Abstract
Purpose
To estimate the normal distance from vascular termini to ora serrata in children’s eyes.
Methods
Clinical records and peripheral fluorescein angiography images of the ora serrata region, taken using scleral indentation and the RetCam system during examination under anesthesia, were retrospectively reviewed from 33 eyes of 31 consecutive patients with presumed normal peripheral retinal vasculature. All patients had ocular disease either only in the fellow eye or if in the study eye, to a degree judged not likely to affect peripheral retinal vascular development.
Results
The mean age at angiography was 3.8 years (range, 2 months to 13 years). Means of 0.9 disk diameters (DD) of nonperfusion temporally (range, 1.5 DD to 0.5 DD; SD 0.3) and 0.6 DD of nonperfusion nasally (range, 1 DD to 0.25 DD; SD 0.2) were found.
Conclusions
In children up to age 13 years, the avascular retina normally extends 1.5 DD or less temporally and 1.0 DD or less nasally from the ora serrata. Conservatively, ≥2 DD of nonperfusion, 3 standard deviations more than normal, should be considered abnormal and a sign of peripheral nonperfusion. These data may serve as preliminary indicators of the range of normal when evaluating diseases with retinal vascular abnormalities in children.
In 1967 Rutnin and Schepens1-3 published a series of papers describing ophthalmoscopic findings in the peripheral fundus. They described the vascular termini in adults to be 0.5 disk diameters (DD) from the ora serrata, with occasional vessels coursing circumferentially and then posteriorly. They did not evaluate the retinal periphery in children. We are unaware of any systematic description of peripheral nonperfusion in children, excluding series of infants with acute retinopathy of prematurity. As the eye grows, there may be areas of anterior nonperfusion present normally. This description of the amount of normal nonperfusion is of critical importance when evaluating children and their potentially affected relatives for diseases that involve the peripheral retinal vasculature, such as Coats disease, familial exudative vitreoretinopathy, Norrie disease, and persistent fetal vasculature.
To our knowledge, description of normal peripheral nonperfusion using fluorescein angiography has not been reported in children, and this testing would enable the identification of the retinal vascular termini more accurately. Although traditional fluorescein angiography with peripheral sweeps or the recently available Optos (Marlborough, MA) wide-field fluorescein angiography system afford imaging of the anterior retina in adults, it is difficult to reliably image the ora serrata in conscious, small children. We have taken advantage of the recent ability to perform fluorescein angiography in conjunction with scleral indentation to image the ora serrata region (FAOSR) using the RetCam system (Clarity Medical Systems, Pleasanton, CA) during examination under anesthesia.
Using FAOSR we have noted peripheral retinal nonperfusion in the presumed normal fellow eye, or eye with normal peripheral vasculature, of patients with pediatric retinal disease. The purpose of this manuscript is to quantify the amount of peripheral nonperfusion in eyes with presumed normal retinal vasculature in children.
Methods
Approval was obtained from the institutional review board for human subjects at the the University of Illinois at Chicago to retrospectively review pediatric retinal databases of patients for pediatric retinal conditions. Retinal drawings, images, and examination records obtained either during clinic visits or examinations under anesthesia were reviewed retrospectively. Age, sex, race, gestational age at birth, laterality of disease, presence of systemic conditions, and family histories of patients were recorded. No patient with a history of premature birth (≤37 weeks) was included. In every patient, the decision to perform fluorsecein angiography was based on need for care of the diseased eye, and images of the less- or nondiseased eye were taken as part of standard clinical practice, particularly in the setting of examination under anesthesia, to evaluate the fellow eye for subtle disease.
In order to quantify the amount of nonperfusion in normal pediatric eyes, children who underwent FAOSR were identified. Given that children without retinal pathology rarely underwent FAOSR, eyes in the study group were generally fellow eyes of patients with unilateral disease. Occasionally eyes with optic nerve disease or only small, posterior retinoblastoma tumors were included, since these conditions would be unlikely to have peripheral retinal vascular abnormalities. For this study, distance from the ora serrata to the vascular termini temporally and nasally was recorded by a single observer (MB) in disk diameters (DD), which is the measurement used in clinical practice and in previous descriptions of the anterior retina.1-3 We believe measurements to be accurate to at most 0.5 DD, because of changes in the globe curvature induced by scleral indentation, except adjacent to the ora serrata, where 0.25 DD can be differentiated. Mean and standard deviation of DD of nonperfusion, as well as correlation with age, were calculated using Excel (Microsoft, Redmond, WA). Mean and standard deviation of nonperfusion were also calculated for the subgroup of eyes with no pathology and for the subgroup of eyes that had ocular pathology. The presence of circumferential vessels near the ora serrata was also recorded.
Results
Patient age, sex, and ocular disease are presented in the Table 1 and in e-Supplement 1 (available at jaapos.org). A total of 33 eyes of 31 consecutive patients (18 females) with adequate peripheral fluorescein angiographic images of ora serrata and vascular termini were identified and had width of nonperfusion measured. Of the 31 patients, 5 had bilateral, asymmetric retinoblastoma, and the eye with less involvement was included. These less-involved eyes had only small, posterior tumors that were not likely to affect the peripheral vasculature. One patient had a sibling with retinoblastoma and was examined for retinoblastoma but had a normal examination. Both eyes of this patient were included. Both eyes of an additional patient with unilateral, posterior choroidal osteoma were included as well. Four patients had fellow eyes with persistent fetal vasculature (PFV), a disease well known to be predominantly unilateral. The diseased eyes all had posterior, PFV-related tent-shaped retinal detachment. None of the study eyes had signs of PFV.
Table 1.
Disease State of Study Eye and Contralateral Eye
| Study Eye Disease | Contralateral Ocular Disease | Number of Eyes |
|---|---|---|
| None | Retinoblastoma | 9 |
| None | Retinocytoma | 1 |
| None | Suspected retinoblastoma (no disease) | 2* |
| None | Persistent Fetal Vasculature | 4 |
| None | Peripapillary Staphyloma | 1 |
| None | Optic Nerve Coloboma | 1 |
| None | Retinochoroidal Coloboma | 1 |
| None | Rhegmatogenous Retinal Detachment | 2 |
| None | Macular scar | 1 |
| None | Trauma | 1 |
| None | Choroidal Osteoma | 1# |
| Choroidal Osteoma (posterior) | None | 1# |
| Retinoblastoma (posterior) | Retinoblastoma | 5 |
| Optic Nerve Edema | Optic Nerve Edema | 1+ |
| Multifocal Choroiditis | Multifocal Choroiditis | 1 |
| Cataract | Cataract | 1 |
both eyes are from the same patient
temporal nonperfusion measured in OS, nasal nonperfusion measured in OD
eyes are from same patient
The mean age at angiography was 3.8 years (range, 2 months to 13 years). Mean averages of 0.9 DD of nonperfusion temporally (range, 1.5 DD to 0.5 DD; SD 0.3) and 0.6 DD of nonperfusion nasally (range, 1 DD to 0.25 DD; SD 0.2) were found. No eyes had a measurement >1.5 DD. Among eyes with no ocular pathology (N = 23), the mean temporal nonperfusion remained 0.9 DD (SD 0.35) and the mean nonperfusion nasally was 0.5 DD (SD 0.22). Among eyes with ocular pathology included in this study (N=10), the mean temporal nonperfusion remained 0.9 DD (SD 0.33) and the mean nonperfusion nasally was 0.6 DD (SD 0.23). Representative images from peripheral fluorescein angiography are presented in Figure 1. There appeared to be no strong correlation (P > 0.05) between age and width of nonperfusion, temporally or nasally (correlation coefficient, 0.27 and 0.32, resp.); 18 eyes had circumferential vessels present near the ora serrata.
FIG 1.
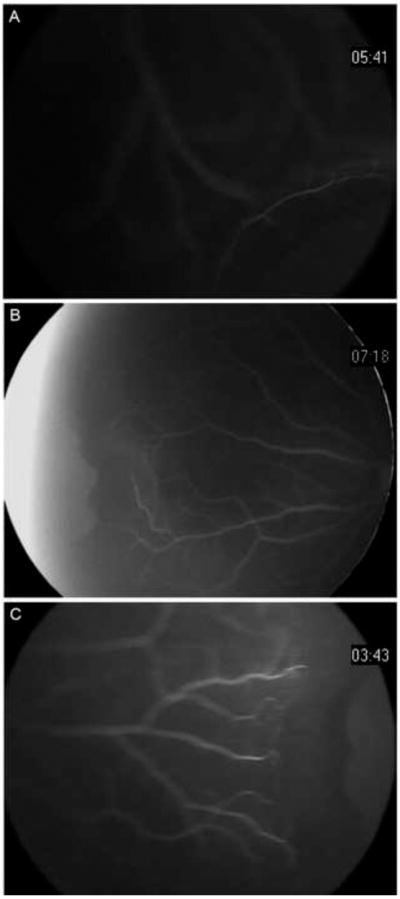
Flourescein angiography of the peripheral retina with scleral indendation. A, vascular termini less than a quarter disk diameter (DD) from the ora serrata in a 7-mont-hold Hispanic girl with contralateral retinoblastoma (80° view). B, vascular termination 1 DD from the ora serrata nasally in a 23-month Hispanic girl with contralateral retinoblastoma (130° view). C, vascular termination 1.5 DD temporally in the same patient as 1B (80° view).
Discussion
Using FAOSR, this study evaluated width of retinal nonperfusion in pediatric eyes with clinically normal appearing periphery and found it to be ≤1.5 DD, with mean of 0.9 DD temporally and 0.6 DD nasally. This is in contrast to the 0.5 DD of peripheral nonperfusion found in normal adults by Rutnin and Schepens1 using ophthalmoscopy. Using astigmatic fluorescein angiography, Asdourian and Goldberg4 reported approximately 1 mm (0.67 DD) of nonperfusion in young adults. These small differences could be real due to age or due to measurement technique. We report differences between nasal and temporal retinal nonperfusion while Rutnin and Schepens reported overall nonperfusion, and may have mentally averaged the nonperfusion during the 360° examination. It is also conceivable that far peripheral vessels are not perfused with fluorescein during scleral indentation, and thus width of nonperfusion would be overestimated with our technique. Performing fluorescein angiography with a mirror contact lens and scleral depression, Zenker5 found nonperfused peripheral vessels in adults, but these vessels were identifiable due to hypofluorescence against choroidal fluorescence. We did not find hypofluorescent vessels during image review. Alternatively, if our data set included eyes with pathologic nonperfusion, mean nonperfusion would be increased.
Similar to adults studies, over half of the eyes in our study had circumferential vessels near the ora serrata.1,5 We believe that circumferential vessels represent a terminal structure of vascular development since retina vessels exist in an end-arterial network. Beginning around 21 weeks and completing by 38-40 weeks gestation, central to peripheral spread of retinal vasculature follows the gradient of retinal ganglion cell maturation and occurs by angiogenesis induced by vascular endothelial growth factor (VEGF) expressed by neuroglia in response to physiologic hypoxia.6 The location of circumferential vessels may mark the area in the far periphery where the retina is thin enough to derive oxygen from the choroidal circulation and thus not produce stimuli for angiogenesis.
Our study group is comprised of fellow “normal” eyes from patients with unilateral or posterior ocular disease, because it was not possible to obtain FAORS on a cohort of children without eye disease due to the requirement of general anesthesia. It is unlikely that similar angiographic data of the ora serrata region could be generated in the clinic in a pediatric population, even with the Optos system. Despite the presence of ocular disease, we believe these results are generalizable to the normal pediatric population. The similarity in mean width of nonperfusion between the subgroup of eyes with no ocular disease and the subgroup with posterior ocular disease strongly suggests a lack of effect on the anterior vascular development by the diseases in the included eyes. Indeed, if any of these included eyes had pathologic nonperfusion, this study would overestimate the degree of nonperfusion in normal eyes, and thus comparisons to a particular disease in which pathologic nonperfusion was suspected would be robust. An eye with more avascular retina than the limit found in our study would certainly have abnormal of nonperfusion.
We recommend 3 standard deviations above the mean nonperfusion as a conservative cutoff for normal. Using a Gaussian distribution, the remainder of the population outside of 3 standard deviations would be less than 0.2%. Practically the threshold value would be 2 DD, since 2.1 DD is not clinically distinguishable from 2 DD. Aside from statistical reasoning, we believe this is a practical and conservative distance to discern normal from abnormal width of peripheral nonperfusion because no eye had more than 1.5 DD of nonperfusion temporally. These data will provide a more scientific basis for determination of abnormal vascular termination for investigation of pedigrees of patients with diseases that affect the peripheral retinal vasculature, such as Coats disease, Norrie disease, familial exudative vitreoretinopathy, PFV, regressed retinopathy of prematurity (ROP), and other disorders. For example, Coats disease may have a genetic component but have variable expressivity.7 Thus identification of candidate genes through linkage analysis in a family of a patient with Coats disease may be possible if ≥2 DD of peripheral nonperfusion is considered a form fruste of the disease. Additionally, a recent publication identified areas of nonperfusion in the unaffected fellow eyes of two patients with Coats disease.8 Knowledge of normal range of nonperfusion would help classify these eyes as either abnormal, which would imply that Coats disease can be bilateral but asymmetric or as having a width of nonperfusion within normal range. Similarly, with recent increasing use of bevacizumab for ROP after the BEAT-ROP trial,9 and given the large areas of avascular retina after secondary vascular arrest, there should be increased interest in the width of residual avascular retina which may be acceptably close to normal.
In our study there was an imbalance in sex of patients, with more females. This is likely due to relatively small sample size, but is unlikely to affect the findings. These data may serve as preliminary indicators of the range of normal and begin to allow differentiation of normal from pathologic peripheral nonperfusion.
Supplementary Material
Acknowledgments
Study conducted at the University of Illinois at Chicago and Retina Consultant, Ltd, Des Plaines, Illinois.
Footnotes
Literature Search
PubMed was searched (1926-2011) for literature in English only using the following search terms: RetCam fluorescein angiography, pediatric fluorescein angiography, peripheral retinal nonperfusion, ora serrata angiography, peripheral retinal vasculature children, peripheral fluorsecein angiography children, vasculature RetCam.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rutnin U, Schepens CL. Fundus appearance in normal eyes. II. The standard peripheral fundus and developmental variations. Am J Ophthalmol. 1967;64:840–52. doi: 10.1016/0002-9394(67)92226-x. [DOI] [PubMed] [Google Scholar]
- 2.Rutnin U, Schepens CL. Fundus appearance in normal eyes. IV. Retinal breaks and other findings. Am J Ophthalmol. 1967;64:1063–78. doi: 10.1016/0002-9394(67)93057-7. [DOI] [PubMed] [Google Scholar]
- 3.Rutnin U, Schepens CL. Fundus appearance in normal eyes. 3. Peripheral degenerations. Am J Ophthalmol. 1967;64:1040–62. doi: 10.1016/0002-9394(67)93056-5. [DOI] [PubMed] [Google Scholar]
- 4.Asdourian GK, Goldberg MF. The angiographic pattern of the peripheral retinal vasculature. Arch Ophthalmol. 1979;97:2316–8. doi: 10.1001/archopht.1979.01020020532003. [DOI] [PubMed] [Google Scholar]
- 5.Zenker HJ. Fluorescein angiography in inflammation of the peripheral fundus: The normal fluorescein angiographic pattern. I. Ophthalmologica. 1985;190:77–82. doi: 10.1159/000309497. [DOI] [PubMed] [Google Scholar]
- 6.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: Roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41:1217–28. [PubMed] [Google Scholar]
- 7.Black GC, Perveen R, Bonshek R, et al. Coats’ disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesis. Hum Mol Genet. 1999;8:2031–5. doi: 10.1093/hmg/8.11.2031. [DOI] [PubMed] [Google Scholar]
- 8.Shane TS, Berrocal AM, Hess DJ. Bilateral fluorescein angiographic findings in unilateral Coats’ disease. Ophthalmic Surg Lasers Imaging. 2011;10:42. doi: 10.3928/15428877-20110203-03. [DOI] [PubMed] [Google Scholar]
- 9.Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


