Abstract
Purpose of Review
Adherence to proven, effective medications remains low, resulting in high rates of clinical complications, hospital readmissions and death. The use of technology to identify patients at risk and to target interventions for poor adherence has increased. This review focuses on research that tests these emerging technologies and evaluates the effect of technology-based adherence interventions on cardiovascular outcomes.
Recent Findings
Recent studies have evaluated technology-based interventions to improve medication adherence by using pharmaceutical databases, tailoring educational information to individual patient needs, delivering technology-driven reminders to patients and providers, and integrating in-person interventions with electronic alerts. Cellular phone reminders and in-home electronic technology used to communicate reminder messages have shown mixed results. Only one study has shown improvement in both adherence and clinical outcome. Current trials suggest that increasing automated reminders will compliment but not replace the benefits seen with in-person communication for medication-taking.
Summary
Integration of in-person contacts with technology-driven medication adherence reminders, electronic medication reconciliation and pharmaceutical databases may improve medication adherence and have a positive effect on cardiovascular clinical outcomes. Opportunities for providers to monitor the quality of care based on new adherence research are evolving and may be useful as standards for quality improvement emerge.
Introduction
Adherence is defined as the extent to which a person’s behavior corresponds to desirable healthcare goals jointly established with the healthcare provider [1, 2]. In cardiovascular disease, adherence to medications is low – over 50% of patients do not take medications as prescribed [3, 4]. The rate of poor adherence has remained stable over two decades [5]: 40% of patients fail to fill an original prescription [6, 7], and over 50% discontinue medications within a year [8-14].
The relationship between adherence to medications and clinical outcomes has been clearly demonstrated [15, 16, 17], particularly in cardiovascular disease. Serious complications, increased hospitalization, and death are associated with poor pill refill and no-fill rates for post-procedural antiplatelet drugs [18], as well as medications for chronic use in hypertension [19], hyperlipidemia [20] and heart failure [21]. Non-adherence to antiplatelet medication (clopidogrel) following intracoronary stent placement, resulted in higher rates of readmission, repeat procedures, and a three-fold greater likelihood of death [12, 22, 23]. Poor medication adherence following myocardial infarction was associated with higher rates of readmission and 30-day mortality [24], while better adherence to evidence-based medications for heart failure mediated event-free survival [25].
Despite the benefits of taking evidence-based medicines as prescribed, poor adherence is a major global public health challenge [1]. Research on its theoretical underpinnings [26], barriers and facilitators [27, 28], and devices and interventions to improve adherence [29-31] serve to illuminate the complexities of medication adherence and difficulties with achievement on a population level. Practical ways to improve adherence, particularly in chronic illness, eludes patients and healthcare providers. Effective interventions are labor intensive [32-34], cost prohibitive [35, 36], and ineffective long-term [13, 14, 30], among a majority of chronic illness patients needing life-long strategies to maintain medication-taking [37]. Most cardiovascular illnesses are accompanied by co-morbid conditions, requiring complex multiple-medication regimens [38, 39], increasing the likelihood of poor adherence [38, 40-42].
New studies are evaluating emerging technology-based approaches to improve medication adherence [43-48]. This paper synthesizes these studies and identifies key lessons learned for clinical practice settings and strategies for integrating quality performance measures for adherence.
Emerging Technologies To Improve Medication Adherence
Existing evidence-based adherence interventions are plagued by high resource intensity, lack of specificity regarding content and delivery, and impracticality for everyday clinical practice settings [33]. Recent trials addressed these issues by testing defined, technology-enhanced interventions with replicable, clearly described components aimed at generalizability and sustainability [49-52]. Despite advances in clarity, replicability, and study design, interventions to improve adherence have produced mixed results. Clinical trials of technology-based interventions can be broadly categorized into two groups: automated detection and reminder systems [42, 53, 54] and in-person systems with an electronic component [20, 52, 55-58] (Table 1). The following section describes differences in intervention components, adherence rates, and clinical outcomes among recent trials of adherence systems.
Table 1.
Summary of Randomized Clinical Trials and Implementation Research for Medication Adherence Interventions
| Author | Design | Patients | Intervention | Aims / Outcomes | Results |
|---|---|---|---|---|---|
| Bosworth, 2011 [55] |
Multicenter, nonrandomized pre-post implementation evaluation |
n=558, Medicaid patients prescribed at least one HTN drug |
Tailored, telephone intervention Delivered by care managers |
Medication adherence – as measured by medication possession ratio (MPR) |
Medication adherence (medication possession ratio) improved from 55% 9-12 months prior to the intervention to 77% 9-12 months after implementation. Sustainability (12 months) of the intervention demonstrated. |
| Chaudhry, 2010 [53] |
Multicenter, randomized controlled trial |
n=1653 patients with HF and recent HF hospitalization |
Randomized to: Group 1: telemonitoring (n=826) Group 2: usual care (n=827) |
Primary: All cause readmission or death within 180 days after enrollment Secondary: HF hospitalization; length of stay; hospitalization frequency |
No difference in primary endpoint No difference in secondary end points or any of its components. |
| Christensen, 2010 [54] |
Multicenter, randomized controlled trial with treatment - control group cross-over at 6 months |
n=398 patients on telmisartan once daily |
Randomized to: Group 1: electronic compliance monitoring with a reminder and monitoring device Group 2: standard therapy Groups crossed over after 6 months. |
Medication adherence – as measured by medication electronic monitoring device Blood pressure control. |
No difference in BP 6% improved medication adherence in intervention group at 6 months 2% improved medication adherence in intervention group at 12 months. |
| Eussen, 2010 [20] |
Multicenter, randomized controlled trial |
n=899 subjects on statin therapy Group 1: n=439 pharmaceutical care Group 2: n=460 usual care |
|
Medication adherence – as measured by drug discontinuation |
Lower discontinuation rate at 6 months in intervention versus usual care (HR 0.66, 95% CI 0.46 to 0.96). No difference between groups at 12 months (HR 0.84, 95% CI 0.65 to 1.10). Median MPR was very high (>99%) in both groups and did not differ between groups. |
| Gazmararian, 2010 [42] |
Multicenter, prospective, nonrandomized controlled trial |
N=275 Group 1: (n=173) intervention group Group 2: (n=102) control group Primarily indigent, minority population |
3-part intervention:
|
Medication adherence – pill refill as measured by cumulative medication gap (CMG) |
No difference in change in medication adherence before and after intervention between intervention and control groups (p = 0.4) |
| Pladevall, 2010 [52] |
Multi-center, clusterrandomized controlled trial |
(n=79) Physician practices (n=877) Patients |
Practices randomized to: Group 1: counted patients’ pills, designated a family member to support adherence behavior, and provided educational information Group 2: usual care |
Primary: blood pressure control at 6 months. Secondary: medication adherence and a composite end point of all-cause mortality and cardiovascularrelated hospitalizations |
Improved blood pressure (odds ratio 0.62, 95% confidence interval 0.50 to 0.78) at 6 months. Improved adherence (odds ratio 1.91, 95% confidence interval 1.19 to 3.05) at 6 months. After 5 years, 16% of the patients in the intervention group and 19% in the control group met the composite end point (hazard ratio 0.97, 95% confidence interval 0.67 to 1.39). |
| Powell, 2010 [56] |
Multiple-hospital, partially blinded behavioral efficacy randomized controlled trial |
n=902 HF patients | Randomized to: Group 1: Education
Management
|
Primary: death or HF hospitalization |
No difference in the education vs. self-management group (163 [40.1%)] vs 171 [41.2%], respectively; odds ratio, 0.95 [95% confidence interval, 0.72-1.26]). No differences on any secondary end points, including death, heart failure hospitalization, all-cause hospitalization, or quality of life. |
| Robinson, 2010 [57] |
Quasi- experimental study (matched intervention and control pharmacies) |
n=18 chain community pharmacies n=180 patients in pharmaceutical care n=196 patients in usual care |
Group 1: Pharmaceutical care groups: Educational training of pharmacists in hypertension therapies, monitoring, and management Group 2: Usual care groups; no pharmacist training |
Medication adherence |
Improved BP: average reduction in systolic BP was 9.9 mm Hg in PC patients compared with 2.8 mm Hg in UC patients (p < 0.05). Based on patient self-report, PC patients were more likely to say that they take their medicines as prescribed compared with UC patients (p < 0.05). Adherence rate was higher in PC patients (0.91 +/− 0.15) compared to UC patients (0.78 +/− 0.30) (p = 0.02) at 6 months No significant difference in adherence rate at 7- to 12-months |
| Tamblyn, 2010 [58] |
Multicenter, randomized controlled trial |
n=2293 primary care patients prescribed lipid-lowering or antihypertensive drugs |
Randomized to: Group 1: adherence tracking and alert system Group 2: active medication list alone |
Drug profile review, changes in cardiovascular drug treatment, and refill adherence in the first 6 months |
Significant increase in drug profile review in the intervention compared to the control group (44.5% v. 35.5%; P < 0.001), No significant increase in drug discontinuations due to side effects (2.3% v. 2.0%; P = 0.61); and a reduction in therapy increases (28.5% v. 29.1%; P = 0.86). There was no significant change in refill adherence after 6 months of follow-up. |
Pharmaceutical database technologies and automated alerts
Pharmaceutical database technology identifies patient-level adherence patterns for filling newly prescribed medications and refills, providing a community-based point of contact with patients beyond the time constraints of hospital or clinic visits. Pharmacists are accessible and convenient for most patients and family caregivers, are knowledgeable information sources, and are able to identify and discuss potential contraindications or concerns regarding active medications. Pharmacy-generated data allows identification and analysis of the proportion of days that patients have access to medication, as either a medication possession ratio (MPR) or cumulative medication gaps (CMG) metric [59]. Besides these metrics, electronic data systems can be programmed to generate phone call reminders to patients regarding the need for pill refill.
A study of hypertensive patients (n=398) using automated reminders in conjunction with electronic monitoring devices showed no significant benefit in either medication adherence or blood pressure control compared to standard therapy controls (Christensen and colleagues [54]). Similarly, in a study by Gazmararian and colleagues [42] using automated reminders alone, patients did not interact consistently with the available technology [42]. In this primarily indigent, inner city population (N=275) using automated pharmaceutical database triggers, telephone reminder calls, and picture cards to address low health literacy, researchers found no significant change in refill adherence in the intervention or control groups. Adherence rates for medication dosing and timing in the intervention versus control groups were 45 and 52%, respectively, in the first 6 months, and 32 and 38% in the second 6 months after randomized groups were crossed-over.
Electronic pharmaceutical data systems have also been studied regarding physician use. Tamblyn and colleagues [58] showed these systems can be programmed to alert physicians to the need for medication reconciliation and are effective in improving the frequency of medication reconciliation by providers. In their study (n=2293 patients), a significant increase in drug profile review occurred in the intervention group compared to controls (44.5% versus 35.5%, respectively), but without significant medication changes (therapeutic optimization of medications) by physicians or improved rates of refill adherence by patients.
Although electronic reminders on pagers, cell phones and text-messages have been studied, randomized clinical trials conducted in 2010 to test interventions based on these technologies suggest that electronic automated triggers alone are ineffective for improving pill refill adherence. Nor do they produce meaningful change in physician actions related to medication reconciliation.
Telemonitoring systems for medication-related self-management
In contrast to pharmacy-generated reminder systems, in-home telemonitoring systems allow patients to generate and respond to their own data. Patients or caregivers record and report symptoms, such as shortness of breath or weight gain, and associated medication dosing, which can be reviewed by the provider for evaluation of trends. Home telemonitoring systems, pioneered by Cleland and colleagues [60], automatically monitor blood pressure, blood glucose, and daily weight changes, among others. In addition, telemonitoring systems offer opportunities for medication-related education, reinforcement, and opportunities for review and reconciliation. Uses of a bi-directional communication strategy supports patient-provider communication and accessibility..
The risk is user error. Patients must choose (and be adequately skilled) to ‘connect’ to the electronic system, and providers must choose (and be adequately available) to respond. New systems obviate the need for conscious provider response by automatically ‘sending’ the information to a central server and generating an alert for values outside of normal range, which is then automatically sent to the provider’s cell phone or computer screen.
In recent randomized trials of new-generation telemonitoring systems, results have been disappointing. In the most recent trial [53], heart failure patients (n=1653) were randomized to telemonitoring (n=826) versus usual care (n=827). Telemonitored patients received symptom education and assessment and reported medications and daily weights from home. Alert values and patient concerns were transmitted electronically to the central server for nurse follow-up within 2-3 days. At the conclusion of the study there was no significant difference between the intervention and control groups in all-cause or heart failure-related hospital readmissions or death. Patients also reported no difference in symptom recognition or medication use. Thus, telemonitoring alone appears to be ineffective in improving outcomes related to medication use and clinical outcomes of self-management in patients with heart failure [61-63].
Combination In-Person and Electronic Technology Interventions
Combinations of in-person with automated reminders or triggers has produced the most effective results for improving medication adherence and clinical outcomes, as well as patient and caregiver satisfaction with information, accuracy of the active medication list, and improvements in patient-provider partnership or person-centeredness of care. The most successful large-scale adherence intervention, the COM99 study, randomized physician practices (n=79) to a 3-part in-person intervention for patients with uncontrolled hypertension [52]. Providers conducted pill counts, designated a family member to support medication adherence behaviors at home, and provided educational information to patients and families. Patient participants (n=877) were more adherent (O.R. 1.91, 95% confidence interval 1.19 to 3.05) and had better controlled blood pressure (O.R. 0.62, 95% confidence interval 0.50-0.78) compared to the control group at 6 months.
Similarly, in a study by Robinson and colleagues [57], personal communication with patients by community-based pharmacists about blood pressure monitoring, hypertensive medication management, and adherence behaviors significantly improved adherence (0.91% vs. 0.78%, p=0.02) and reduced systolic blood pressure (by 9.9mmHg in intervention group versus 2.8 mmHg in controls, p<.05) at 6 months; 12 month outcomes showed no significant difference, demonstrating lack of sustainability of the effects. Eussen and colleagues [20] also showed that in-person communications by community pharmacists (n= 26 pharmacies randomized; n=899 intervention subjects, n=460 control) effectively improved medication adherence to statin therapy at 6 months but was not sustained to 12 months.
By contrast, a multidisciplinary implementation study by Bosworth and colleagues among Medicaid patients (N=588) with uncontrolled hypertension and poor adherence (55% baseline MPR) showed improved medication adherence for 12 months [55]. Using a pre-post observational cohort design, the study evaluated MPR 12 months prior to implementing an in-person telephone intervention by care managers to a stable cohort of patients prescribed at least one anti-hypertensive agent. Following implementation, the MPR was 77% and was sustained at 12-months post-implementation.
Resource Intensity and impracticality of administering interventions in a single clinical setting
The interventions described above are labor intensive, time-consuming, and expensive. Follow-up phone calls, frequent patient contact, and unlimited provider access are financially burdensome for the healthcare system. Although investigators have demonstrated the cost-effectiveness of such interventions [64, 65], many community hospitals and physician practice groups find the cost prohibitive [66, 67].
Interventions are also difficult to sustain for patients with chronic illness. Many interventions have failed to show long-term effectiveness, in part because of unproven community-based follow-up and reinforcement in a sustainable, system-wide delivery format [68]. Self-care interventions for late-stage chronic illness are particularly problematic in terms of standardization since patients who do not feel well are increasingly and unavoidably dependent on others to facilitate care, including medication-taking [37].
Moving Toward Adherence-Dependent Quality and Performance Measures
Consistent with current emphases on quality, access, and equity of care [69], the cardiovascular literature emphasizes performance measures in which medication adherence contributes to quality score cards, affecting not only mortality but also more intermediate treatment targets: blood pressure and lipid control and decreased symptom-associated readmissions [70-74]. The link between medication adherence and treatment targets is robust;, although practical approaches that help patients improve medication adherence are lacking. For this reason, the World Health Organization has distinguished between modifiable and non-modifiable risk determinants [2]. In a clinical setting, nonmodifiable factors might serve as ‘flags’ to alert providers to communicate especially carefully with patients, whereas modifiable factors might serve to trigger specific patient-problem interventions.
Adherence to medications is increasingly recognized as requiring shared goal setting between patients and providers [75]. Tailored, telephone-scripted nursing interventions have shown success in improving medication adherence, alleviating the time commitment of the primary care provider and lowering system and provider costs [50, 76]. However, these programs have not been tested in real-world settings and are only now being implemented in large, prospective, population-based cohorts [55].
Implications for Provider-Based Performance Measures
Quality of care is evaluated using performance measures established by the Centers for Medicare and Medicaid Services (CMS)[72], derived from evidence-based practice guidelines, such as the American Heart Association and American College of Cardiology guidelines for care [77], and supported by national healthcare policy and quality groups such as National Quality Forum [78]. Performance measures include providing optimal evidence-based treatments at the correct dose and time and providing adequate information to patients at discharge to the home setting. Patient and provider indicators of medication adherence are tied to these performance measures and are publicly reported at patient, provider, and healthcare system levels [79-81].
Provider compliance with clinical guidelines has differed by treatment. For example, compliance with ACE-I in heart failure was 80-90%, but beta-blocker use was far less optimal, ranging from 27% in usual care groups to 55%-67% in intervention groups [82, 83]. Provider performance in achieving evidence-based guideline targets has improved [84], and interventions that improve provider compliance improve clinical patient outcomes [85], including decreased hospital readmission rates and hospitalized days.
New recommendations for improvement of provider compliance with guidelines include use of checklists and information technology upgrades to enable electronic documentation systems to provide guideline-based prompts [86, 87]. A similar approach may help providers in ambulatory and community clinic settings, and may also improve ‘self-care’ for patients and family caregivers in the home.
Implications for Patient-Centered Performance Measures
Treatment targets for selected cardiovascular patients include blood pressure, blood glucose levels (HgA1C), smoking status, and daily weight fluctuations. Providers’ ability to influence these targets is based on patients’ ability to adhere to evidence-based, prescribed medications. Thus, provider interventions are under increasing scrutiny to include adherence interventions, and performance scores are increasingly dependent on physicians’ ability to affect medication-taking, even for patients with long-term chronic illness.
One approach to integrating adherence interventions into standard patient care is to assess medication-taking behaviors during each initial patient assessment [70]. A short series of questions can help to assess patients’ medication-taking behaviors and plan effective communication and intervention [88].
New multidisciplinary and home-based interventions to improve CMS measures of patient outcomes, cost, and efficiency of care include early recognition of poor adherence, decreased rates of complications, and prevention of hospital readmission [34, 50]. Strategies to facilitate adherence have included using reminders, reinforcements, rewards, and feedback on patient progress; providing education; reinforcing cognitive cues through use of question/answer sessions; improving process variables, such as waiting time, transportation to center or clinic, and the convenience of parking; improving system variables, such as carefully evaluating the frequency of scheduled follow-up; and improving the availability of and access to healthcare providers. Although these interventions have improved monitoring of early signs and symptoms and decreased hospitalizations and mortality, mortality remains an impractical performance measure for most cardiovascular diseases, particularly at the local clinical level. While early heart failure management and home-based nursing intervention programs showed improved mortality [89-91], in recent trials, their effect on mortality was not significant [92, 93].
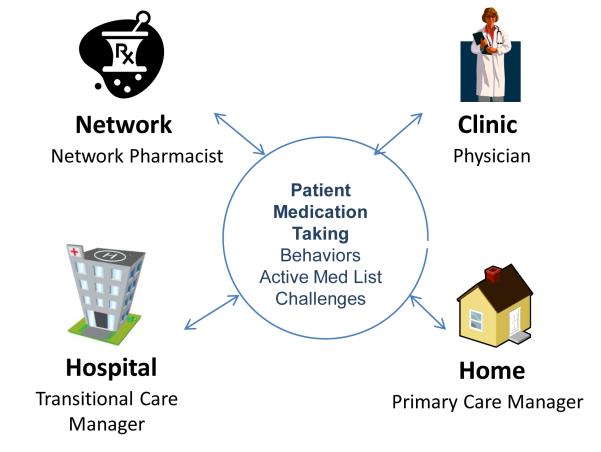
More practical measures focus on Institute of Medicine quality indicators other than mortality, such as patient satisfaction with information given and the safety, quality and efficiency of care transitions from one setting to another [68, 94, 95]. Transitions between settings (Figure 1) offer opportunities for improving medication adherence when health systems integrate dedicated personnel and time for medication reconciliation, communication of prescription changes, tele-home monitoring and in-person follow-up calls. Such strategies implemented over time and across settings and episodes of care help patients meet treatment targets more effectively [34, 94, 96, 97]. They go beyond single-setting applications or automated telephone reminders to intervene at each episode of chronic care, using technology to maintain continuous communication between providers, monitor and track prescriptions and refills, decrease early discontinuation of beneficial medications, and improve adherence. These new interventions also include pharmaceutical cost-sharing programs and incentive-based insurance programs.
Figure 1.
Example of Pharm Assist intervention: a multiple-step pathway representing key components of behavior reinforcement for medication adherence. Modified from the Community Care of North Carolina Pharmacy Home Project. Troy Trygstad, PharmD, PhD
Conclusion
Although many variables are associated with adherence, few are consistently strong predictors, and a predictive profile of poor adherence has not been established. Nevertheless, recent findings regarding adherence to cardiovascular medications show a consistent relationship between medication adherence and clinical outcomes, and therefore have implications for quality-based performance measures that target clinical outcomes. Starting with a simple checklist and an assessment of adherence on first clinical contact, and integrating technology-based reminders with in-person communication may help both patients and providers to get started [86].
Key points.
Poor adherence to medications is associated with higher rates of clinical complications, re-hospitalizations and death in cardiovascular disease.
New interventions to improve adherence use emerging technology, such as automated electronic alerts and reminders for refill of prescriptions.
Passive delivery or use of technology-based interventions alone, without an active, in-person component, are not effective in improving adherence rates or patient outcomes.
Feasible clinical strategies to improve adherence include routine assessment of patient adherence and use of in-person interventions to reinforce and support technology-based triggers.
Reference List
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Sabate E. World Health Organization Report: Adherence to Long-Term Therapies - Evidence for Action. World Health Organziation; Switzerland: 2003. [Google Scholar]
- 2.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. ValueHealth. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 3.Monane M, Bohn RL, Gurwitz JH, et al. Noncompliance with congestive heart failure therapy in the elderly. ArchInternMed. 1994;154(4):433–437. [PubMed] [Google Scholar]
- 4.Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. ArchInternMed. 1990;150(7):1509–1510. [PubMed] [Google Scholar]
- 5.Hodges P. Heart failure: epidemiologic update. Crit Care NursQ. 2009;32(1):24–32. doi: 10.1097/01.CNQ.0000343131.27318.36. [DOI] [PubMed] [Google Scholar]
- 6.Murray MD, Tu W, Wu J, et al. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. ClinPharmacolTher. 2009;85(6):651–658. doi: 10.1038/clpt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiner JF, Ho PM, Beaty BL, et al. Sociodemographic and clinical characteristics are not clinically useful predictors of refill adherence in patients with hypertension. CircCardiovascQualOutcomes. 2009;2(5):451–457. doi: 10.1161/CIRCOUTCOMES.108.841635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanelli M, Pedan A, Liu N, et al. The role of patient inexperience in medication discontinuation: a retrospective analysis of medication nonpersistence in seven chronic illnesses. ClinTher. 2009;31(11):2628–2652. doi: 10.1016/j.clinthera.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Ali RC, Melloni C, Ou FS, et al. Age and persistent use of cardiovascular medication after acute coronary syndrome: results from medication applied and sustained over time. JAmGeriatrSoc. 2009;57(11):1990–1996. doi: 10.1111/j.1532-5415.2009.02483.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah ND, Dunlay SM, Ting HH, et al. Long-term medication adherence after myocardial infarction: experience of a community. AmJMed. 2009;122(10):961–913. doi: 10.1016/j.amjmed.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinacty CM, Adams AS, Soumerai SB, et al. Racial differences in long-term adherence to oral antidiabetic drug therapy: a longitudinal cohort study. BMCHealth ServRes. 2009;9:24. doi: 10.1186/1472-6963-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moussa ID, Colombo A. Antiplatelet therapy discontinuation following drug-eluting stent placement: dangers, reasons, and management recommendations. CatheterCardiovascInterv. 2009;74(7):1047–1054. doi: 10.1002/ccd.22167. [DOI] [PubMed] [Google Scholar]
- 13.Melloni C, Alexander KP, Ou FS, et al. Predictors of early discontinuation of evidence-based medicine after acute coronary syndrome. AmJCardiol. 2009;104(2):175–181. doi: 10.1016/j.amjcard.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113(2):203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 15.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366(9502):2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 16.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. ArchInternMed. 2006;166(17):1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 17.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. ArchInternMed. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 18.Burzotta F, Trani C, Todaro D, et al. Outcome of patients treated by a novel thin-strut cobalt-chromium stent in the drug-eluting stent era: Results of the SKICE (Skylor in real world practice) registry. Catheter Cardiovasc Interv. 2009;73(4):457–465. doi: 10.1002/ccd.21882. [DOI] [PubMed] [Google Scholar]
- 19.Bailey JE, Wan JY, Tang J, et al. Antihypertensive medication adherence, ambulatory visits, and risk of stroke and death. J Gen Intern Med. 2010;25(6):495–503. doi: 10.1007/s11606-009-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eussen SR, van der Elst ME, Klungel OH, et al. A pharmaceutical care program to improve adherence to statin therapy: a randomized controlled trial. Ann Pharmacother. 2010;44(12):1905–1913. doi: 10.1345/aph.1P281. [DOI] [PubMed] [Google Scholar]
- 21.Lamb DA, Eurich DT, McAlister FA, et al. Changes in adherence to evidence-based medications in the first year after initial hospitalization for heart failure: observational cohort study from 1994 to 2003. Circ Cardiovasc Qual Outcomes. 2009;2(3):228–235. doi: 10.1161/CIRCOUTCOMES.108.813600. [DOI] [PubMed] [Google Scholar]
- 22.Steinwender C, Hartenthaler B, Lambert T, et al. Incidence of stent thrombosis in patients with drug eluting stents and short-term dual antiplatelet therapy. EuroIntervention. 2009;4(5):593–599. doi: 10.4244/eijv4i5a100. [DOI] [PubMed] [Google Scholar]
- 23.Collet JP, Montalescot G, Steg PG, et al. Clinical outcomes according to permanent discontinuation of clopidogrel or placebo in the CHARISMA trial. ArchCardiovascDis. 2009;102(6-7):485–496. doi: 10.1016/j.acvd.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Tuppin P, Neumann A, Danchin N, et al. Evidence-based pharmacotherapy after myocardial infarction in France: adherence-associated factors and relationship with 30-month mortality and rehospitalization. Arch Cardiovasc Dis. 2010;103(6-7):363–375. doi: 10.1016/j.acvd.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Wu JR, Lennie TA, De Jong MJ, et al. Medication adherence is a mediator of the relationship between ethnicity and event-free survival in patients with heart failure. JCard Fail. 2010;16(2):142–149. doi: 10.1016/j.cardfail.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosworth HB, editor. Improving Patient Treatment Adherence: A Clinician Guidebook. Springer; New York: 2010. [Google Scholar]
- 27.Lehane E, McCarthy G. Medication non-adherence--exploring the conceptual mire. IntJNursPract. 2009;15(1):25–31. doi: 10.1111/j.1440-172X.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 28.Ngoh LN. Health literacy: a barrier to pharmacist-patient communication and medication adherence. JAmPharmAssoc(2003) 2009;49(5):e132–e146. doi: 10.1331/JAPhA.2009.07075. [DOI] [PubMed] [Google Scholar]
- 29.Christensen A, Osterberg LG, Hansen EH. Electronic monitoring of patient adherence to oral antihypertensive medical treatment: a systematic review. JHypertens. 2009;27(8):1540–1551. doi: 10.1097/HJH.0b013e32832d50ef. [DOI] [PubMed] [Google Scholar]
- 30.De Bleser L, Matteson M, Dobbels F, et al. Interventions to improve medication-adherence after transplantation: a systematic review. TransplInt. 2009;22(8):780–797. doi: 10.1111/j.1432-2277.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 31.Julius RJ, Novitsky MA, Jr., Dubin WR. Medication adherence: a review of the literature and implications for clinical practice. JPsychiatrPract. 2009;15(1):34–44. doi: 10.1097/01.pra.0000344917.43780.77. [DOI] [PubMed] [Google Scholar]
- 32.Fried TR, Tinetti ME, Iannone L. Primary care clinicians’ experiences with treatment decision making for older persons with multiple conditions. Arch Intern Med. 2011;171(1):75–80. doi: 10.1001/archinternmed.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. CochraneDatabaseSystRev. 2008;(2) doi: 10.1002/14651858.CD000011.pub3. CD000011. [DOI] [PubMed] [Google Scholar]
- 34.Cooper LA, Roter DL, Bone LR, et al. A randomized controlled trial of interventions to enhance patient-physician partnership, patient adherence and high blood pressure control among ethnic minorities and poor persons: study protocol NCT00123045. ImplementSci. 2009;4:7. doi: 10.1186/1748-5908-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapman RH, Kowal SL, Cherry SB, et al. The modeled lifetime cost-effectiveness of published adherence-improving interventions for antihypertensive and lipid-lowering medications. Value Health. 2010;13(6):685–694. doi: 10.1111/j.1524-4733.2010.00774.x. [DOI] [PubMed] [Google Scholar]
- 36.Esposito D, Bagchi AD, Verdier JM, et al. Medicaid beneficiaries with congestive heart failure: association of medication adherence with healthcare use and costs. AmJManagCare. 2009;15(7):437–445. [PubMed] [Google Scholar]
- 37.Granger BB, Sandelowski M, Tahshjain H, et al. A qualitative descriptive study of the work of adherence to a chronic heart failure regimen: patient and physician perspectives. JCardiovascNurs. 2009 doi: 10.1097/JCN.0b013e3181a4be30. [DOI] [PubMed] [Google Scholar]
- 38.Dunbar-Jacob J, Bohachick P, Mortimer MK, et al. Medication adherence in persons with cardiovascular disease. JCardiovascNurs. 2003;18(3):209–218. doi: 10.1097/00005082-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Bohachick P, Burke LE, Sereika S, et al. Adherence to angiotensin-converting enzyme inhibitor therapy for heart failure. ProgCardiovascNurs. 2002;17(4):160–166. doi: 10.1111/j.0889-7204.2002.01643.x. [DOI] [PubMed] [Google Scholar]
- 40.Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure. BMJ. 2003;327(7414):513–514. doi: 10.1136/bmj.327.7414.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson SH, Johnson JA, Farris KB, Tsuyuki RT. Development and validation of a survey to assess barriers to drug use in patients with chronic heart failure. Pharmacotherapy. 2002;22(9):1163–1172. doi: 10.1592/phco.22.13.1163.33512. [DOI] [PubMed] [Google Scholar]
- 42.Gazmararian J, Jacobson KL, Pan Y, et al. Effect of a pharmacy-based health literacy intervention and patient characteristics on medication refill adherence in an urban health system. AnnPharmacother. 2010;44(1):80–87. doi: 10.1345/aph.1M328. [DOI] [PubMed] [Google Scholar]
- 43.Shah BR, Adams M, Peterson ED, et al. Secondary Prevention Risk Interventions Via Telemedicine and Tailored Patient Education (SPRITE): A Randomized Trial to Improve Postmyocardial Infarction Management. Circ Cardiovasc Qual Outcomes. 2011;4(2):235–242. doi: 10.1161/CIRCOUTCOMES.110.951160. [DOI] [PubMed] [Google Scholar]
- 44.Vervloet M, van Dijk L, Santen-Reestman J, et al. Improving medication adherence in diabetes type 2 patients through Real Time Medication Monitoring: a randomised controlled trial to evaluate the effect of monitoring patients’ medication use combined with short message service (SMS) reminders. BMC Health Serv Res. 2011;11:5. doi: 10.1186/1472-6963-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du HY, Newton PJ, Zecchin R, et al. An intervention to promote physical activity and self-management in people with stable chronic heart failure. The Home-Heart -Walk study: study protocol for a randomized controlled trial. Trials. 2011;12(1):63. doi: 10.1186/1745-6215-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koehler F, Winkler S, Schieber M, et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: study design. Eur J Heart Fail. 2010;12(12):1354–1362. doi: 10.1093/eurjhf/hfq199. [DOI] [PubMed] [Google Scholar]
- 47.Feldman PH, McDonald MV, Mongoven JM, et al. Home-based blood pressure interventions for blacks. CircCardiovascQualOutcomes. 2009;2(3):241–248. doi: 10.1161/CIRCOUTCOMES.109.849943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svarstad BL, Kotchen JM, Shireman TI, et al. The Team Education and Adherence Monitoring (TEAM) trial: pharmacy interventions to improve hypertension control in blacks. CircCardiovascQualOutcomes. 2009;2(3):264–271. doi: 10.1161/CIRCOUTCOMES.109.849992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. ArchInternMed. 2009;169(21):1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. AmHeart J. 2009;157(3):450–456. doi: 10.1016/j.ahj.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Thorpe CT, Bryson CL, Maciejewski ML, Bosworth HB. Medication acquisition and self-reported adherence in veterans with hypertension. MedCare. 2009;47(4):474–481. doi: 10.1097/mlr.0b013e31818e7d4d. [DOI] [PubMed] [Google Scholar]
- 52.Pladevall M, Brotons C, Gabriel R, et al. Multicenter cluster-randomized trial of a multifactorial intervention to improve antihypertensive medication adherence and blood pressure control among patients at high cardiovascular risk (the COM99 study) Circulation. 2010;122(12):1183–1191. doi: 10.1161/CIRCULATIONAHA.109.892778. **This cluster-randomized trial of physician practices showed that a simple, in-person intervention and use of family member support improved both medication adherence and blood pressure at 6 months.
- 53.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. ** This randomized clinical trial of home-based telemonitoring of patients with heart failure showed patient engagement with the device to be poor. Though automated reminders were sent after 2 days of non-use, 45% of patients were not using the device at 6 months. Expecting patients to initiate and engage with automated, technology-based monitoring was not effective in improving medication adherence or adherence-related clinical outcomes.
- 54.Christensen A, Christrup LL, Fabricius PE, et al. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. J Hypertens. 2010;28(1):194–200. doi: 10.1097/HJH.0b013e328331b718. [DOI] [PubMed] [Google Scholar]
- 55.Bosworth HBD CA, Ruppenkamp J, Trygstad, et al. Evaluation of a self-management implementation intervention to improve hypertension control among patients in Medicaid. Translational Behavioral Medicine: Practice, Policy, Research. 2011;1(1):191–199. doi: 10.1007/s13142-010-0007-x. *This was the first population-based implementation evaluation of an enhanced medical home model with a community pharmacist intervention to improve medication adherence to anti-hypertensive medications. The notable feature of this study is 12 month sustainability of a 25% improvement in medication possession ratio and refill adherence among this asymptomatic population of patients.
- 56.Powell LH, Calvin JE, Jr., Richardson D, et al. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304(12):1331–1338. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson JD, Segal R, Lopez LM, Doty RE. Impact of a pharmaceutical care intervention on blood pressure control in a chain pharmacy practice. AnnPharmacother. 2010;44(1):88–96. doi: 10.1345/aph.1L289. [DOI] [PubMed] [Google Scholar]
- 58.Tamblyn R, Reidel K, Huang A, et al. Increasing the detection and response to adherence problems with cardiovascular medication in primary care through computerized drug management systems: a randomized controlled trial. Med Decis Making. 2010;30(2):176–188. doi: 10.1177/0272989X09342752. * This was the first study to test an automated alert intervention linked to an electronic medical record to trigger medication reconciliation by primary care physicians. Though drug profile review increased in patients with uncontrolled hypertension, the automated reminder did not translate into an increase in therapeutic dosing changes or improved adherence to prescription refill.
- 59.Cramer JA, Benedict A, Muszbek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. IntJ ClinPract. 2008;62(1):76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cleland JG, Louis AA, Rigby AS, et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45(10):1654–1664. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 61.Inglis SC, Clark RA, McAlister FA, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev. 2010;(8) doi: 10.1002/14651858.CD007228.pub2. CD007228. [DOI] [PubMed] [Google Scholar]
- 62.Inglis SC, Clark RA, Cleland JG. Telemonitoring in patients with heart failure. N Engl J Med. 2011;364(11):1078–1079. doi: 10.1056/NEJMc1100395. author reply 1079-1080. [DOI] [PubMed] [Google Scholar]
- 63.Clark RA, Inglis SC, McAlister FA, et al. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334(7600):942. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parry C, Mahoney E, Chalmers SA, Coleman EA. Assessing the quality of transitional care: further applications of the care transitions measure. MedCare. 2008;46(3):317–322. doi: 10.1097/MLR.0b013e3181589bdc. [DOI] [PubMed] [Google Scholar]
- 65.Naylor MD. Transitional care for older adults: a cost-effective model. LDIIssueBrief. 2004;9(6):1–4. [PubMed] [Google Scholar]
- 66.Balu S, Simko RJ, Quimbo RM, Cziraky MJ. Impact of fixed-dose and multi-pill combination dyslipidemia therapies on medication adherence and the economic burden of sub-optimal adherence. CurrMedResOpin. 2009;25(11):2765–2775. doi: 10.1185/03007990903297741. [DOI] [PubMed] [Google Scholar]
- 67.Peterson ED, Albert NM, Amin A, et al. Implementing critical pathways and a multidisciplinary team approach to cardiovascular disease management. AmJCardiol. 2008;102(5A):47G–56G. doi: 10.1016/j.amjcard.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med. 2010;363(24):2364–2367. doi: 10.1056/NEJMe1011769. [DOI] [PubMed] [Google Scholar]
- 69.Committee on Quality of Health Care in America IoM . Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; Washington, DC: 2001. [Google Scholar]
- 70.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 71.Fonarow GC, Peterson ED. Heart failure performance measures and outcomes: real or illusory gains. JAMA. 2009;302(7):792–794. doi: 10.1001/jama.2009.1180. [DOI] [PubMed] [Google Scholar]
- 72.Patterson ME, Hernandez AF, Hammill BG, et al. Process of care performance measures and long-term outcomes in patients hospitalized with heart failure. MedCare. 2010;48(3):210–216. doi: 10.1097/MLR.0b013e3181ca3eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peterson ED, Roe MT, Rumsfeld JS, et al. A call to ACTION (acute coronary treatment and intervention outcomes network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. CircCardiovascQualOutcomes. 2009;2(5):491–499. doi: 10.1161/CIRCOUTCOMES.108.847145. [DOI] [PubMed] [Google Scholar]
- 74.Pillittere-Dugan D, Nau DP, McDonough K, Pierre Z. Development and testing of performance measures for pharmacy services. JAmPharmAssoc(2003) 2009;49(2):212–219. doi: 10.1331/JAPhA.2009.09012. [DOI] [PubMed] [Google Scholar]
- 75.Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev. 2010;(3) doi: 10.1002/14651858.CD004371.pub3. CD004371. [DOI] [PubMed] [Google Scholar]
- 76.Bosworth HB, Olsen MK, Oddone EZ. Improving blood pressure control by tailored feedback to patients and clinicians. AmHeart J. 2005;149(5):795–803. doi: 10.1016/j.ahj.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 77.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 78.National Quality Forum . Measurement Framework: Evaluating Efficiency Across Patient-Focused Episodes of Care. NQF; Washington, D.C.: 2009. pp. 1–18. [Google Scholar]
- 79.Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. JManagCare Pharm. 2009;15(9):728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naylor MD. Advancing the science in the measurement of health care quality influenced by nurses. MedCare ResRev. 2007;64(2 Suppl):144S–169S. doi: 10.1177/1077558707299257. [DOI] [PubMed] [Google Scholar]
- 81.Bosworth HB, Powers BJ, Oddone EZ. Patient self-management support: novel strategies in hypertension and heart disease. Cardiol Clin. 2010;28(4):655–663. doi: 10.1016/j.ccl.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ansari M, Shlipak MG, Heidenreich PA, et al. Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation. 2003;107(22):2799–2804. doi: 10.1161/01.CIR.0000070952.08969.5B. [DOI] [PubMed] [Google Scholar]
- 83.Tsuyuki RT, Ackman ML, Montague TJ. Effects of the 1994 Canadian Cardiovascular Society clinical practice guidelines for congestive heart failure. CanJCardiol. 2002;18(2):147–152. [PubMed] [Google Scholar]
- 84.Ambardekar AV, Fonarow GC, Dai D, et al. Quality of care and in-hospital outcomes in patients with coronary heart disease in rural and urban hospitals (from Get With the Guidelines-Coronary Artery Disease Program) AmJCardiol. 2010;105(2):139–143. doi: 10.1016/j.amjcard.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Crespo Leiro MG, Murga N, Matali A, Lopez-Sendon JL. Medication adherence in patients with heart failure and a depressed ejection fraction attending cardiology clinics. RevEspCardiol. 2009;62(4):454–455. doi: 10.1016/s1885-5857(09)71676-7. [DOI] [PubMed] [Google Scholar]
- 86.Gawande A. The checklist: if something so simple can transform intensive care, what else can it do? New Yorker. 2007:86–101. [PubMed] [Google Scholar]
- 87.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. NEnglJMed. 2009;360(5):491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 88.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive Validity of a Medication Adherence Measure in an Outpatient Setting. J ClinHypertens(Greenwich) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Hernandez AF. A centralised telephone intervention reduced combined all cause mortality or admission for worsening HF in chronic heart failure. EvidBasedMed. 2006;11(2):50. doi: 10.1136/ebm.11.2.50. [DOI] [PubMed] [Google Scholar]
- 90.Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. JAmGeriatrSoc. 2004;52(5):675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 91.Stewart S, Horowitz JD. Home-based intervention in congestive heart failure: long-term implications on readmission and survival. Circulation. 2002;105(24):2861–2866. doi: 10.1161/01.cir.0000019067.99013.67. [DOI] [PubMed] [Google Scholar]
- 92.Assyag P, Renaud T, Cohen-Solal A, et al. RESICARD: East Paris network for the management of heart failure: absence of effect on mortality and rehospitalization in patients with severe heart failure admitted following severe decompensation. ArchCardiovascDis. 2009;102(1):29–41. doi: 10.1016/j.acvd.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 93.Hugtenburg JG, Borgsteede SD, Beckeringh JJ. Medication review and patient counselling at discharge from the hospital by community pharmacists. PharmWorld Sci. 2009;31(6):630–637. doi: 10.1007/s11096-009-9314-z. [DOI] [PubMed] [Google Scholar]
- 94.Parry C, Min SJ, Chugh A, et al. Further application of the care transitions intervention: results of a randomized controlled trial conducted in a fee-for-service setting. HomeHealth Care ServQ. 2009;28(2-3):84–99. doi: 10.1080/01621420903155924. [DOI] [PubMed] [Google Scholar]
- 95.Naylor MD, Sochalski JA. Scaling up: bringing the transitional care model into the mainstream. Issue Brief (Commonw Fund) 2010;103:1–12. [PubMed] [Google Scholar]
- 96.DeWalt DA, Broucksou KA, Hawk V, et al. Comparison of a one-time educational intervention to a teach-to-goal educational intervention for self-management of heart failure: design of a randomized controlled trial. BMCHealth ServRes. 2009;9:99. doi: 10.1186/1472-6963-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naylor MD. Transitional care: a critical dimension of the home healthcare quality agenda. JHealthcQual. 2006;28(1):48–54. doi: 10.1111/j.1945-1474.2006.tb00594.x. [DOI] [PubMed] [Google Scholar]