Summary
Classic experiments have shown that ovulation and estrous cyclicity are under circadian control, and surgical ablation of the suprachiasmatic nuclei (SCN) results in estrous acyclicity in rats [1-3]. Here, we characterized reproductive function in the circadian Clock mutant mouse,[4, 5] and found that the circadian Clock mutation both disrupts estrous cyclicity and interferes with the maintenance of pregnancy. Clock mutant females have extended and irregular estrous cycles, lack a coordinated LH surge on the day of proestrus, exhibit increased fetal reabsorption during pregnancy, and have a high rate of full-term pregnancy failure. Clock mutants also show an unexpected decline in progesterone levels at mid-pregnancy and a shortened duration of pseudopregnancy, suggesting that maternal prolactin release may be abnormal. In a second set of experiments, we interrogated the function of each level of the hypothalamic-pituitary-gonadal (HPG) axis in order to determine how the Clock mutation disrupts estrous cyclicity. We report that Clock mutants fail to show an LH surge following estradiol priming, in spite of the fact that hypothalamic levels of gonadotropin-releasing hormone (GnRH), pituitary release of luteinizing hormone (LH), and serum levels of estradiol and progesterone are all normal in Clock/Clock females. These data suggest that Clock mutants lack an appropriate circadian daily timing signal required to coordinate hypothalamic hormone secretion. Defining the mechanisms by which the Clock mutation disrupts reproductive function offers a model for understanding how circadian genes affect complex physiological systems.
Results and Discussion
Clock mutant females exhibit defects in estrous cyclicity and proestrus LH release
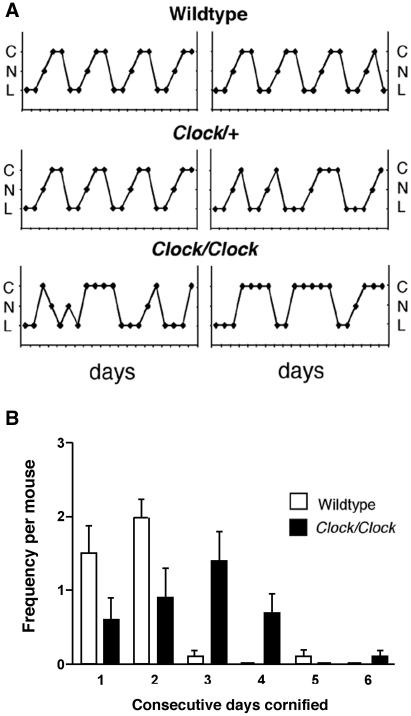
The suprachiasmatic nuclei (SCN) of the hypothalamus coordinate circadian physiology and behavior by functioning as the master pacemaker in a hierarchical system of multiple circadian oscillators: the SCN receive photic input from retinal ganglion cells and subsequently phase-coordinate the activity of tissue-specific oscillators via neuronal and humoral output [6]. Circadian output from the SCN plays a major role in the regulation of female reproduction. Previous studies have shown that, in rats, disruption of communication between the SCN and the GnRH neurons responsible for regulating reproductive function—either by ablating the SCN or by severing the neuronal pathways between the SCN and the preoptic area—results in estrous acyclicity and infertility [2, 3]. Circadian output from the SCN can also be altered by disrupting core gene components of the molecular pacemaker, including Clock [4], Bmal1 [7], mPer1 and mPer2 [8, 9] and mCry1 and mCry2 [10, 11]. Therefore, analysis of mice with ‘clock gene’ mutations provides a way to dissect genetically the role of circadian rhythms in reproduction. Here, we characterized reproductive function in the female Clock mutant mouse, which carries a 51-amino acid deletion in the transcriptional activation domain of the CLOCK protein [5, 12]. Wildtype, Clock/+, and Clock/Clock females were examined daily for the onset of vaginal opening and, upon reaching 10 weeks of age, were monitored by vaginal lavage to record estrous cyclicity (see Supplemental Data for a detailed description of all Materials and Methods). Although the timing of vaginal opening was normal (Supplemental Table 1), there were significant differences in the characteristics of estrous cycles among genotypes (Figure 1A): while wildtype females and the majority of Clock/+ females exhibited multiple consecutive 4-5 day long cycles, Clock/Clock females had prolonged and irregular cycles characterized by significantly fewer days of proestrus and more days of estrus (Figure 1B, Supplemental Figure 1).
Figure 1. Clock/Clock females display lengthened and irregular estrous cycles.
A, Representative estrous cycles as measured by vaginal cytology in wildtype (top), Clock/+ (middle), and Clock/Clock (bottom) females. C = cornified, N = nucleated, L = leukocytic. B, Clock/Clock females have significantly more days of cornified smears compared to wildtype females, as determined by an unpaired t-test for the number of consecutive days cornified (* indicates p < 0.05, ** indicates p < 0.01).
Abnormal estrous cycles could be due to either central or peripheral defects. Therefore, we began by examining ovarian function in intact cycling females. Wildtype, Clock/+, and Clock/ Clock females were sacrificed on the afternoon of diestrus or proestrus, serum levels of estradiol and progesterone were measured by radioimmunoassay (RIA), and ovaries were fixed for histological analysis. In all three genotypes, estradiol (Supplemental Figure 2A) and progesterone (Supplemental Figure 2B) levels were low on diestrus and significantly elevated on the afternoon of proestrus, and histological analysis of ovarian tissue from proestrus Clock/Clock mice showed no gross morphological abnormalities (Supplemental Figure 3, Supplemental Table 2). Follicles at all stages of development were present, and both corpora lutea and Graafian follicles were present in numbers comparable to those observed in wildtype tissue. These results suggest that the estrous cycle irregularities we observed in Clock/Clock females do not stem from an effect of the Clock mutation acting at the level of the ovary.
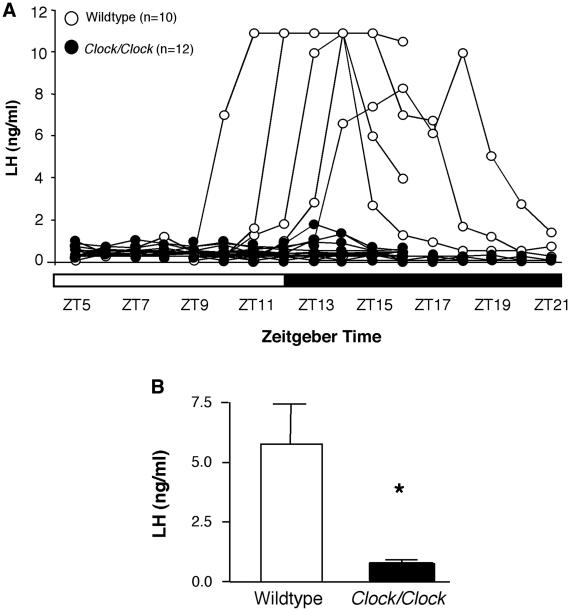
We then investigated the timing and amplitude of the LH surge by collecting serial blood samples from ovary-intact wildtype and Clock/Clock mice on the morning, afternoon, and evening of proestrus, as defined by a nucleated vaginal smear. The proportions of wildtype (50%) and Clock/Clock (0%) mice exhibiting an LH peak of 10 ng/ml or greater differed significantly (chi square, p < 0.01, Figure 2A). None of the Clock/Clock females exhibited LH concentrations exceeding 1.8 ng/ml, and concentrations in the majority of mutants never varied from baseline (0.2 ng/ml). Maximum serum LH concentrations were significantly lower in Clock/Clock than wildtype females (Student’s t-test. p < 0.05, Figure 2B).
Figure 2. Clock/Clock mutants fail to have a coordinated LH surge on the day of proestrus.
A, Individual LH traces from all wildtype (open circles) and Clock/Clock (closed circles) females sampled from either ZT5-16 or ZT9-21. B, Individual peak LH values in serum obtained from serially sampled mice. Peak Clock/Clock LH values were compared using an unpaired t-test and found to be significantly lower than peak wildtype values (** indicates p < 0.01). Due to the limited sample volume collected, samples with LH values exceeding the range of the RIA could not be re-assayed at lower sample concentrations, preventing absolute measurements of serum concentrations above 10 ng/ml. Therefore, for analysis and presentation purposes, these values were set at 10 ng/ml, and we defined the minimum “surge” value as 14% of the maximum, or 1.4 ng/ml, as previous studies in rats have shown that 14 percent of the peak surge is the minimum value of LH required for ovulation [38]. Using these criteria, only one Clock mutant reached the minimum surge level at any time point.
It is possible that Clock mutants have a normal LH surge that is not temporally associated with a nucleated smear, but the hormonal profiles we observed make this unlikely. A minimum of 30 hours of exposure to estradiol is required to induce GnRH release [13]; both vaginal cytology and serum sampling show that Clock mutants have low estradiol levels prior to the day of a nucleated smear. Therefore, a large LH surge is probably not occurring before the initial sampling time (ZT5). It is also unlikely that a surge is occurring long after the morning of a nucleated smear, as extended elevation of progesterone results in inhibition of LH release [14], and Clock mutants exhibit elevated progesterone coincident with a nucleated smear. Thus, the most parsimonious explanation is that the small elevations in LH observed throughout the day of proestrus in individual Clock mutants are sufficient to induce ovulation.
Clock mutant females exhibit elevated rates of fetal reabsorption and pregnancy failure
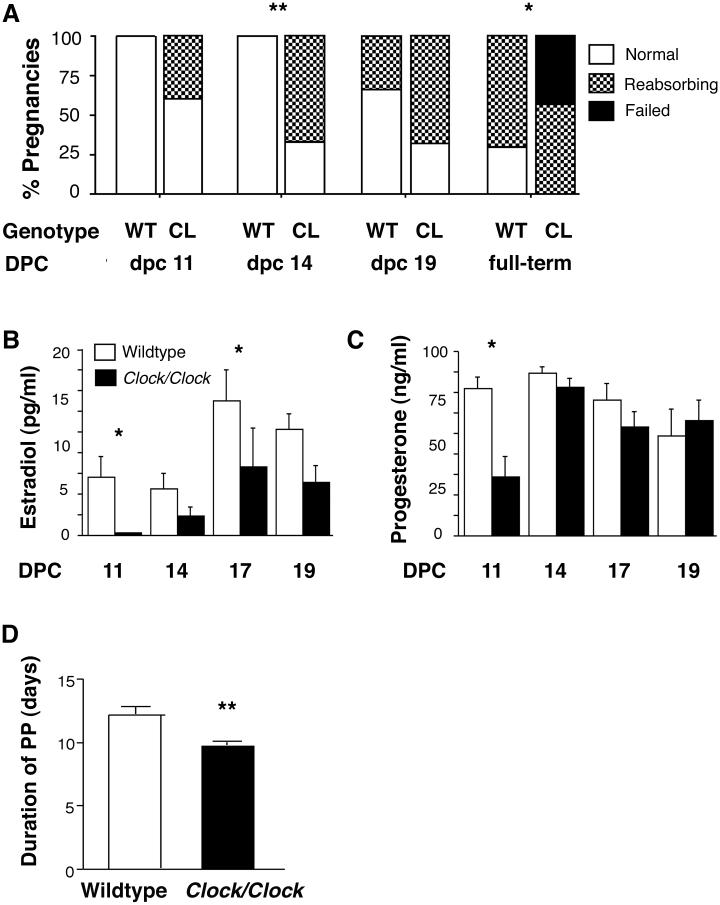
To our knowledge, circadian rhythm defects have never been implicated in the abnormal progession of pregnancy, although some researchers have suggested a connection [15], and anecdotal evidence from our laboratory suggested that Clock mutant females regularly failed to produce offspring. To verify and characterize this defect, wildtype and Clock/Clock females were mated with wildtype males, and the initiation and progression of pregnancy was measured by observing fetal reabsorption at 11, 14, and 17 days post copulation (dpc), and at full-term. Although the early stages of pregnancy were normal in Clock mutants, Clock/Clock females showed an increased rate of midgestation fetal reabsorption, and pregnancy failure at full-term, compared to wildtype females. The percentage of Clock/Clock pregnancies showing signs of fetal reabsorption was significantly different from wildtype pregnancies at two time points, dpc 14 and full-term, and the percentage of fetuses being reabsorbed by Clock mutant dams was elevated at dpc 14 and full-term (chi square. p < 0.05. Figure 3A, Supplemental Figure 4). All of the wildtype females and 57% (4 of 7) of the Clock/Clock females allowed to carry their pregnancies to full term delivered live litters on dpc 20. However, 43% (3 of 7) of the Clock/Clock females either went into an extended but non-productive labor or failed to enter labor and instead fully reabsorbed the full-term fetuses.
Figure 3. Clock/Clock pregnancies are characterized by increased rates of fetal reabsorption, reduced serum levels of estradiol and progesterone, and possible dysregulation of prolactin release.
A, The percentage of Clock mutant dam pregnancies showing any signs of fetal reabsorption is significantly greater than wildtype at dpc 14 (** indicates p < 0.01) and full-term (* indicates p ≤ 0.05), as determined by chi square analysis. In many cases, both wildtype and Clock pregnancies showed some signs of reabsorption by full-term, but reabsorbing fetuses occurred more frequently in Clock pregnancies. While all wildtype pregnancies delivered normally, 40% of Clock mutant dams carried to full-term but failed to deliver. Importantly, Clock mutant females exhibited copulatory plugs as frequently as wildtype females, indicating that mating behavior was intact in the mutants. Additionally, the average number of fetuses per genotype at dpc 11 was almost identical between genotypes (7.6 fetuses per wildtype dam, 7.75 fetuses per Clock/Clock dam), indicating that the early stages of pregnancy, including ovulation, fertilization, implantation, and early fetal development, occur normally in Clock mutants. WT = wildtype, CL = Clock/Clock, DPC = days post copulation. B, Estradiol levels are significantly reduced in Clock/Clock (closed circles) mice compared to wildtypes (open circles) (p < 0.001). Estradiol in both genotypes is elevated in late pregnancy (dpc 17, 19) compared to mid-pregnancy (dpc 11, 14) (p < 0.01). C, Progesterone is significantly lower in Clock/Clock females compared to wildtypes (p < 0.05) due to very low progesterone levels in Clock/Clock females at dpc 11. In both genotypes, progesterone levels at dpc 14 are elevated compared to dpc 11 and dpc 19 values (p ≤ 0.01). For both B and C, * indicates p < 0.05. Prior to testing the hypotheses, data were checked to verify that they met the assumptions of normality and equality of variance required for analysis of variance. Because the data violated one or more of the assumptions, data were transformed using logarithms and statistical analysis (two-way ANOVA and Fisher’s LSD) was performed on the log transformed data. D, The duration of pseudopregnancy is significantly shorter in Clock/Clock females compared to wildtype controls as determined by t-test (** indicates p ≤ 0.001). Wildtype (n = 10) and Clock/Clock (n = 26) females were mated with vasectomized CD-1 males and the length of pseudopregnancy, as indicated by leukocytic vaginal cytology, was measured by vaginal lavage. The presence of a copulatory plug was designated as dpc 1, and the final day of leukocytic smears was designated as the last day of pseudopregnancy.
Estradiol and progesterone are vital for maintaining uterine receptivity to developing fetuses during pregnancy and promoting parturition [16]. Therefore, we measured estradiol (Figure 3B) and progesterone (Figure 3C) levels at dpc 11, 14, 17, and 19 (the day before expected parturition) to determine whether altered ovarian hormone levels could explain the pregnancy phenotype in Clock/Clock females. Clock mutants exhibited significantly lower estradiol levels compared to wildtypes throughout pregnancy, although both genotypes displayed an increase in estradiol levels from mid-pregnancy (dpc 11, dpc 14) to late pregnancy (dpc 17, dpc 19). At full-term, estradiol in Clock mutants was only one-third the level of estradiol in wildtype females. Because estradiol is important for enhancing uterine contractility [17], it is likely that low estradiol levels were at least partially responsible for the failure of some Clock/Clock females to initiate labor.
Progesterone levels were also reduced in Clock mutants, most notably at dpc 11. Mid-gestational levels of progesterone are particularly important for maintaining blood flow to developing fetuses [18], and previous studies have shown a quantitative relationship between progesterone levels and maintenance of pregnancy [19]. Therefore, the reduced progesterone levels observed in Clock mutant dams at dpc 11 may explain the fetal reabsorption observed by dpc 14. By dpc 14, progesterone levels in Clock mutants had risen, and the rate of fetal reabsorption in Clock/Clock females remained similar to that in wildtypes until full-term.
The duration of pseudopregnancy is shortened in Clock mutant mice
We hypothesized that the observed abnormalities in fetal reabsorption and ovarian hormones in pregnant Clock/Clock females could be explained by abnormal prolactin release during early and/or mid-pregnancy. Prolactin release is induced by the copulatory stimulus, and thereafter occurs in two daily surges that are roughly coordinated to lights-on and lights-off, suggesting that release is under circadian control and is therefore vulnerable to gene mutations that alter the circadian pacemaker [20, 21]. Prolactin is the only factor required to rescue and maintain the ovarian corpora lutea (CL), which produce the elevated serum progesterone levels characteristic of and necessary for pregnancy and pseudopregnancy [22]. At mid-gestation, around dpc 10, maternal prolactin release tapers off and the CL are supported for the remainder of pregnancy by the prolactin-like placental lactogen (PL-1) produced by fetuses [23]. If the mating was infertile, there is no PL-1 to rescue the CLs, and the female resumes estrous cyclicity. Thus, the termination of pseudopregnancy is a reliable indicator of the termination of mating-induced PRL release. Therefore, in order to determine whether a defect in maternal prolactin release might be responsible for low Clock/Clock progesterone levels at dpc 11 and subsequent elevated fetal reabsorption at dpc 14, we measured the duration of pseudopregnancy. This functional assessment was used rather than measurement of PRL levels because the expected variability in the timing of PRL secretion and prevalence of PRL release in response to stress would have made interpretation of the results difficult.
Pseudopregnancy was significantly shortened in Clock/Clock females compared to wildtype controls (Figure 3D), suggesting that PRL secretion may cease earlier in Clock/Clock mice than in wildtype females. The functional consequences of premature cessation of prolactin release are partial or complete CL regression, a drop in progesterone levels, and either abortion or an increase in fetal reabsorption [18]. These consequences are consistent with our observations. Although it is possible that there is a failure on the part of the Clock/+ and Clock/Clock fetuses to begin to support the CLs at the appropriate developmental stage, the cessation of pseudopregnancy in mutant females often occurs several days prior to the onset of fetal PL-1 production. We have also observed that, when a litter of Clock/Clock embryos is transplanted into a pseudopregnant wildtype female, the pregnancy progresses normally, indicating that Clock mutant fetuses are capable of sustaining pregnancy in normal females (BHM, personal observation). Therefore, at least some of the Clock mutant pregnancy abnormalities are likely due to abnormal circadian control of maternal prolactin release. Our hypothesis is consistent with the findings of other groups that have described a link between the circadian system and prolactin secretion [24].
Abnormal LH release in Clock mutants is due to a hypothalamic defect
The role of circadian rhythms in LH release and the estrous cycle is more thoroughly described than in pregnancy. Therefore, we focused on the estrous cycle to determine how the Clock mutation could result in altered reproductive physiology. We hypothesized that Clock/Clock females display irregular estrous cycles and fail to have a coordinated LH surge due to a disruption of the daily timing signal from the SCN to the GnRH neurons, rather than as a result of pituitary defects or inappropriate feedback from ovarian hormones.
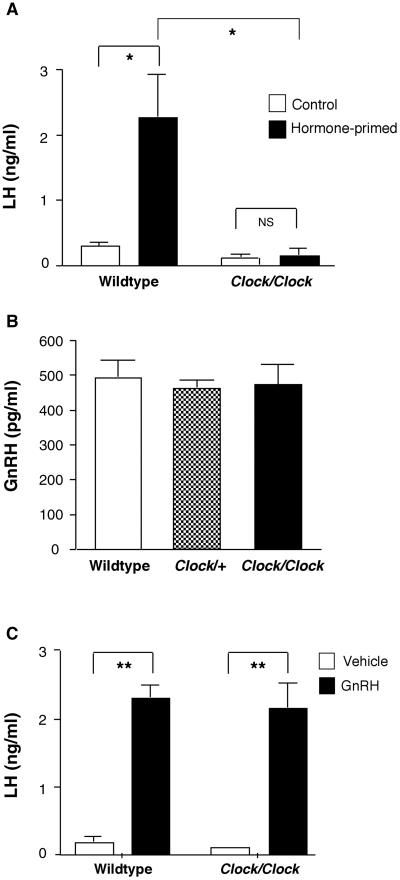
In rodents, coordinated GnRH release on the afternoon of proestrus requires both a daily timing signal originating in the SCN and permissive levels of estrogen and progesterone [25]. To determine whether the observed defect in LH release in Clock mutants was due to inappropriate steroid feedback, ovariectomized mutant and wildtype mice received low-dose estradiol capsules followed by an injection of estradiol benzoate; a paradigm that induces an LH surge during the circadian-timed window. Estradiol capsules alone resulted in baseline levels of LH in both genotypes due to negative feedback on GnRH neurons (Figure 4A). Injection with estradiol benzoate resulted in positive feedback and a subsequent LH surge in wildtype mice, but failed to induce an LH surge in Clock/Clock mice. These data are consistent with the hypothesis that Clock mutants lack a coordinated daily timing signal triggering GnRH release, and rules out the possibility that improper levels of ovarian hormones prevent ovary-intact Clock mutants from mounting a coordinated LH surge.
Figure 4. Hypothalamic-pituitary gonadal axis function in Clock mutants.
A, Estradiol benzoate treatment resulted in a significant elevation in serum LH in wildtype ovariectomized females (* indicates p < 0.01), but did not produce elevated LH levels in Clock/Clock females. NS = not significant. Two-way ANOVA. Treatment (F = 8.9, df (1,24) p < 0.01); Genotype (F = 11.8, df (1,24), p < 0.01). Mice were ovariectomized and implanted with estradiol capsules. Six days following implantation, mice received an injection of either estradiol benzoate or sesame oil at 0800h, and samples were collected the following evening at ZT13. B, There is no difference in hypothalamic GnRH peptide content among wildtype, Clock/+, and Clock/Clock females on the afternoon of proestrus. One-way ANOVA (F = 0.1, df (2,11), p = 0.9). C, GnRH treatment (400 ng/kg, sc) resulted in significantly increased serum LH compared to control levels in both wildtype and Clock/Clock females that had been hormone primed as described above (** indicates p < 0.001).
Several studies show that GnRH transcription is under circadian control, and the Clock mutation results in the down-regulation of transcription of a number of genes in addition to the core clock genes, introducing the possibility that the Clock mutation alters GnRH synthesis [26, 27]. To test this hypothesis, we measured GnRH by RIA in hypothalami from wildtype, Clock/+, and Clock/Clock mice on the afternoon of proestrus. Hypothalamic GnRH content was normal in Clock/+ and Clock/Clock females (Figure 4B); thus, the mutation does not interfere with the production of GnRH, and Clock mutants have an adequate supply of the peptide available for release on proestrus.
Finally, we evaluated pituitary function in vivo by measuring serum LH following treatment with GnRH in wildtype and mutant females. Expression of the core clock gene Period2 is rhythmic in pituitaries in culture, raising the possibility that the Clock might interfere directly with either LH production or release at the level of the pituitary [28]. However, pituitary release of LH following a GnRH challenge was identical in wildtype and Clock/Clock females (Figure 4C), indicating that pituitary function is normal in Clock mutants.
Since all aspects of the HPG axis other than GnRH/LH release are normal in Clock mutants, estrous cycle defects in the Clock mutants appear to result from a disruption of the timing and/or coordination of GnRH release on proestrus. This disruption may be due to either altered output from the SCN to GnRH neurons or an effect of the Clock mutation on GnRH neurons themselves. Several groups have shown that core clock genes, including Bmal1 and Per1, are expressed and cycle in an immortalized GnRH cell line (GT1-7), suggesting that intracellular pacemakers within GnRH neurons may be important for the circadian regulation of GnRH release [29, 30]. It is possible that expression of the Clock mutation within GnRH neurons may disrupt the expression of other proteins necessary for GnRH release, such as hormone or ion channel receptors. In support of this, Chappell and colleagues found that transfection of GT1-7 cells with the CLOCK-Δ19 dominant negative mutation decreases mean GnRH pulse frequency, whereas overexpression of Cry1, which inhibits CLOCK-induced transcription, increases GnRH pulse frequency [29]. However, the ultimate relationship between GnRH neuron pulsatility and the regulation of proestrus GnRH release remains undefined. It is therefore unclear whether the extent of the disruption of GnRH pulsatility induced by the Clock mutation in GT1-7 cells would be sufficient to cause the defects in LH release and estrous cyclicity that we observed in Clock/Clock mice.
Although a direct effect of the Clock mutation on GnRH neurons in vivo remains to be examined, we hypothesize that Clock mutants fail to have an LH surge because the SCN in mutant animals does not provide a coordinated time-of-day signal to GnRH neurons. Likely candidates for conveying the SCN-GnRH signal are the neuropeptides vasopressin and vasoactive intestinal peptide (VIP), both of which are expressed in neurons projecting from the SCN to the preoptic area [31]. VIP-containing fibers make direct projections to the population of GnRH neurons that expresses c-fos on the afternoon of proestrus [32, 33], and suppression of VIP synthesis in the SCN attenuates peak LH levels during estradiol-induced surges [34]. Vasopressin-containing neurons project to interneurons adjacent to GnRH fibers [35], and, in rats, inhibition of vasopressin signaling on the morning of a hormone-induced surge significantly attenuates LH release [36]. Furthermore, vasopressin expression is rhythmically controlled by CLOCK-induced transcription, and vasopressin content in the SCN is drastically reduced in Clock mutants, suggesting a molecular mechanism for the missing time-of-day signal in Clock/Clock females [37]. Further studies are necessary to refine our understanding of the roles these peptides play in controlling GnRH release. More generally, characterization of possible reproductive defects in other circadian mutants will help to clarify the role of the Clock gene specifically, as opposed to the role of the molecular pacemaker, in the circadian control of reproductive function.
Supplementary Material
Acknowledgements
We thank Brigitte Mann for performing the RIAs and Drs. Neena B. Schwartz and Teresa K. Woodruff for helpful advice. This work was supported by a predoctoral fellowship from the National Science Foundation (B.H.M.), and NIH awards AG-18200 and HL-59598 (F.W.T). Joseph S. Takahashi is an Investigator in the Howard Hughes Medical Institute.
Abbreviations
- SCN
suprachiasmatic nucleus
- GnRH
gonadotropin-releasing hormone
- HPG
hypothalamic-pituitary-gonadal
- LH
luteinizing hormone
- FSH
follicle stimulating hormone
- CL
corpus luteum
- DPC
days post copulation
- RIA
radioimmunoassay
- VIP
vasoactive intestinal peptide
References
- 1.Hoffman JC. The influence of photoperiod on reproductive functions in female mammals. In: Knobil E, Neill J, editors. Physiology of Reproduction. Raven Press; 1988. pp. 57–77. [Google Scholar]
- 2.Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci. 1977;198:279–296. doi: 10.1098/rspb.1977.0098. [DOI] [PubMed] [Google Scholar]
- 3.Weigand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 4.Vitaterna MH, King DP, Chang A, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TDL, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian Clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi JS, Turek FW, Moore RY, editors. Handbook of Behavioral Neurobiology: Circadian Clocks, edn. Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- 7.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sheng Sun Z., Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 9.Bae K, Xiaowei J, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 10.Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenan D, Buijs RM, Bootsma D, Hoeijmakers JHJ, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 12.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 13.Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104:1247–1255. doi: 10.1210/endo-104-5-1247. [DOI] [PubMed] [Google Scholar]
- 14.Girmus RL, Wise ME. Progesterone directly inhibits pituitary luteinizing hormone secretion in an estradiol-dependent manner. Biol Reprod. 1992;46:710–714. doi: 10.1095/biolreprod46.4.710. [DOI] [PubMed] [Google Scholar]
- 15.Endo A, Watanabe T. Effects of non-24-hour days on reproductive efficacy and embryonic development in mice. Gamete Res. 1989;22:435–441. doi: 10.1002/mrd.1120220409. [DOI] [PubMed] [Google Scholar]
- 16.Hodgen GD, Itskovitz J. Recognition and maintenance of pregnancy. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press, Ltd.; New York: 1988. [Google Scholar]
- 17.Thorburn GD, Challis JRG. Endocrine control of parturition. Physiological Reviews. 1979;59:863–918. doi: 10.1152/physrev.1979.59.4.863. [DOI] [PubMed] [Google Scholar]
- 18.Milligan SR, Finn CA. Minimal progesterone support required for the maintenance of pregnancy in mice. Human Reproduction. 1997;12:602–607. doi: 10.1093/humrep/12.3.602. [DOI] [PubMed] [Google Scholar]
- 19.Csapo I, Wiest WG. An examination of the quantitative relationship between progesterone and the maintenance of pregnancy. Endocrinology. 1969;85:735–746. doi: 10.1210/endo-85-4-735. [DOI] [PubMed] [Google Scholar]
- 20.Freeman ME, Smith MS, Nazian SJ, Neill JD. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology. 1973;94:875–882. doi: 10.1210/endo-94-3-875. [DOI] [PubMed] [Google Scholar]
- 21.Stirland JA, Hastings MH, Loudon AS, Maywood ES. The tau mutation in the Syrian hamster alters the photoperiodic responsiveness of the gonadal axis to melatonin signal frequency. Endocrinology. 1996;137:2183–2186. doi: 10.1210/endo.137.5.8612567. [DOI] [PubMed] [Google Scholar]
- 22.Smith MS. Role of prolactin in mammalian reproduction. In: Greep RO, editor. Reproductive Physiology III. University Park Press; Baltimore: 1980. pp. 249–276. [PubMed] [Google Scholar]
- 23.Linzer DIH, Fisher SJ. The placenta and the prolactin family of hormones: regulation of the physiology of pregnancy. Mol Endo. 1999;13:837–840. doi: 10.1210/mend.13.6.0286. [DOI] [PubMed] [Google Scholar]
- 24.Palm IF, Van der Beek EM, Swarts HJM, Van der Vliet J, Wiegant VM, Buijs RM, Kalsbeek A. Control of the estradiol-induced prolactin surge by the suprachiasmatic nucleus. Endocrinology. 2001;142:2296–2302. doi: 10.1210/endo.142.6.8219. [DOI] [PubMed] [Google Scholar]
- 25.Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56:239–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- 26.Gore AC. Diurnal rhythmicity of gonadotropin-releasing hormone gene expression in the rat. Neuroendocrinology. 1998;197:257–263. doi: 10.1159/000054373. [DOI] [PubMed] [Google Scholar]
- 27.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 28.Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neuroscience. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillespie JMA, Chan BPK, Roy D, Cai F, Belsham DD. Expression of circadian rhythm genes in gonadotropin-releasing hormone-secreting GT1-7 neurons. Endocrinology. 2003;144:5285–5292. doi: 10.1210/en.2003-0802. [DOI] [PubMed] [Google Scholar]
- 31.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 32.Smith MJ, Jennes L, Wise PM. Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology. 2000;141:4317–4320. doi: 10.1210/endo.141.11.7876. [DOI] [PubMed] [Google Scholar]
- 33.Krajnak K, Rosewell KL, Wise PM. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod. 2001;64:1160–1164. doi: 10.1095/biolreprod64.4.1160. [DOI] [PubMed] [Google Scholar]
- 34.Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- 35.Watson RE, Jr, Langub MC, Jr, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res. 1995;689:254–264. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- 36.Palm IF, Van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience. 1999;93:659–666. doi: 10.1016/s0306-4522(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 37.Jin X, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 38.Greig F, Weisz J. Preovulatory levels of luteinizing hormone, the critical period and ovulation in rats. J Endocrinol. 1973;57:235–245. doi: 10.1677/joe.0.0570235. [DOI] [PubMed] [Google Scholar]
- 39.Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O’Malley BW, Levine JE. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140:3653–3658. doi: 10.1210/endo.140.8.6895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.