Abstract
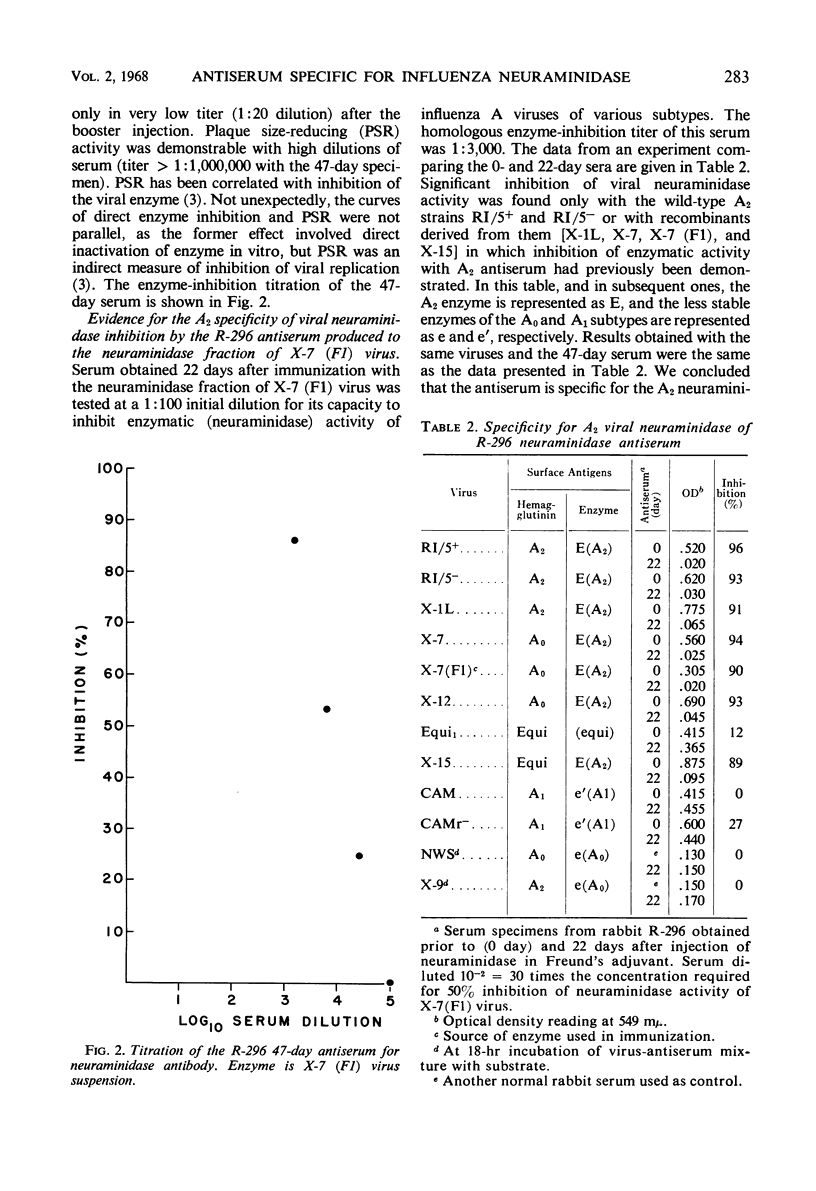
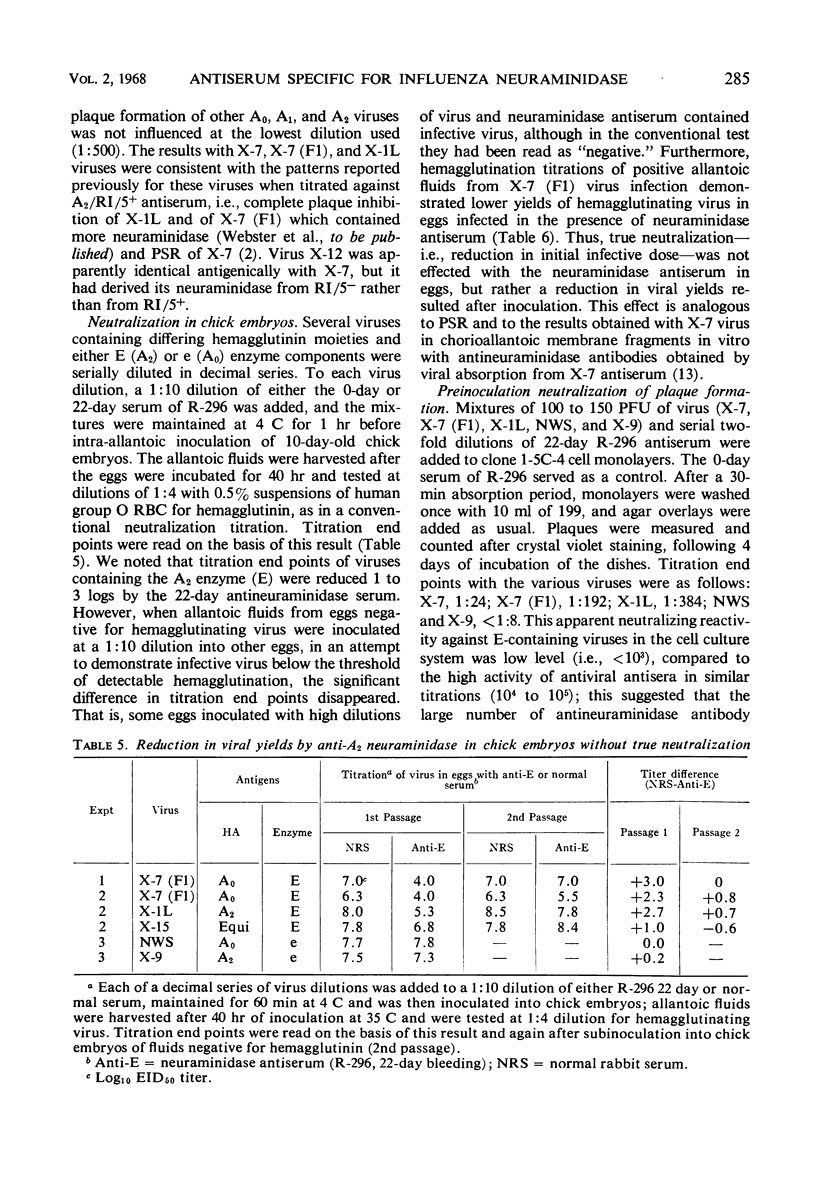
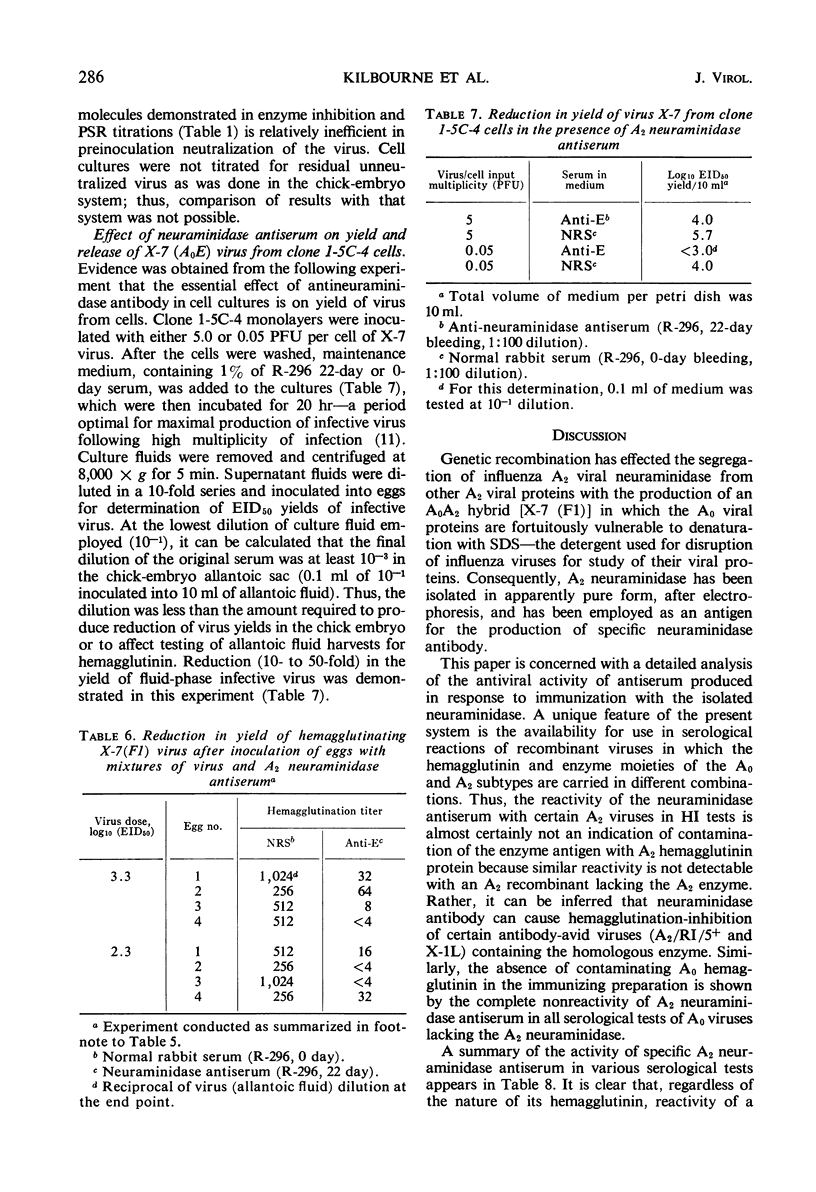
Antiserum specific for influenza A2 neuraminidase was produced by immunization of rabbits with the purified enzyme which had been isolated by electrophoresis from the proteins of a detergent-disrupted A0A2 influenza virus recombinant [X-7 (F1)]. This recombinant contained hemagglutinin of the A0 subtype and A2 neuraminidase. Antiserum to the isolated A2 neuraminidase did not react in any of four serological tests with A0 or A2 subtype viruses that lacked the A2 enzyme. In contrast, the antiserum inhibited the neuraminidase activity only of wild-type and recombinant viruses containing the A2 enzyme, regardless of the nature of their hemagglutinin proteins. The antiserum caused hemagglutination-inhibition of some, but not all, viruses bearing the A2 enzyme, and it reduced the plaque size or plaque number of all viruses tested that contained A2 neuraminidase. In the chick embryo and in cell culture, low dilutions of antiserum reduced the yield of virus. True neutralization of virus in the chick embryo did not occur. We conclude that an antiserum specific for A2 neuraminidase influenced the yield and release of virus from influenza virus-infected cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HOWE C., LEE L. T., ROSE H. M. Collocalia mucoid: a substrate for myxovirus neuraminidase. Arch Biochem Biophys. 1961 Dec;95:512–520. doi: 10.1016/0003-9861(61)90184-9. [DOI] [PubMed] [Google Scholar]
- Jahiel R. I., Kilbourne E. D. Reduction in plaque size and reduction in plaque number as differing indices of influenza virus-antibody reactions. J Bacteriol. 1966 Nov;92(5):1521–1534. doi: 10.1128/jb.92.5.1521-1534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILBOURNE E. D., MURPHY J. S. Genetic studies of influenza viruses. I. Viral morphology and growth capacity as exchangeable genetic traits. Rapid in ovo adaptation of early passage Asian strain isolates by combination with PR8. J Exp Med. 1960 Mar 1;111:387–406. doi: 10.1084/jem.111.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- MAYRON L. W., ROBERT B., WINZLER R. J., RAFELSON M. E., Jr Studies on the neuraminidase of influenza virus. I. Separation and some properties of the enzyme from Asian and PR8 strains. Arch Biochem Biophys. 1961 Mar;92:475–483. doi: 10.1016/0003-9861(61)90387-3. [DOI] [PubMed] [Google Scholar]
- SUGIURA A., KILBOURNE E. D. GENETIC STUDIES OF INFLUENZA VIRUSES. II. PLAQUE FORMATION BY INFLUENZA VIRUSES IN A CLONE OF A VARIANT HUMAN HETEROPLOID CELL LINE. Virology. 1965 Jul;26:478–488. doi: 10.1016/0042-6822(65)90010-3. [DOI] [PubMed] [Google Scholar]
- Seto J. T., Rott R. Functional significance of sialidose during influenza virus multiplication. Virology. 1966 Dec;30(4):731–737. doi: 10.1016/0042-6822(66)90178-4. [DOI] [PubMed] [Google Scholar]
- Sugiura A., Kilbourne E. D. Genetic studies of influenza viruses. 3. Production of plaque-type recombinants with A0 and A1 strains. Virology. 1966 May;29(1):84–91. doi: 10.1016/0042-6822(66)90198-x. [DOI] [PubMed] [Google Scholar]
- WONG S. C., KILBOURNE E. D. Changing viral susceptibility of a human cell line in continuous cultivation. I. Production of infective virus in a variant of the Chang conjunctival cell following infection with swine or N-WS influenza viruses. J Exp Med. 1961 Jan 1;113:95–110. doi: 10.1084/jem.113.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Preparation and properties of antibody directed specifically against the neuraminidase of influenza virus. J Immunol. 1967 Jul;99(1):49–55. [PubMed] [Google Scholar]



