Abstract
Objective
To develop a simple, reproducible model of disc degeneration in rabbits through percutaneous annular puncture and to confirm the degree of degeneration over time.
Methods
Fifteen New Zealand white rabbits (4 to 5 months old and weighing approximately 3 to 3.5 kg each) underwent annular puncture of the L2-L3, L3-L4, and L4-L5 discs. Rabbits were sacrificed at 4, 8, or 20 weeks after puncture. For a longitudinal study to assess changes in disc height over time, serial X-rays were performed at 0, 2, 4, 8, and 20 weeks for rabbits in the 20-week group. Upon sacrifice, the whole spinal column and discs were extracted and analyzed with magnetic resonance imaging (MRI), real time reverse transcriptase-polymerase chain reaction, and histological staining.
Results
The X-rays showed a slow, progressive decrease in disc height over time. Significant disc space narrowing compared to preoperative disc height was observed during the time period (p<0.001). The MRI grade, aggrecan, and matrix metalloprotease-13 mRNA expression and hematoxylin and eosin/safranin O/anti-collagen II staining were consistently indicative of degeneration, supporting the results of the X-ray data.
Conclusion
Percutaneous annular puncture resulted in slow, reproducible disc degeneration that was confirmed by radiology, biochemistry, and histology. This in vivo model can be used to study and evaluate the safety and efficacy of biologic treatments for degenerative disc disease.
Keywords: Model, animal; Intervertebral disc degeneration; Minimally invasive procedure
INTRODUCTION
Low back pain is a common cause of disability with major socioeconomic consequences, affecting millions of individuals each year. Although the pathophysiology of this condition is not yet fully known, intervertebral disc (IVD) degeneration has been shown to be a major cause of low back pain and lumbar disc herniation2).
Since the exact pathogenesis and pathophysiology of disc degeneration is still unknown, most treatments for disc disease have focused on treating the numerous pathologic conditions arising from disc degeneration itself rather than on directly preventing, slowing, or reversing disc degeneration. Recently, a number of novel therapeutic approaches have been proposed for direct management of the underlying causes of IVD degeneration. These approaches include synthetic peptide or growth factor injections1,5,10), gene therapy13,14), cell therapy3,11), and tissue engineering4).
Further advances in the treatment of disc degeneration will require improved understanding of the pathogenesis and pathophysiology of disc degeneration, together with testing the safety and efficacy of new approaches using suitable animal models that mimic disc degeneration in humans.
Previous studies have described animal models in which the IVD is stabbed to induce disc degeneration. These stab models can be classified into two groups. The classic stab model by Lipson and Muir7) uses a full-thickness, ventral stab of the annulus fibrosus (AF) to induce disc degeneration relatively quickly in rabbit lumbar discs. This model appears to produce biochemical and histological changes that are similar to those seen in disc degeneration in humans. However, the full-thickness stab model creates a macroscopic injury that induces rapid and severe disc degeneration; thus, it does not mimic the slow, progressive disc degeneration seen in humans. The superficial disc injury model15) creates a superficial stab wound at the AF. Degeneration after superficial annular injury is much less rapid than with the classic stab model. However, the degeneration is also less reproducible and may therefore be less useful for the assessment of treatment safety and efficacy. Although both stab models result in disc degeneration, neither creates a relatively slow, progressive, and reproducible disc degeneration that allows researchers to study the safety and efficacy of new therapeutic interventions.
The newly developed annulus puncture models9,17), use hypodermic needles with controlled depth to produce a slower, more progressive, and more reproducible decrease in disc height than the classic stab procedure. However, this model requires an open surgical approach to the lumbar spine that is costly and bothersome to perform.
The aim of the present study was to establish a simple, reproducible model of disc degeneration in rabbits through percutaneous puncturing of the annulus with needles in a minimally invasive manner. Another aim was to confirm that this approach results in the slow, progressive disc degeneration that occurs in humans. To achieve these objectives, the author performed radiologic, magnetic resonance imaging (MRI), histological, and biological studies to examine the progress of disc degeneration.
MATERIALS AND METHODS
With approval from the Institutional Animal Care and Use Committee, fifteen New Zealand white rabbits (4-5 months old and weighing approximately 3-3.5 kg each) underwent annular puncture of the L2-L3, L3-L4, and L4-L5 discs. Five rabbits were allocated to 3 different groups according to the time at which they were sacrificed : 4, 8, or 20 weeks postoperatively. For the rabbits in the 20-week group, serial X-rays were performed at 0, 2, 4, 8, and 20 weeks to obtain longitudinal data on disc height changes over time. At each sacrifice time, the five rabbits were killed, and the whole lumbar spinal column was extracted ex vivo for MRI analysis. After the scans, the discs were removed from the punctured and control levels for biochemistry and histology.
Surgical technique
Each rabbit was anesthetized with intramuscular injection of xylazine (5 mg/kg) and ketamine (35 mg/kg), and the fur was shaved from the mid back and right flank. After anesthesia, a lateral plain X-ray was obtained to establish the pre-injection baseline height of the IVDs. The rabbit was then placed in the lateral oblique prone position, and the injection field was sterilized with an alcohol sponge. Initially, the L5-L6 disc was identified through manual palpation of the interspinous space from the mid back and pelvic rim with fluoroscopy (VPX-200; Toshiba Corporation, Tokyo, Japan). After confirmation of the exact level, an 18-gauge angiography needle was inserted 3-4 cm ventral from the midline into the disc space under fluoroscopic control (Fig. 1A). After brief confirmation of the needle position in the center of the disc space by anteroposterior and lateral fluoroscopy, the needle was held in the disc space for 10 seconds. Before removal of the punctured needle, the needle was rotated 180 degrees. A constant amount of nucleus was leaked within the needle and identified by pushing the needle with an empty syringe after removal of the stylet (Fig. 1B). In each rabbit, each of three discs (L2-L3, L3-L4, and L4-L5) was punctured. The L1-L2 and L5-L6 levels were designated as the non-punctured, internal controls. For each level, all procedures for identification and puncture were performed within 20 seconds. Special care was taken to minimize contact with the periosteal tissues of the vertebrae because this could cause hypertrophy of the soft tissues and bony structures around the discs. No antibiotics and analgesic were used after puncture. No rabbits showed neurological symptoms, and all tolerated the procedure well. The rabbits were placed in their cages after observation for recovery. At the planned time, they were sacrificed with intramuscular injection of ketamine (25.0 mg/kg) followed by intravenous injection of sodium pentobarbital (1.2 g/kg).
Fig. 1.
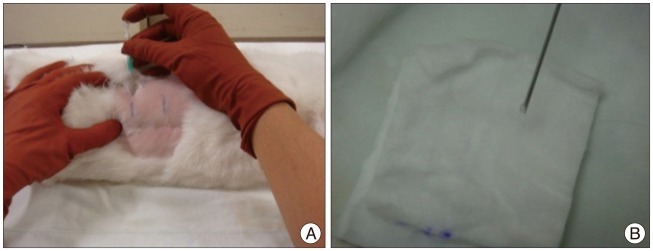
Percutaneous needle puncture into the rabbit discs. A : An angiography needle is inserted 3-4 cm ventral from the midline into the disc space using a fluoroscope. B : Constant amount of nucleus material is checked during the puncture to create even disc degeneration.
Radiologic analyses
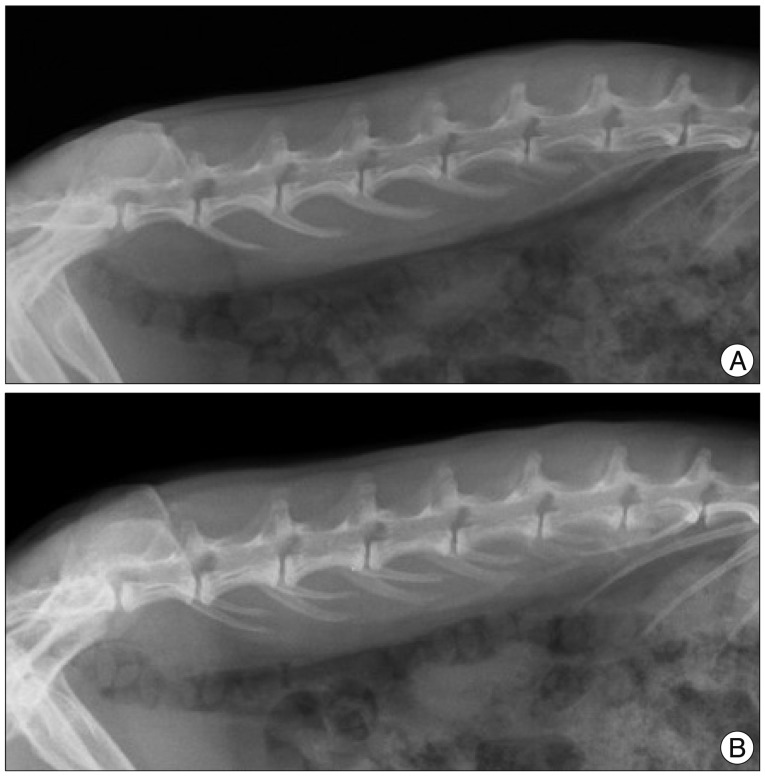
After rabbits were anesthetized, lateral plain radiographs of the lumbar spine were taken with a radiograph machine (collimator-to film distance, 50 cm; exposure, 5 mAs; penetration power, 44 kVp). During the radiographs, special care was taken to minimize axial rotation of the disc space by holding rabbits in the lateral decubitus position while ensuring the X-ray beam was straight. The lateral film was confirmed to be straight by checking for overlap of both transverse processes of the spine (Fig. 2). The X-ray was repeated if a straight film was not obtained. To decrease the error from beam divergence, the beam was centered at 4 cm from the iliac crest. In addition, each rabbit was treated with a consistent amount of anesthesia in order to provide a similar degree of muscle relaxation to minimize differences in disc height. Radiographs were sent to a PACS server and analyzed with the PiViewSTAR program (INFINITT Healthcare, Seoul, Korea).
Fig. 2.
Lumbar lateral X-rays of the rabbit spine. A : Overlapping of transverse processes of the spine is noted in a straight lateral X-ray. B : Distortion of alignment of transverse processes suggests axial rotation of the disc space, which may cause the error in calculating disc height.
The IVD height was expressed as the disc height index (DHI) and was analyzed quantitatively by two independent observers using the methods of Lü et al.8) with modification9) for evidence of changes in the vertebral body adjacent to the disc spaces of the stabbed and intact control discs. To avoid possible magnification error, changes in the DHI of injected discs were normalized to the preinjected IVD height and expressed as percent DHI (% DHI).
MRI scans were performed on ex vivo spinal columns harvested from the rabbits. A 7-T MRI machine (BIOSPEC 70/20 USR; Bruker-biospin, Billerica, MA, USA) with a quadrature extremity coil was used. T2-weighted sections in the sagittal plane were obtained with the following settings : fast spin echo sequence with time to repetition of 3000 ms and time to echo of 70 ms; 512×256 matrix; 100×36 mm field of view; and signal averaging of 2. Section thickness was 1.5 mm with a 0-mm gap. The MRIs were analyzed quantitatively using the modified Thompson grade, in which changes in the degree and area of signal intensity were graded from 1 to 4 (1, normal; 2, minimal decrease in signal intensity but obvious narrowing of the high signal area; 3, moderate decrease in signal intensity; and 4, severe decrease in signal intensity). At the three time points, the MRI data for the punctured discs were pooled to assess the degree of degeneration over time.
Biochemical analyses
The L2-L3 and L3-L4 levels were extracted for real time quantitative polymerase chain reaction (PCR) analysis. The L1-L2 disc was also extracted as a non-punctured control. From each disc, the nucleus pulposus (NP) was carefully removed from AF and stored separately. The tissues were immediately placed into liquid nitrogen and frozen at -80℃ in preparation for PCR analysis.
Genes were selected from the representative forms related to the ECM component (aggrecan) and catabolic enzymes (matrix metalloprotease-13, MMP-13). The frozen NP samples were homogenized with a homogenizer (Mini-Beadbeater; Bio Spec, Bartlesville, OK, USA) in 1 mL Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was extracted in accordance with the manufacturer's instructions. cDNA was generated with Moloney murine leukemia virus reverse transcriptase (RT)(Invitrogen). The RT reaction was amplified in triplicate with real-time PCR (ABI PRISM 7900) in a final volume of 10 µL using SYBR Green Master Mix reagent at a final concentration of 1× (Applied Biosystems, Foster City, CA, USA). Glyceraldehyde phosphate dehydrogenase (GAPDH), a constitutively expressed housekeeping gene, was also amplified under the same conditions and used to normalize reactions. To ensure the specificity of the reaction, the size of the PCR product for each gene was verified by 2.0% agarose gel electrophoresis. Results were obtained with sequence detection software (ABI PRISM 7900) and evaluated with Microsoft Excel.
For quantitation of gene expression, the comparative Ct method was used. The differences between the mean Ct values of the gene of interest and GAPDH were denoted (ΔCt), and the difference between ΔCt of the degenerated sample and ΔCt of the normal control sample was labeled ΔΔCt. Log2 (ΔΔCt) gave the relative quantitation value of gene expression between the degenerated disc and the control disc in each animal.
The primers for the rabbit-specific genes were designed in accordance with published sequences available in GenBank. The sequences were as follows : aggrecan (5' GCTACGGAGACAAG GATGAGTTC 3' and 5' CGTAAAAGACCTCACCCTCCAT 3'), MMP-13 (5' TGCCCCTCCTCAACAGTAAC 3' and 5' GAGCCCGCTGCATTCTTCTT 3'), GAPDH (5' AAGGCCATCACCATCTTCCA 3' and 5' GGATGCGTTGCTGACAATCT 3').
Histologic and immunohistochemical analyses
The punctured disc L4-5 and the control disc L5-6 were harvested for histologic analyses. An electric saw was used to cut each disc together with the adjacent vertebral body. Tissues were fixed with 10% neutral buffered formalin for 48 hours, decalcified in decalcification solution (National Diagnostics, Atlanta, GA, USA) for 3 days, and processed for paraffin sectioning. Blocks embedded in paraffin were cut into mid-sagittal sections (4 µm in thickness) with a microtome. Sections were stained with hematoxylin and eosin (H&E) and safranin O to determine the shape and number of cellular constituents as well as the amount of ECM, and then, analyzed under a light microscope (Nikon Eclipse E800; Nikon, Melville, NY, USA) at magnifications ranging from 40× to 200×. For immunohistochemical staining, the sections were incubated with anti-collagen type II mouse monoclonal antibody (1 : 100 dilution; #II-4C11; Calbiochem, Frankfurt, Germany) and then with HRP-labeled polymer-conjugated secondary antibody against mouse immunoglobulin G. The sections were stained with ready-to-use 3,3'-diaminobenzidine substrate-chromogen solution (Dako, Produktionsvej, Denmark) and then counterstained with Mayer's hematoxylin. Nonimmune mouse immunoglobulin G (Dako) was used for a negative control.
Histologic findings included the number and nature of cells, matrix amount, and integrity of the border between the NP and AF. The findings were quantitatively evaluated with a previously published grading system9). The grades were assigned 1 to 3 points for each of 4 categories, according to the severity of degeneration. Thus, the grades ranged from 4 to 12. The non-punctured disc at the L5-L6 level was also observed as a control in each rabbit to evaluate normal, age-related changes.
Statistical analyses
Longitudinal X-ray data were analyzed with repeated measures analysis of variance (ANOVA) with multiple comparisons, adjusted by Bonferroni method. The effect of time after puncture was analyzed with the Kruskal-Wallis test. Mann-Whitney U tests were used to compare degeneration for the MRI, gene expression, and histology data. Data were analyzed with PASW Statistics 18.0 (IBM, Armonk, NY, USA). Data are presented as mean±standard deviation. p-values less than 0.05 were considered statistically significant.
RESULTS
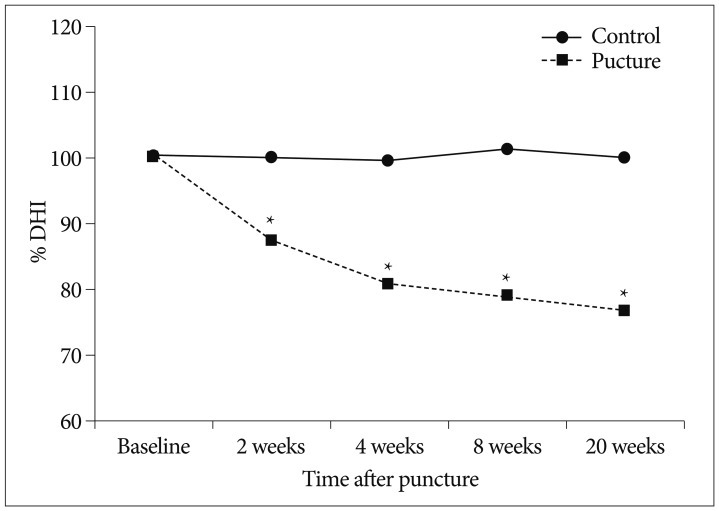
To assess disc height change over time, % DHI was calculated in the 20-week group. When compared to baseline % DHI values obtained before the experiment, control discs showed no significant change in % DHI at any time point during the experiment. In contrast, the punctured discs showed significant narrowing of disc space at 2 weeks (87.2%,) and at 4 weeks (80.5%) (p<0.001, respectively) (Fig. 3). Thereafter, the % DHI changed to 76.5% at 20 weeks. Degeneration progressed gradually and was maintained over the study period (p<0.001, repeated measures ANOVA).
Fig. 3.
Degeneration progress a slow and gradual pattern over time. The punctured discs shows significant narrowing compared to the control discs at each time (*p<0.001). DHI : disc height index.
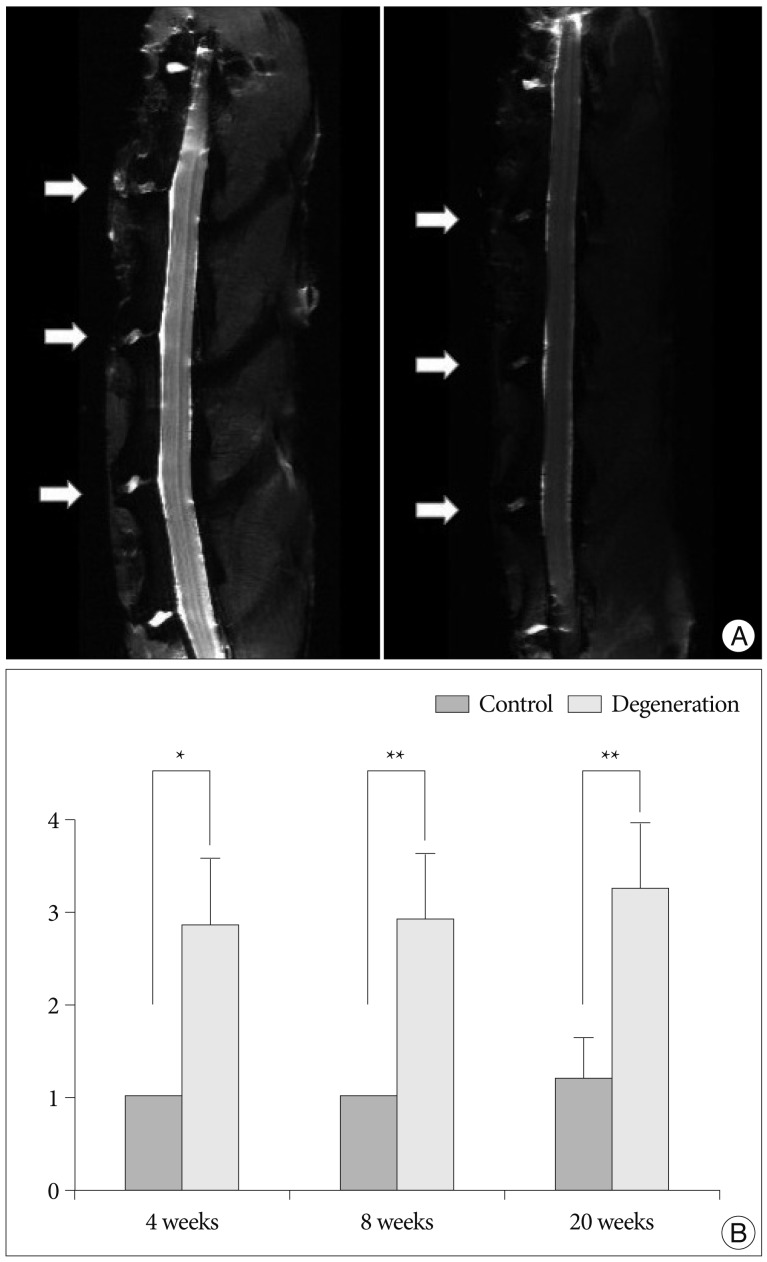
The MRI data showed a loss of signal intensity and a decrease in area from mild to severe for each punctured disc (L2-3, L3-4, and L4-5) on the T2-weighted midsagittal images (Fig. 4A). The two non-punctured control discs (L1-2 and L5-6) maintained a consistent, normal grade over the same time period. At 4 weeks after puncture, the MRI grade of the punctured discs had significantly increased compared to that of the control discs (p<0.01). The MRI grade for each of the punctured discs also tended to increase over time, although this tendency was not statistically significant (p=0.274, the Kruskal-Wallis test) (Fig. 4B).
Fig. 4.
Ex vivo analyses of MRI of the rabbit spine. A : Representative T2-weighted sagittal image of lumbar spine shows that moderate degenerated discs (arrows; left) at L2-3, L3-4, and L4-5. Severe degenerated disc demonstrates more decreased in signal intensity and area (arrows; right). B : The MRI grade of the punctured discs is significantly increased compared to that of the control disc (L5-6) at each time period (*p<0.01, **p<0.001).
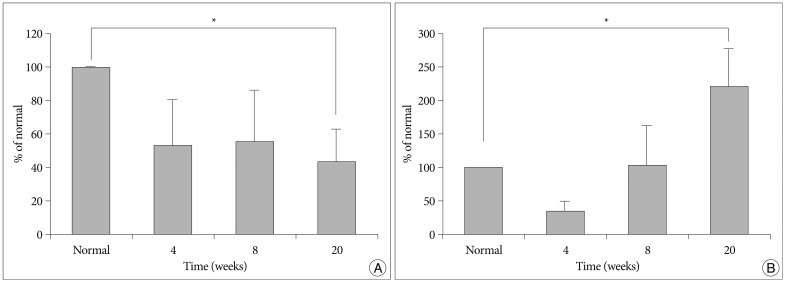
The gene expression results during the course of degeneration were calculated as relative percentages compared with the intact, non-punctured levels of mRNA for each respective gene (Fig. 5). Representative ECM component aggrecan decreased to 45.5% at 4 weeks and to 30.7% at 20 weeks (Fig. 5A). The relative expression levels were not significantly different between time periods after puncture. For the catabolic enzyme MMP-13, the levels of mRNA decreased slightly at 4 weeks, increased at 8 weeks, and remained increased through week 20 (Fig. 5B). The gene expression levels were significantly increased over the time period (p<0.05). For both genes, expression levels in the punctured discs were significantly different from those of normal control discs at 20 weeks (p<0.05).
Fig. 5.
mRNA level of aggrecan and MMP-13 in the punctured degenerated discs calculated as relative percentages compared with the intact, non-punctured level. A : Aggrecan mRNA level in degenerated discs is significantly decreased than that in the control disc (L1-2) at 20 weeks post-puncture (*p<0.05). B : MMP-13 mRNA levels gradually increase over time. Significant difference between the control and the punctured level is noted at 20 weeks (*p<0.05). MMP : matrix metalloprotease.
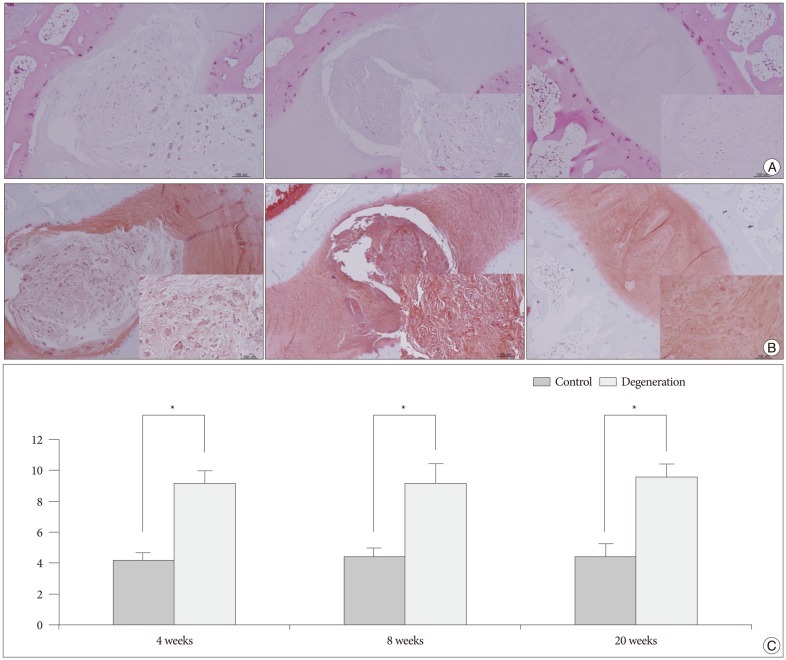
Representative H&E staining and safranin O staining of histologic sections from the rabbit discs showed various stages of degeneration (Fig. 6A, B). Each healthy, non-punctured disc displayed an AF with a normal pattern of fibrocartilage lamellas and a normal border between the AF and NP. The NP was round and bloated in appearance and consisted of numerous large, notochordal cells (Fig. 6A, B; left column). The middle column of Fig. 6A, B depicts a disc with intermediate degeneration from a rabbit sacrificed at 4 weeks. In this disc, the annulus had a more wavy appearance, and the NP was reduced in the extracellular matrix and the large vacuolated cells. The border between the annulus and nucleus was less distinct than in the normal disc. The right column of Fig. 6A, B represents a severely degenerated disc at the 20-week time point; in this disc, most of the contents of the NP had been lost, and the disc showed wavy fibrocartilage lamellas of the AF with abundant associated fibroblasts. The immunohistochemical expression of type II collagen in degenerated discs was decreased in the ECM and pericellular area of the NP, compared with that of a normal control disc (Fig. 7). The histologic score of the degenerated discs was significantly higher than that of control discs at each time point (p<0.01) (Fig. 6C).
Fig. 6.
Histologic analyses of the rabbit discs. 40× and 200× (inner rectangle) power images. A : Normal non-punctured disc shows a clear boundary between the NP and AP with a normal pattern of fibrocartilage lamellas in AF. The NP cells are numerous large, vacuolated notochordal cells (left). In moderate degenerated disc, the border between the NP and AF is less distinct than in the normal disc. The notochordal cells are decreased in number and replaced with chondrocyte-like cells (middle). In a severely degenerated disc, the NP tissue is lost and replaced with fibrocartilage lamellas of the AF with abundant associated fibroblasts (right). B : Serial sections of the discs stained with Safranin O. C : Significantly higher grades are noted in the degenerated discs when compared with the controls (*p<0.01). NP : nucleus pulposus, AF : annulus fibrosus.
Fig. 7.
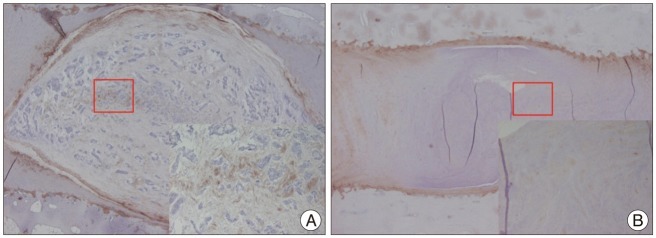
Immunohistochemical staining of the rabbit discs. 40× and 200× (inner rectangle) power images. Expression of type II collagen is prominent in the ECM and pericellular region of the NP within the control disc. B : The expression is scanty in the degenerated disc. NP : nucleus pulposus.
DISCUSSION
This paper describes a minimally invasive percutaneous puncture model of disc degeneration in rabbits. The author found that the posterolateral puncture of rabbit lumbar discs with an 18-gauge angiography needle resulted in slow, progressive, and reproducible disc degeneration over 20 weeks, as confirmed by X-ray, MRI, gene expression, and histologic findings. This needle puncture model appeared to induce disc degeneration more slowly than does the stab model7), in which disc degeneration is achieved with a full-thickness, ventral annular lesion from a No. 11 blade. Needle puncture with the selected angiography needle led to small, controlled leakage of the NP and slow degeneration over time. In contrast, the classic stab model results in immediate herniation of a large bulk of the NP, and studies have found almost no nuclear material in situ 1 to 2 days post stab7) and rapid narrowing of the disc space in 2 weeks9).
Lü et al.8) devised a simple radiographic technique for measurement of disc height : % DHI. For more accurate measurements, the author used Masuda et al.'s9) modification of this technique. In addition, the author tried to properly position each rabbit, exactly align the X-ray beam, and maintain a consistent level of anesthesia in order to minimize any errors in disc height calculation. The film was repeatedly checked until both transverse processes of the spine were overlapped, suggesting that the true lateral view had been obtained (Fig. 2). These methods permitted to obtain consistent results showing progressive degeneration of the discs over time. Data from the rabbits in the 20-week group were used to examine serial changes in disc height over time. Moreover, a repeated measures design was used to minimize any inter-subject variability in % DHI after disc puncture. A statistically significant decrease of % DHI, compared to that of control disc was observed over the time period. This result was comparable to other annular puncture model9), which studied with various sized needles.
The results of ex vivo MRI assessment with the modified Thompson grade were consistent with the radiographic findings. The results were also consistent with those of previous studies that used MRI to verify disc degeneration in rabbits9,17). The use of a simple numeric scale from 1 to 4 has shortcomings in terms of quantitative data analysis. This limitation can be minimized by using a quantitative, automated technique to define the region of interest with computer software17).
The biochemical changes associated with IVD degeneration include degradation of the ECM and an increase in catabolic enzymes. The major components of the ECM are proteoglycans and collagens, and aggrecan is the major proteoglycan in the disc. Among the collagens in the disc, type II collagen is a major constituent, particularly in the NP. The catabolic involvement of MMP-13 on the ECM, especially for collagen type II, has previously been demonstrated during degeneration of the IVD6,12,16). The results of this study for changes in the aggrecan and MMP-13 are typical of those that occur during degeneration of the IVD.
Typical changes in histology were also noted in the present study. After early loss of vacuolated, notochordal cells within the NP at 4 weeks after puncture, the AF underwent infolding, the nucleus was replaced with fibrocartilage, and the ECM severely condensed. Safranin O stains the proteoglycan content in ECM. In the degenerated discs, the intensity of safranin O was decreased. The expression of type II collagen, a major ECM component in the NP, decreased in degenerated discs. The histologic scale enabled the quantification of disc degeneration, and the results were consistent with those from the X-rays and MRI. Thus, this scale can be used in the assessment of biological treatments for disc degeneration.
Recently, open surgical needle puncture models have been developed, and these models appear to induce slower disc degeneration than does the classic stab model because they cause controlled leakage of the nuclear contents by annular puncture from needles of various sizes9,17). The anterior surface of the disc is exposed from a posterolateral retroperitoneal approach, and the anterolateral AF is punctured with a 16- or 18-gauge hypodermic needle at a controlled depth of penetration.
However, the open puncture models require more technical support, time, and costs related to surgery and postsurgical care. In addition, excessive dissection or irritation of the ligaments in the perivertebral area can lead to osteophyte formation around the disc space. Osteophytes make it difficult to inject therapeutic agents, such as proteins and cells. In general, when using a disc degeneration model to verify the safety and efficacy of new biological treatments, the delivery of new pharmaceutical agents should be simple and repeatable. Unfortunately, in the open model, second delivery requires another open surgery, which is usually performed on the contralateral side1,10). Doing a repeated delivery is more complicated than an initial delivery due to the surgical stress to the experimental animals. However, in this percutaneous needle puncture model, the delivery of agents is simple and controllable through the use of fluoroscopy, and repeated injection can be performed easily at a predetermined time interval with minimal stress to the animals.
To ensure that disc degeneration is reproducible and even, it is important to control the depth and location of the needle stab in the disc. Previous open puncture models have controlled the depth at 5 mm with a handmade stopper9) or a locking forceps17), but the location of the needle tip is determined by the researcher's feel in the operative field. This model confirms the depth of the needle in real time using fluoroscopy, so obtaining a controlled depth is easy and reproducible. In addition, the exact location of the needle tip in the disc space is confirmed in the anteroposterior and lateral fluoroscopic views.
Preliminary experiments revealed the importance of extracting an equivalent amount of nucleus material during each puncture to create even disc degeneration (data not shown). After puncture, the holding time in the disc should be constant. Longer holding times result in greater extraction of NP. Moreover, as the degree of needle rotation increases, a greater amount of disc degeneration develops. On the basis of these preliminary results, the holding time and degree of rotation of the punctured needle were maintained at 10 seconds and 180 degrees, respectively, in the present study. Controlling the degree of disc degeneration is easily accomplished with this model and makes it possible to assess various hypotheses related to the grade of disc degeneration.
One of the drawbacks of image-guided puncture is that it may cause repetition of the puncture. It is also technically demanding because the operator must avoid unnecessary contact with or damage to the ligamentous structures around the disc and the AF itself in order to prevent uneven disc degeneration. Thus, adequate training in this technique is required. Excessive exposure to radiation is another concern, especially for researchers performing a large series of studies. Thus, the use of protective devices, such as a lead gown, lead gloves, thyroid protector, and goggles, is mandatory when performing these procedures. Disc degeneration from the annular puncture model has an issue that may not reflect an actual degenerative process as seen in clinics. However, controlled leakage of NP using this fluoroscopic-guided needle puncture adjusted by holding time and degree of rotation may mimic various stages of disc herniation and its consequent degenerative changes. Future studies using larger animals such as dogs, pigs, and non-human primates could support these findings.
CONCLUSION
The author developed a rabbit model of degenerative disc disease that uses a novel percutaneous technique. The model was simple and minimally invasive and resulted in slow, progressive, and reproducible disc degeneration confirmed by radiology, biochemistry, and histology. This model can be used to study the pathogenesis of IVD degeneration and to evaluate the safety and efficacy of new biologic treatments for degenerative disc disease.
Acknowledgements
Hae-Jin Kim, Ph.D., Eun-Joung Moon, Ph.D., Yang-Seon Kim, Ph.D., and Hyeon-Jeong Kim, M.S., provided the technical support and thoughtful comments on the design for gene expression study. The technical support for in vivo studies was provided by the Laboratory Animal Research Center in the Samsung Biomedical Research Institute (SBRI). SBRI is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) accredited facility and abide by the Institute of Laboratory Animal Resources (ILAR) guide.
References
- 1.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, et al. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976) 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 2.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 3.Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008;17(Suppl 4):492–503. doi: 10.1007/s00586-008-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc : the challenges. Eur Spine J. 2008;17(Suppl 4):480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ. Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976) 2013;38:E49–E58. doi: 10.1097/BRS.0b013e31827aa896. [DOI] [PubMed] [Google Scholar]
- 6.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 7.Lipson SJ, Muir H. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine (Phila Pa 1976) 1981;6:194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lü DS, Shono Y, Oda I, Abumi K, Kaneda K. Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine (Phila Pa 1976) 1997;22:1828–1834. doi: 10.1097/00007632-199708150-00006. discussion 1834-1835. [DOI] [PubMed] [Google Scholar]
- 9.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture : correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 10.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976) 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 11.Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics : disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon SH, Gilbertson LG, Nishida K, Knaub M, Muzzonigro T, Robbins PD, et al. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer : implications for the clinical management of intervertebral disc disorders. Spine (Phila Pa 1976) 2000;25:2573–2579. doi: 10.1097/00007632-200010150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy : an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976) 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976) 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 2005;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]