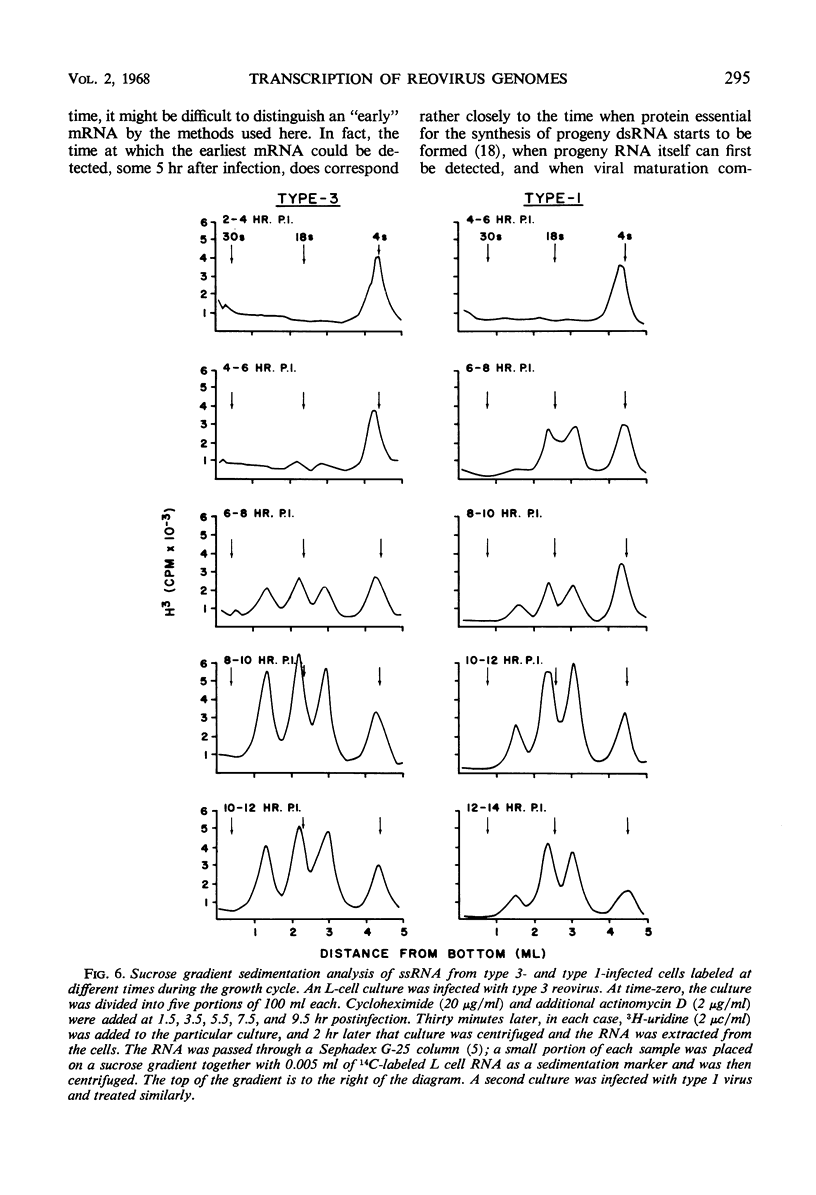
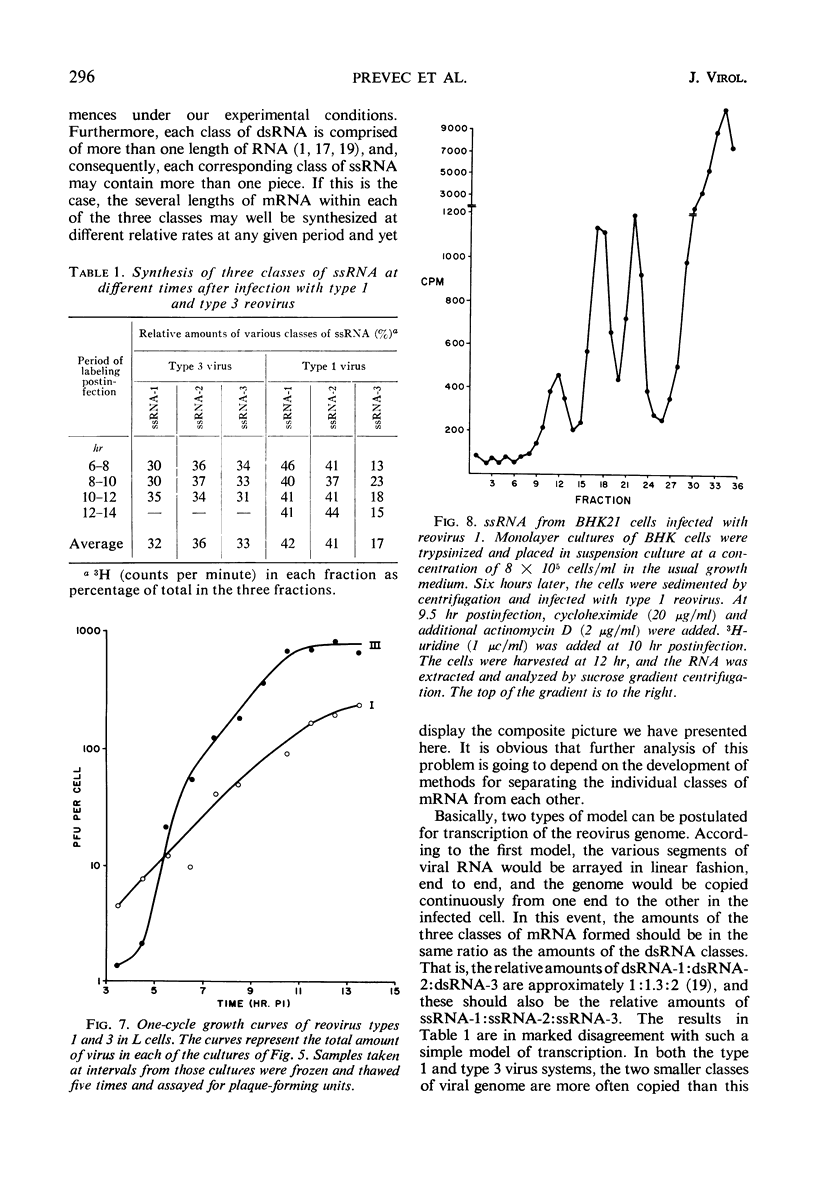
Abstract
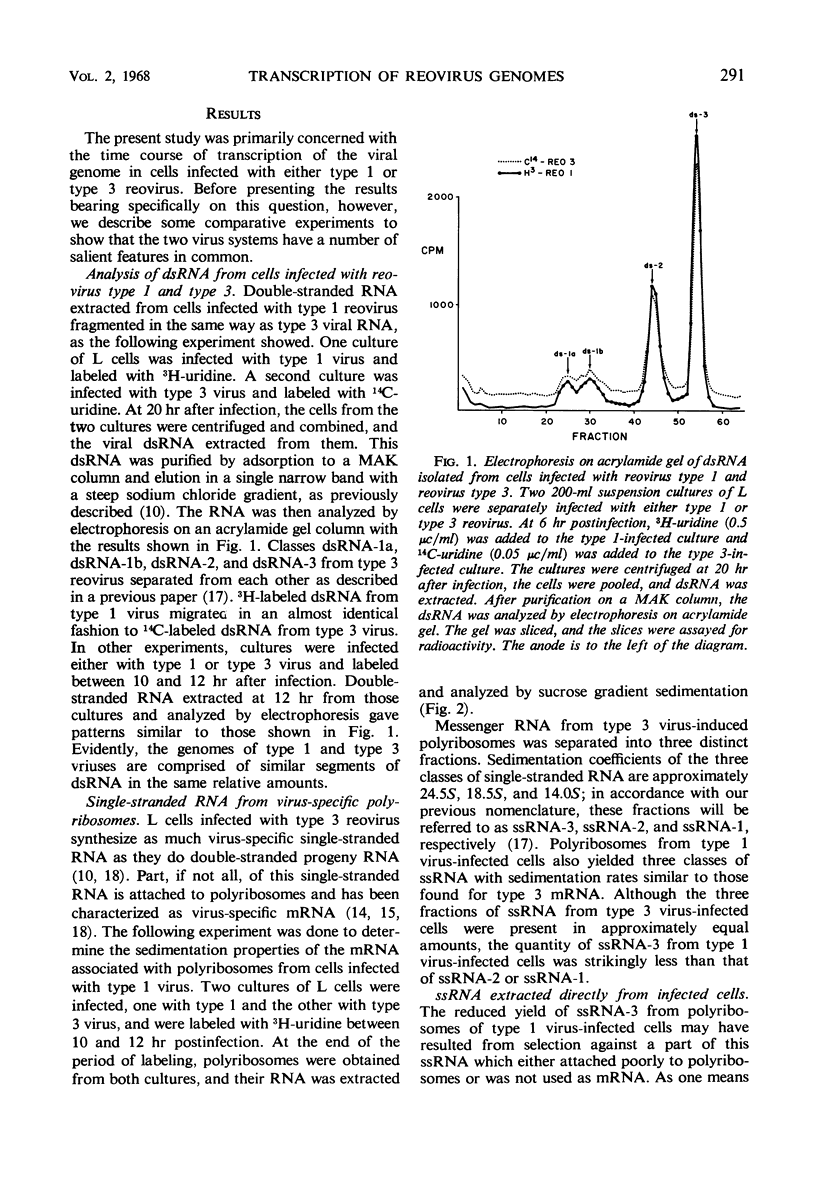
The double-stranded ribonucleic acid (RNA) genome of type 1 reovirus was fragmented into three size classes on extraction, as has already been shown for reovirus type 3. The relative amounts and molecular weights of the three classes were the same for the two viruses. Cells infected with type 1 virus synthesized three classes of messenger RNA. Each class of messenger RNA hybridized exclusively with a denatured double-stranded RNA fragment of equivalent length, as had also been found for type 3 reovirus. The double-stranded RNA segments thus act as specific units for transcription of messenger RNA in the infected cells. In cells infected with type 3 reovirus, the three classes of messenger RNA are made in equal amounts throughout the course of multiplication. In contrast, cells infected with type 1 virus produced only half as much of the largest messenger RNA as they did of the other two classes at all times during the replicative cycle. This finding suggests that transcription of the largest segments of type 1 viral genome is restricted.
Full text
PDF

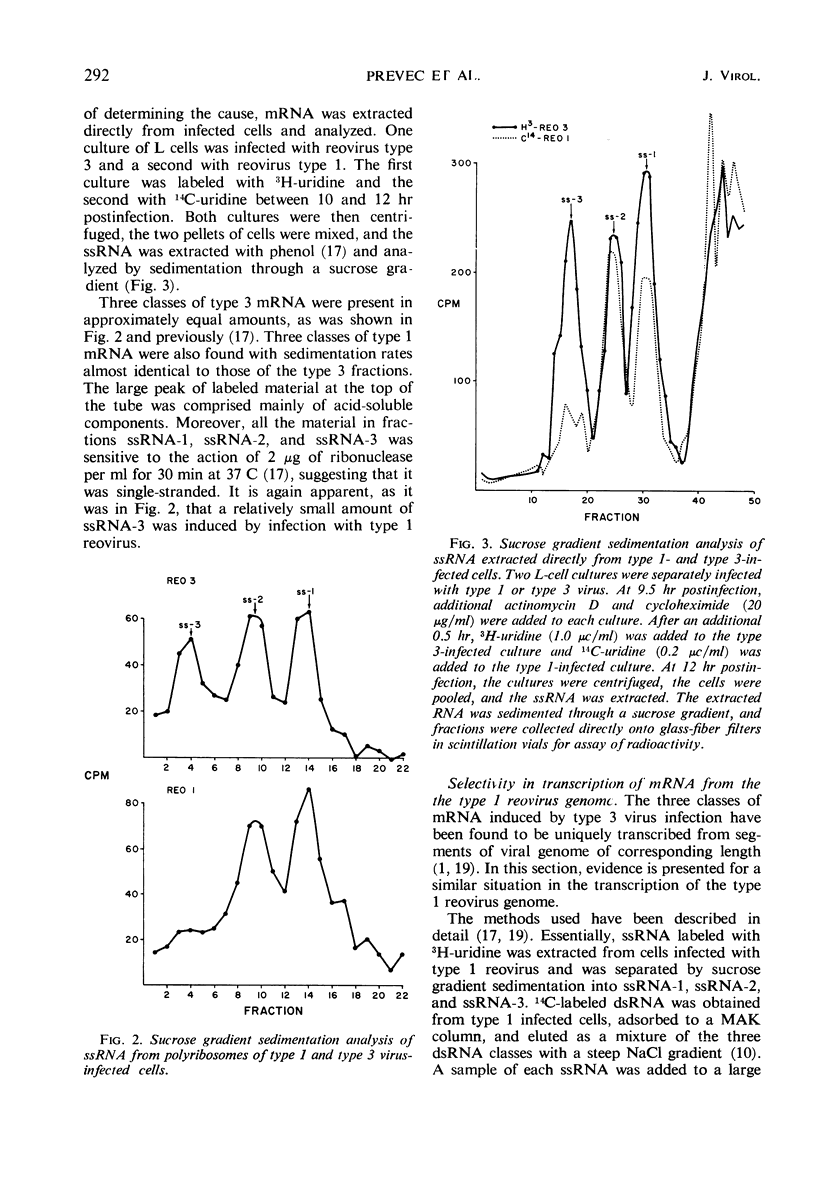

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy A. R., Joklik W. K. Studies on reovirus RNA. II. Characterization of reovirus messenger RNA and of the genome RNA segments from which it is transcribed. J Mol Biol. 1967 Oct 14;29(1):19–26. doi: 10.1016/0022-2836(67)90178-7. [DOI] [PubMed] [Google Scholar]
- Bellamy A. R., Shapiro L., August J. T., Joklik W. K. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. doi: 10.1016/0022-2836(67)90177-5. [DOI] [PubMed] [Google Scholar]
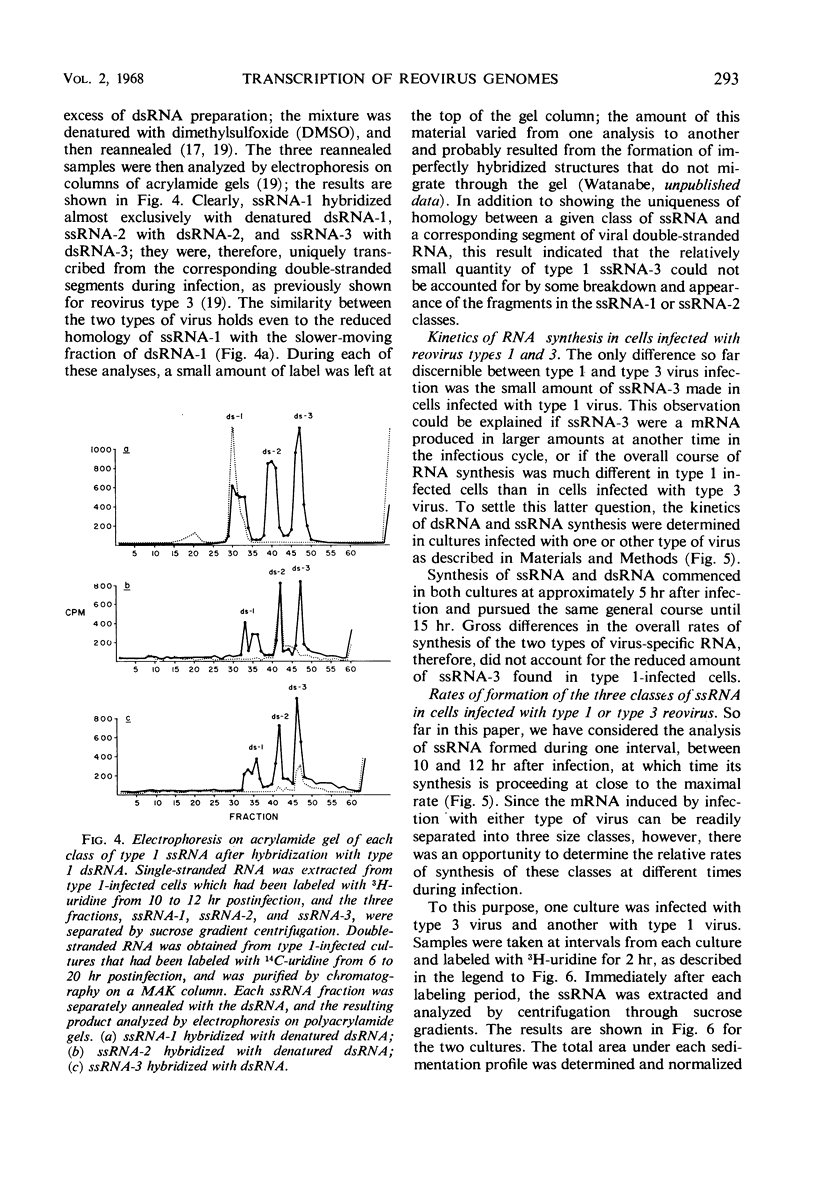
- Dunnebacke T. H., Kleinschmidt A. K. Ribonucleic acid from reovirus as seen in protein monolayers by electron microscopy. Z Naturforsch B. 1967 Feb;22(2):159–164. doi: 10.1515/znb-1967-0210. [DOI] [PubMed] [Google Scholar]
- EGGERS H. J., GOMATOS P. J., TAMM I. Agglutination of bovine erythrocytes: a general characteristic of reovirus type 3. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:879–881. doi: 10.3181/00379727-110-27679. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., STOECKENIUS W. ELECTRON MICROSCOPE STUDIES ON REOVIRUS RNA. Proc Natl Acad Sci U S A. 1964 Dec;52:1449–1455. doi: 10.1073/pnas.52.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Snyder L., Colvill A. J., Sarnat M. Selective synthesis of T-even bacteriophage early messenger in vitro. J Mol Biol. 1966 Aug;19(2):541–547. doi: 10.1016/s0022-2836(66)80021-9. [DOI] [PubMed] [Google Scholar]
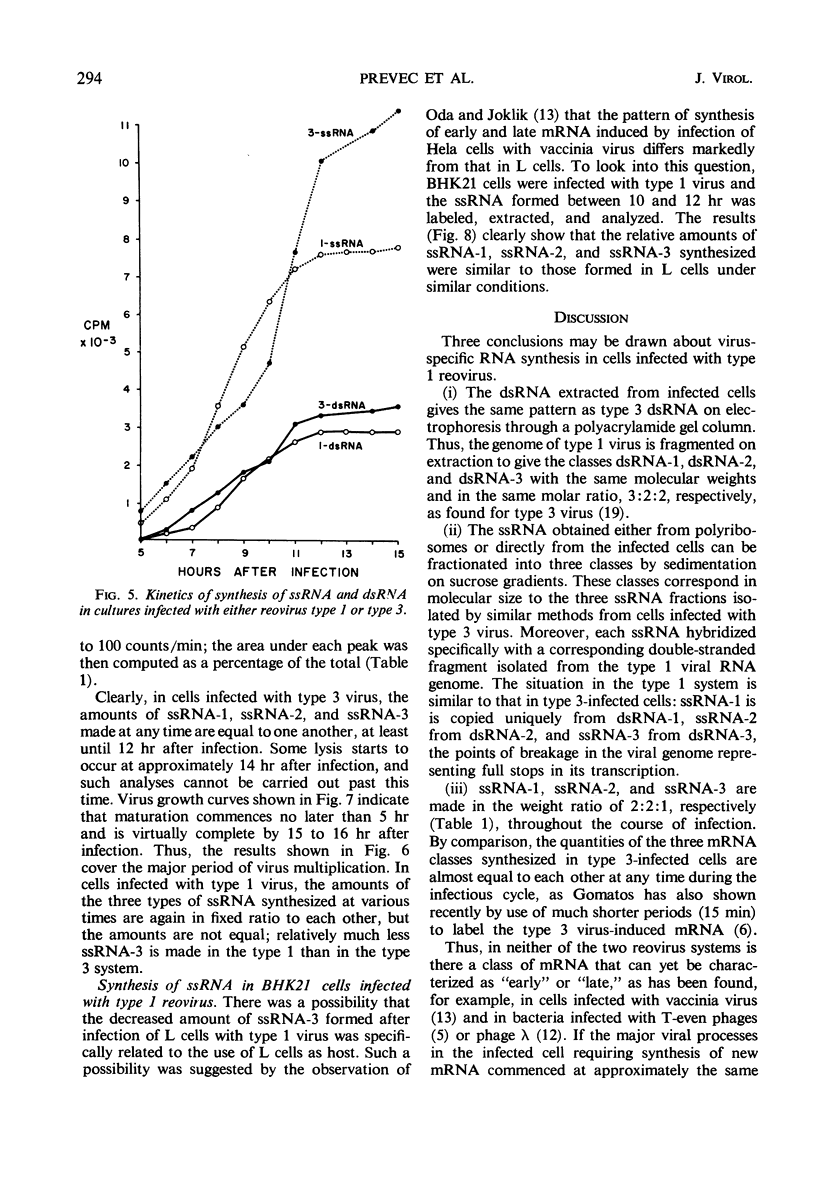
- Gomatos P. J. RNA synthesis in reovirus-infected L929 mouse fibroblasts. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1798–1805. doi: 10.1073/pnas.58.4.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., DUNNEBACKE T. H., SPENDLOVE R. S., SCHAFFER F. L., WHITCOMB R. F. ELECTRON MICROSCOPY OF RNA FROM REOVIRUS AND WOUND TUMOR VIRUS. J Mol Biol. 1964 Nov;10:282–288. doi: 10.1016/s0022-2836(64)80046-2. [DOI] [PubMed] [Google Scholar]
- Kudo H., Graham A. F. Synthesis of reovirus ribonucleic acid in L cells. J Bacteriol. 1965 Oct;90(4):936–945. doi: 10.1128/jb.90.4.936-945.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGRIDGE R., GOMATOS P. J. The structure of RNA. Reovirus RNA and transfer RNA have similar three-dimensional structures, which differ from DNA. Science. 1963 Aug 23;141(3582):694–698. doi: 10.1126/science.141.3582.694. [DOI] [PubMed] [Google Scholar]
- Naono S., Gros F. On the mechanism of transcription of the lambda genome during induction of lysogenic bacteria. J Mol Biol. 1967 May 14;25(3):517–536. doi: 10.1016/0022-2836(67)90203-3. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Prevec L., Graham A. F. Reovirus-specific polyribosomes in infected L-cells. Science. 1966 Oct 28;154(3748):522–523. [PubMed] [Google Scholar]
- Shatkin A. J., Rada B. Reovirus-directed ribonucleic acid synthesis in infected L cells. J Virol. 1967 Feb;1(1):24–35. doi: 10.1128/jvi.1.1.24-35.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P. The effect of cycloheximide on polyribosomes from hamster cells. Biochem Biophys Res Commun. 1966 Sep 8;24(5):758–764. doi: 10.1016/0006-291x(66)90390-1. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Graham A. F. Structural units of reovirus ribonucleic acid and their possible functional significance. J Virol. 1967 Aug;1(4):665–677. doi: 10.1128/jvi.1.4.665-677.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kudo H., Graham A. F. Selective inhibition of reovirus ribonucleic acid synthesis by cycloheximide. J Virol. 1967 Feb;1(1):36–44. doi: 10.1128/jvi.1.1.36-44.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Prevec L., Graham A. F. Specificity in transcription of the reovirus genome. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1040–1046. doi: 10.1073/pnas.58.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]



