Error bars reflect standard errors of the mean.
Response capture
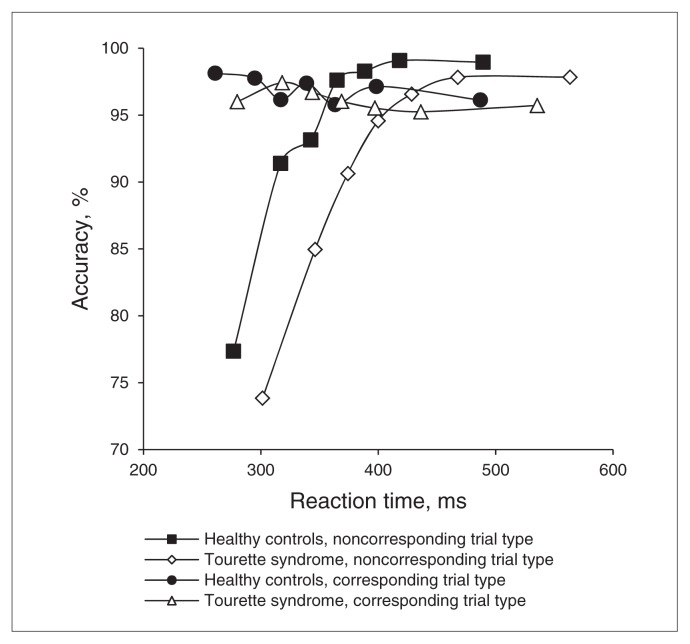
The conditional accuracy functions shown in Figure 2 reveal that fast errors are predominant on noncorresponding trials. Slow responses on noncorresponding trials as well as both fast and slow responses on corresponding trials were associated with near-perfect accuracy. The Tourette syndrome and control groups showed similar patterns of fast errors. We first analyzed accuracy rates across all bins of the CAF as a function of correspondence and group. Similar to the mean analyses, accuracy was reduced for noncorresponding compared with corresponding trials, (correspondence, F1,53 = 34.07, p < 0.001), but did not differ between groups or as a function of correspondence across groups (group, F1,53 = 2.22, p = 0.14; group × correspondence, F1,53 = 2.15, p = 0.15). Accuracy rates varied across bins of the RT distribution, (bins, F6,318 = 46.56, p < 0.001), and the pattern of more fast errors for noncorresponding than for corresponding trials was confirmed (bins × correspondence, F6,318 = 47.43, p < 0.001). Notably, the factor group did not differentially affect these patterns, (group × bin, F6,318 = 0.88, p = 0.46; group × bins × correspondence, F6,318 = 0.76, p = 0.52). To measure the strength of prepotent response capture, we focused our next analysis on a comparison of accuracy rates from the first bin of corresponding and noncorresponding trials according to the a priori theoretical rationale provided by the DPAS model. More fast errors occurred on noncorresponding than on corresponding trials from the fastest bin (correspondence, F1,53 = 85.83, p < 0.001). However, the groups showed a similar percentage of fast response errors, (group, F1,53 = 1.54, p = 0.22), that did not vary by correspondence (group × correspondence, F1,53 = 0.22, p = 0.64). According to the DPAS model, Tourette syndrome and control groups experienced similar levels of initial capture from the involuntary activation of prepotent, incorrect response urges.
Fig. 2.
Conditional accuracy functions. To compute the conditional accuracy function (CAF), all reaction times (RTs) for corresponding and noncorresponding trial types are rank-ordered separately and then partitioned into equal-sized bins representing the fastest to the slowest RTs. For each bin, an accuracy rate is calculated and plotted against the mean RT for that bin, creating a CAF that spans the entire distribution of reactions. The figure depicts the CAFs for corresponding and noncorresponding trial types in Tourette syndrome and control groups. As expected, errors were predominantly associated with the fastest RTs (i.e., the fastest RT bin) on noncorresponding trials, confirming that participants were susceptible to capture by the incorrect motor urge. The Tourette syndrome and control groups showed similar patterns of fast errors, indicating that the strength of initial capture by the prepotent motor action was equivalent across groups.
Selective suppression
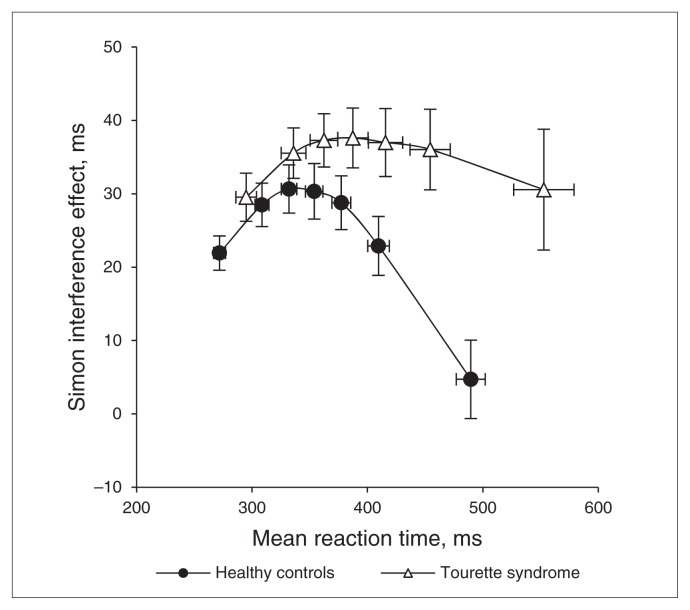
The Δ plots for the Tourette syndrome and control groups shown in Figure 3 illustrate the dynamic change in the Simon effect across the RT distribution, (bins, F6,318 = 10.03, p < 0.001). As predicted by the DPAS model, the hypothesized buildup of inhibitory control results in a precipitous reduction of the Simon effect for the slowest RTs. Importantly, this pattern varied by group (bins × group, F6,318 = 3.45, p = 0.033), with the clear difference emerging in the final bins of the Δ plot where the effect of suppression is predicted to be most pronounced. The suppression dynamics are best measured by the slope of the reduction of the Simon effect across the slowest segment of the Δ plot, with a more negative-going slope associated with more proficient suppression (see Forstmann and colleagues15). As the figure depicts, the final Δ slope is more steeply negative-going among controls than among participants with Tourette syndrome (m = −0.23 v. m = −0.07; F1,53 = 5.53, p = 0.022). According to the DPAS model, this slope difference indicates that participants with Tourette syndrome were less effective than controls at suppressing the interference produced by prepotent responses.
Fig. 3.
Reaction time (RT) Δ plots. To compute a Δ plot, RTs for correct responses to corresponding and noncorresponding trial types are rank-ordered separately and then partitioned into equal-sized bins representing the fastest to the slowest RTs. For each bin, an interference effect is computed (mean RT for noncorresponding trials minus mean RT for corresponding trials) and plotted against the mean RT for that bin. This allows for visualization of the magnitude of interference from the incorrect prepotent motor action across the entire distribution of RTs. A Δ plot is depicted separately for Tourette syndrome and control groups. As expected, the magnitude of interference increases across fast and intermediate response latencies, but then reverses as inhibition of the interfering motor action builds up. The slope between the 2 slowest RT bins provides the most sensitive measure of the inhibition process (i.e., a more negative-going slope indicates more proficient suppression). This slope is significantly less negative-going in the Tourette syndrome than the control group, suggesting that patients with Tourette syndrome are less proficient at suppressing prepotent motor actions.
Association of performance variables with clinical features and treatment of Tourette syndrome
Ratings of tic severity (i.e., total YGTSS tic severity score), OCD, ADHD, anxiety and depression did not correlate with the strength of prepotent response capture (i.e., fast errors) or the proficiency of suppression (i.e., final Δ slope; all p > 0.10). Eleven of the patients with Tourette syndrome were taking medication to reduce tics. To rule out this potential confound, we confirmed that response suppression (i.e., final Δ slope) remained less proficient among the 17 patients with Tourette syndrome who were not taking tic medication (m = −0.04) than the control group (m = −0.23; F1,42 = 7.84, p = 0.008). To further investigate these medication subgroups of patients with Tourette syndrome, a comparison of those taking versus not taking medications to reduce tic symptoms indicated that patients taking medications showed stronger capture by pre-potent responses (i.e., issued more fast response errors) than patients not taking medications (F1,26 = 4.35, p = 0.047). However, the 2 groups of patients did not differ in the proficiency of suppressing prepotent responses, as reflected in the final Δ slope value (F1,26 = 0.51, p = 0.48). Notably, these subgroups also did not differ in tic severity (p = 0.47) or in age (p = 0.42). In a separate subgroup analysis, there also were no differences between patients taking selective serotonergic reuptake inhibitors/tetracyclic medications (n = 11) versus those not taking these medications (n = 17) in terms of overall reaction time (p = 0.59), response capture (p = 0.18) or response suppression (p = 0.31).
Given the differences in anxiety ratings between the groups, we performed additional analyses to rule out the potential influence of anxiety. We addressed this potential confound in 2 ways. First, we matched healthy controls to patients with Tourette syndrome by anxiety levels; 21 of 27 controls and 8 of 28 patients with Tourette syndrome rated anxiety as absent or minimal. Thus, we subgrouped 16 controls (with BAI scores > 2) with participants with Tourette syndrome who had similar levels of anxiety (p = 0.19 for t test comparing BAI scores) and reanalyzed group differences in the patterns of response capture (errors from the fastest RT bin) and suppression (final slope from the Δ plot). Consistent with the main analyses, compared with the healthy control subgroup, the Tourette syndrome group showed significantly less proficient suppression (p = 0.028) but similar response capture (p = 0.60). As an alternative approach, we also trimmed the Tourette syndrome group by eliminating 8 patients with the most extreme anxiety and OCD scores (i.e., values that arguably reflected mild to moderate symptoms) and eliminated 4 extreme controls who reported no symptoms of anxiety and OCD. Again, compared with this subgroup of 22 controls, the subgroup of 20 patients with Tourette syndrome showed no differences in BAI or OCD ratings (p = 0.09 and p = 0.10, respectively), but less proficient suppression (p = 0.026).
Discussion
The Simon task produced clear interference effects in Tourette syndrome and control groups, as evidenced by slowing of mean RT and reduction in mean accuracy when a prepotent response conflicted with rather than facilitated the goal-driven response. Moreover, RT slowing on conflict trials was more pronounced among patients with Tourette syndrome, suggesting greater interference from the activation of a conflicting prepotent response (see Georgiou and colleagues21). Importantly, the DPAS model and distributional analyses provided greater precision in specifying whether patients with Tourette syndrome experienced stronger initial capture by the conflicting prepotent response or had greater difficulties suppressing this response activation due to impaired top–down inhibitory control. Conditional accuracy functions indicated that both groups showed similar patterns of fast errors on conflict (noncorresponding) trials, suggesting that patients with Tourette syndrome did not experience stronger stimulus-driven activation of prepotent responses than healthy controls. The Δ plots also conformed to the predictions of the DPAS model, with interference effects increasing over fast to intermediate response latencies, but reversing dramatically at the slowest response latencies, consistent with the proposed temporal effects of inhibitory control. The slope of interference reduction was significantly less pronounced among patients with Tourette syndrome, indicating a reduced ability to suppress interference from strong, prepotent action tendencies or urges.
Contemporary models propose that alterations in prefrontal–basal ganglia circuits underlie theorized inhibitory control deficits in patients with Tourette syndrome.29 These circuits are also linked empirically to inhibitory action control. For example, the measure of inhibitory control used here, the final Δ slope, correlates inversely with selective activation in the right inferior frontal cortex (rIFC), a key node in inhibitory control circuits.15,16 The subthalamic and caudate nuclei of the basal ganglia, both of which putatively receive input from the rIFC, give rise to hyperdirect and indirect pathways that have also been linked to inhibitory action control.30 Moreover, patient groups with basal ganglia dysfunction (e.g., ADHD, Parkinson disease) also show less negative-going final Δ slopes consistent with poor suppression of prepotent responses.12,13 Finally, stimulation of the subthalamic nucleus modulates inhibitory control in patients with Parkinson disease when performing the Simon task used in our study,14 and imaging studies also suggest caudate nucleus involvement during conflict trials of the Simon task.17,31 Given that patients with Tourette syndrome show morphometric and functional changes in these prefrontal and basal ganglia structures,32,33 the circuits formed by these basal ganglia and prefrontal areas may be particularly important in understanding failures in inhibitory control over prepotent actions.
Maturational age is an important mediator of Tourette syndrome symptoms and frontal–basal ganglia integrity. There has been some suggestion that in children compensatory neuronal changes develop in frontal–basal ganglia circuitries as a result of chronic efforts at suppressing tics, but this pattern is generally contrasted by evidence for hypotrophy of these circuits and diminished inhibitory control among adults with active Tourette syndrome.18,34–36 The Tourette syndrome sample in our study was primarily an adult population, and the pattern of effects remained unchanged even if older adolescents were excluded from the analyses. The persistence of tic behaviour into adulthood may reveal deficient maturation of prefrontal–basal ganglia inhibitory control circuits in a vulnerable subset of patients.33–36 In support of this idea, at least 1 study reported that reduced caudate nucleus volume in childhood was predictive of more severe tic symptoms in early adulthood.37 Thus, participants with Tourette syndrome in our sample may be representative of this vulnerable subset of patients who continue to experience reduced inhibitory control over prepotent responses into adulthood. Longitudinal studies are needed to track the progression of inhibitory control processes from adolescence into adulthood and compare structural or functional neural differences between adults whose symptoms persist or remit/reduce in adulthood.
Limitations
Comorbidities, particularly ADHD and/or OCD, have been argued to be potential mediators of executive cognitive deficits in patients with Tourette syndrome.38 Since we studied a Tourette syndrome group with subclinical psychiatric symptoms, our findings do not address the role of psychiatric comorbidities on cognitive control. However, the findings support the existence of inhibitory control deficits that cannot be directly attributable to comorbid psychiatric conditions. Interestingly, we found no associations between ADHD and OCD ratings and measures of response activation and suppression in the Simon task. Tic severity did not correlate with inhibitory control, suggesting that individual differences in tic intensity and disability capture an element of Tourette syndrome that is distinguishable from the inhibitory control deficit measured here. This is not necessarily surprising given that extremely heterogeneous tic behaviours that range from simple to complex movement patterns involve both manual and vocal response modalities, vary in intensity across developmental stages and vary owing to many contextual and social factors across individuals.39
It may seem surprising that the Tourette syndrome group did not show stronger response activation by the prepotent response (i.e., make more fast response errors) given their difficulty controlling responses to premonitory urges. However, prepotent response activation due to processing of an external stimulus that is unrelated to tic phenomena may be qualitatively different from the activation of prepotent tic responses that arise from internal somatosensory and sensorimotor urges. This idea is also supported by studies of patients with Tourette syndrome performing the go/no-go task, which requires a speeded response to a “go” stimulus presented frequently and an occasional withholding of a response to a less frequently occurring “no-go” stimulus. The development of a prepotent response tendency to frequent “go” stimuli leads to commission errors with less frequently presented “no-go” stimuli, which is used as a putative measure of inhibitory control (i.e., a higher rate of commission errors indicates poorer inhibitory control). Interestingly, studies consistently report no differences in commission error rates between Tourette syndrome and control groups, a pattern that is similar to our finding that patients with Tourette syndrome do not show greater susceptibility to acting on strong prepotent, stimulus-driven response impulses.33,40–43 This further argues for differences between stimulus-driven action impulses and behavioural impulses associated with premonitory urges in patients with Tourette syndrome.
Our findings do not address directly the influence of tic-related medications. Notably, the finding of poor inhibitory control was preserved even after excluding the subset of participants with Tourette syndrome taking medications to reduce tic symptoms. However, patients taking these medications were significantly more susceptible to acting erroneously on strong prepotent responses than those not taking medications. Although speculative, this finding may reflect differences in tic severity such that patients taking medications do so because they had been experiencing stronger tic symptoms. It should be noted that any conclusions based on the analysis of medication effects are tentative, as the study was neither designed to assess this effect nor powered to address this issue. Future studies that assess the impact of tic-related medications on inhibitory control and prepotent response activation are clearly warranted. There has been some suggestion that medications for tics do not affect neuropsychological test performance adversely;44 however, medication effects might be detectable using more sensitive measures of inhibitory action control.
Conclusion
Our results provide empirical evidence that adults with persistent Tourette syndrome show a reduced ability to suppress prepotent motor actions that conflict with goal-directed behaviour. Whether this deficit in cognitive control contributes to tic expression requires additional investigation. Nonetheless, these findings bolster the postulated link between Tourette syndrome and disruption to prefrontal–basal ganglia circuits involved in inhibitory action control.45
Acknowledgements
This work was supported by a Tourette Syndrome Association (TSA) grant awarded to S.A. Wylie, W. van den Wildenberg and D.O. Claassen. We thank Bert van Beek for programming the computer task. We thank Laura Wegner for study coordination and Dr. Fred Wooten for assistance in participant recruitment.
Footnotes
Competing interests: None declared for K.E. Kanoff and K.R. Ridderinkhof. As above for S.A. Wylie, W.P.M. van den Wildenberg and D.O. Classen. S.A. Wylie also declares institutional grant support from the National Institute on Aging (National Institutes of Health). D.O. Classen also declares a speaker honorarium from Teva Pharmaceutical Industries.
Contributors: S.A. Wylie, D.O. Claassen, K.R. Ridderinkhof and W.P.M. van den Wildenberg designed the study and wrote the article. S.A. Wylie, D.O. Claassen and K.E. Kanoff acquired the data. All authors analyzed the data and approved the article’s publication. S.A. Wylie, K.E. Kanoff, K.R. Ridderinkhof and W.P.M. van den Wildenberg reviewed the article.
References
- 1.Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–37. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- 2.Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette’s syndrome. Mov Disord. 2003;18:1530–3. doi: 10.1002/mds.10618. [DOI] [PubMed] [Google Scholar]
- 3.Stern E, Silbersweig DA, Chee KY, et al. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57:741–8. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- 4.Thomalla G, Siebner HR, Jonas M, et al. Structural changes in somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain. 2009;132:765–77. doi: 10.1093/brain/awn339. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Maia TV, Marsh R, et al. The neural circuits that generate tics in Tourette’s syndrome. Am J Psychiatry. 2011;168:1326–37. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JR, Troiano AR, Calne DB. Functional imaging in Tourette’s syndrome. J Neural Transm. 2004;111:1495–506. doi: 10.1007/s00702-004-0173-4. [DOI] [PubMed] [Google Scholar]
- 7.Moretto G, Schwingenschuh P, Katschinig P, et al. Delayed experience of volition in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 2011;82:1324–7. doi: 10.1136/jnnp.2010.221143. [DOI] [PubMed] [Google Scholar]
- 8.Heise CA, Wanschura V, Albrecht B, et al. Voluntary motor drive: possible reduction in Tourette syndrome. J Neural Transm. 2008;115:857–61. doi: 10.1007/s00702-007-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon JR. Reactions toward the source of stimulation. J Exp Psychol. 1969;81:174–6. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- 10.Ridderinkhof KR, Wylie SA, van den Wildenberg WPM. Action control in times of conflict: analysis of reaction time distributions in healthy and clinical populations. In: Posner M, editor. Cognitive neuroscience of attention. 2nd ed. New York: Guilford Press; 2011. pp. 409–20. [Google Scholar]
- 11.van den Wildenberg WP, Wylie SA, Forstmann BU, et al. To head or to heed? Beyond the surface of selective action inhibition: a review. Front Hum Neurosci. 2010;4:222. doi: 10.3389/fnhum.2010.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridderinkhof KR, Scheres A, Oosterlaan J, et al. Distribution-analytical techniques in the study of ADHD: delta plot analyses reveal deficits in response suppression that are eliminated by methylphenidate treatment. J Abnorm Psychol. 2005;114:197–215. doi: 10.1037/0021-843X.114.2.197. [DOI] [PubMed] [Google Scholar]
- 13.Wylie SA, Ridderinkhoff KR, Bashore TR, et al. The effect of Parkinson’s disease on the dynamics of on-line and proactive cognitive control during action selection. J Cogn Neurosci. 2010;22:2058–73. doi: 10.1162/jocn.2009.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wylie SA, Ridderinkhof KR, Elias WJ, et al. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson’s disease. Brain. 2010;133:3611–24. doi: 10.1093/brain/awq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forstmann BU, van den Wildenberg WPM, Ridderinkhof KR. Neural mechanisms, temporal dynamics, and individual differences in interference control. J Cogn Neurosci. 2008a;20:1854–65. doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- 16.Forstmann BU, Jahfari S, Scholte HS, et al. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci. 2008b;28:9790–6. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson BS. Neuroimaging studies of Tourette syndrome: a decade of progress. Adv Neurol. 2001;85:179–96. [PubMed] [Google Scholar]
- 18.Jahfari S, Waldorp L, van den Wildenberg WP, et al. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci. 2011;31:6891–9. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Channon S, Gunning A, Frankl J, et al. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20:58–65. doi: 10.1037/0894-4105.20.1.58. [DOI] [PubMed] [Google Scholar]
- 20.Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioral inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46:1327–36. doi: 10.1111/j.1469-7610.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 21.Georgiou N, Bradshaw JL, Phillips JG, et al. The Simon effect and attention deficits in Gilles de la Tourette’s syndrome and Huntington’s disease. Brain. 1995;118:1305–18. doi: 10.1093/brain/118.5.1305. [DOI] [PubMed] [Google Scholar]
- 22.Thibault G, O’Connor KP, Stip E, et al. Electrophysiological manifestation of stimulus evaluation, response inhibition and motor processing in Tourette syndrome patients. Psychiatry Res. 2009;167:202–20. doi: 10.1016/j.psychres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–49. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Steer RA. Beck Anxiety Inventory. San Antonio (TX): Psychological Corp; 1993. [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio (TX): Psychological Corp; 1996. [Google Scholar]
- 26.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive-Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 27.Conners CK, Erhardt D, Sparrow E. Conners’ adult ADHD rating scales. Technical manual. New York: Multi-Health Systems; 1999. [Google Scholar]
- 28.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–73. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Mink JW. Neurobiology of basal ganglia circuits in Tourette syndrome: Faulty inhibition of unwanted motor patterns? Adv Neurol. 2001;85:113–22. [PubMed] [Google Scholar]
- 30.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Peterson BS, Kane MJ, Alexander GM, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13:427–40. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 32.Peterson BS, Thomas P, Kane MJ, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–24. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 33.Stern ER, Blair C, Peterson BS. Inhibitory deficits in Tourette’s syndrome. Dev Psychobiol. 2008;50:9–18. doi: 10.1002/dev.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baym CL, Corbett BA, Wright SB, et al. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain. 2008;131:165–79. doi: 10.1093/brain/awm278. [DOI] [PubMed] [Google Scholar]
- 35.Draganski B, Martino D, Cavanna AE, et al. Multispectal brain morphometry in Tourette syndrome persisting into adulthood. Brain. 2010;133:3661–75. doi: 10.1093/brain/awq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson SR, Parkinson A, Jung J, et al. Compensatory neural reorganization in Tourette syndrome. Curr Biol. 2011;21:580–5. doi: 10.1016/j.cub.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloch MH, Leckman JF, Zhu H, et al. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–8. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eddy CM, Rizzo R, Cavanna AE. Neuropsychological aspects of Tourette syndrome: a review. J Psychosom Res. 2009;67:503–13. doi: 10.1016/j.jpsychores.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord. 2011;26:1149–56. doi: 10.1002/mds.23618. [DOI] [PubMed] [Google Scholar]
- 40.Hershey T, Black KJ, Hartlein J, et al. Dopaminergic modulation of response inhibition: an fMRI study. Brain Res Cogn Brain Res. 2004;20:438–48. doi: 10.1016/j.cogbrainres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Roessner V, Albrecht B, Dechent P, et al. Normal response inhibition in boys with Tourette syndrome. Behav Brain Funct. 2008;4:29. doi: 10.1186/1744-9081-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichele H, Eichele T, Hammar A, et al. Go/NoGo performance in boys with Tourette syndrome. Child Neuropsychol. 2010;16:162–8. doi: 10.1080/09297040903150182. [DOI] [PubMed] [Google Scholar]
- 43.Serrien DJ, Orth M, Evans AH, et al. Motor inhibition in patients with Gilles de la Tourette syndrome: functional activation patterns as revealed by EEG coherence. Brain. 2005;128:116–25. doi: 10.1093/brain/awh318. [DOI] [PubMed] [Google Scholar]
- 44.Bornstein RA, Yang V. Neuropsychological performance in medicated and unmedicated patients with Tourette’s disorder. Am J Psychiatry. 1991;148:468–71. doi: 10.1176/ajp.148.4.468. [DOI] [PubMed] [Google Scholar]
- 45.Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–82. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]