Abstract
Cytomegalovirus (CMV) induces strong and long-lasting immune responses, which make it an attractive candidate for a cancer vaccine vector. In this study, we tested whether a tumor antigen expressed in CMV can induce a strong anti-tumor effect. We expressed an unmodified melanoma antigen, mouse tyrosinase-related protein 2 (TRP2), in mouse cytomegalovirus (MCMV). Prophylactic vaccination of the mice with a single dose of MCMV-TRP2 induced rejection of B16 melanoma challenge; therapeutic vaccination with MCMV-TRP2 prolonged the survival of the mice challenged with B16 cells. Additionally, vaccination with MCMV-TRP2 five months before tumor challenge still induced tumor rejection, which indicated that the vaccine induced long-term protection. Furthermore, MCMV-TRP2 protected mice against B16 melanoma challenge regardless of the pre-existing CMV infection. We found that vaccination with MCMV-TRP2 induced long-lasting TRP2 specific antibodies but not CD8 T cells. In addition, depletion of CD4 and CD8 T cells did not compromise the antitumor effect by MCMV-TRP2; while in B cell deficient (μMT) mice, the vaccine lost its antitumor effect. These results indicate that antibodies, not T cells, are important in mediating the antitumor effect during the effector phase by the vaccine. We also made a spread deficient MCMV-TRP2 lacking the essential glycoprotein gL, which showed a similar antitumor effect. In conclusion, our study indicates that tumor antigen (TRP2) expressed in MCMV induces a strong and long-lasting anti-melanoma effect through an antibody-dependent mechanism. Our findings demonstrate that CMV might be a promising vector for the development of cancer vaccines.
Keywords: Cytomegalovirus, Melanoma, Tyrosinase related protein 2, Cancer vaccine
Introduction
Cytomegalovirus (CMV) infection drives long-lived, high-level humoral and cellular immune responses. It is particularly notable for the CD8 T cell response it elicits, which persists at high levels and even increases over time, a phenomenon known as “memory inflation” [1]. In healthy humans, CMV-specific T cells permanently comprise an average of 5% of total CD4 and CD8 T cell compartments[2]; high T cell responses are also seen in experimentally infected mice [3,4,5]. Support for the idea of using CMV as a vaccine vector was obtained when Karrer et al reported that recombinant CMV expressing viral antigens drove similarly high-level T cell responses and was protective against viral challenge in mice[6]. Notably, protective immunity in that study actually increased with time post vaccination. Strong and long-lasting antigen specific immune responses are highly desirable for a cancer vaccine; a vaccine that maintains high level responses long term may guard against late recurrence. Therefore, CMV is an attractive vector for the development of cancer vaccines. Interest in CMV as a vaccine vector has intensified recently, when a CMV-based vaccine provided dramatic protection against SIV in the monkey models of AIDS[7,8].
Unlike SIV antigens, tumor antigens are usually self-antigens, which normally don’t induce strong immune responses. However, it has been shown that CMV expressing an ovarian antigen, Zona Pellucida 3 (ZP3), was able to break self-tolerance and induce profound autoimmune infertility[9]. Based on this precedent, we thought that tumor antigens delivered by CMV might be able to overcome self-tolerance and induce antitumor immune responses.
In this study, we sought to determine whether CMV expressing a melanoma antigen could induce anti-tumor immunity in the B16 mouse melanoma model. We constructed a murine CMV (MCMV) vaccine containing an expression cassette of the mouse TRP2 gene. We also generated a spread deficient version of the vaccine. We report here our initial characterization of the CMV-based cancer vaccines.
Methods and materials
Mice, cell lines and antibodies
Six to eight-week old female C57BL/6 mice and breeders of B cell deficient mice (μMT) were obtained from Jackson Laboratories (Bar Harbor, ME). All animal studies were approved by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at Oregon Health and Sciences University. B16F10 cells were from Dr Hongming Hu at Providence Hospital, Portland, Oregon. Cells were passaged and maintained in complete DMEM medium supplemented with 5% FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. TRP2 antibody was from Abcam (Cambridge, MA). Anti-CD4 (GK1.5) and anti-CD8 (2.43) were from Bio-X-Cell (West Lebanon, NH). Eflu450 conjugated anti-mouse CD8 and PE conjugated anti-mouse IFNγ were from eBiosciences (San Diego, CA).
Tumor challenge and vaccinations
In the prophylactic setting, mice were intraperitoneally (i.p.) immunized with MCMV or MCMV-TRP2 and one week later the mice were subcutaneously (s.c) challenged with 2×105 B16/F10 cells in 100ul PBS in the shaved right flank. In the therapeutic setting, mice were s.c challenged with 2×105 B16/F10 cells in 100ul PBS and 3 days later the mice were immunized with MCMV or MCMV-TRP2. Unless otherwise indicated, each group comprised 5 mice and each mouse was immunized i.p. with 4 × 106 pfu of MCMV or MCMV-TRP2. Tumor size was monitored with a caliper every 2–3 days by measuring two perpendicular tumor diameters and presented as the mean of the two diameters.
Western blot
To analyze protein expression, whole cell protein was extracted from MCMV-TRP2 infected NIH3T3 cells and immunoblotting was conducted. Briefly, protein extract was added to 3x sample buffer (125 mM Tris-HCl, pH 6.8, 1% SDS, 2% 2-ME, and 0.01% bromophenol blue), boiled for 5 min, and loaded onto a 10% SDS-polyacrylamide gel. After separation, proteins were transferred to a PVDF membrane by electroblotting (Bio-Rad). The membrane was blocked with 5% nonfat milk in TBST [TBS with 0.1% (w/v) Tween 20] for 1 h at room temperature, probed for overnight at 4°C with TRP2 specific antibody. After three washes, the membranes were incubated with an HRP-linked secondary antibody for 1 h at room temperature. The membranes were then washed 3 times with 1xTBST, and protein bands were visualized by chemiluminescence (Amersham Biosciences).
Flow cytometry
For measurement of intracellular IFN-γ, blood was withdrawn from the mice through tail bleeding and red blood cell lysis was performed with ammonium chloride (150 mM NH4Cl, 10 mM NaHCO3), and the remaining cells were stimulated with TRP2 peptide (TRP2 180-188: SVYDFFVWL; synthesized by Neobioscience, Cambridge, MA) at a final concentration of 1 μM in the presence of brefeldin A for 6 h at 37°C, 5% CO2. After that, cells were harvested, washed once with PBS, and stained with eflu450-conjugated anti-CD8 for 30 min at room temperature, and then washed three times with PBS. Cells were incubated in Fix/Perm buffer (BD biosciences) for 20 min at 4°C. After washing, cells were stained with PE-conjugated anti-IFNγ in Perm/wash buffer for 30 min at room temperature. Cells were then acquired using a LSR-II cytometer (BD Immunocytometry), with Flowjo software for data analysis.
TRP2 specific antibodies in mouse sera were assessed by a flow cytometry adapted methodology reported previously[10]. Serum (1:100 dilution) from vaccine-immunized mice was incubated with B16F10 cells, and then stained with FITC-anti mouse IgG and measured by flow cytometer. The level of antibodies was presented as mean fluorescent intensity (MFI).
Cell depletion
CD4, CD8 T cells were depleted by intrapeitoneal adminstration of antiCD4 (GK1.5), anti-CD8 (2.43) or a control antibody (rat IgG) at a dose of 300 μg every 3 days for a total of 5 times. The injection of antibodies was started 2 days before the tumor challenge. Cell depletion efficiency was over 95% in the blood three days after the first injection as determined by flow cytometry analysis.
Sera transfer
Sera from mice vaccinated with MCMV-TRP2 were pooled. 500 μl of the pooled sera was transferred intraperitoneally to naïve B6 mice every 3–4 days for a total of five times. Mice were challenged subcutaneously with B16 cells (2×105 cells/mouse) on the day of the second sera transfer.
Statistics
Difference in tumor incidence and survival were evaluated by log-rank test. P<0.05 was defined as significant difference.
Results
MCMV-TRP2 induces anti-tumor immunity in prophylactic and therapeutic settings
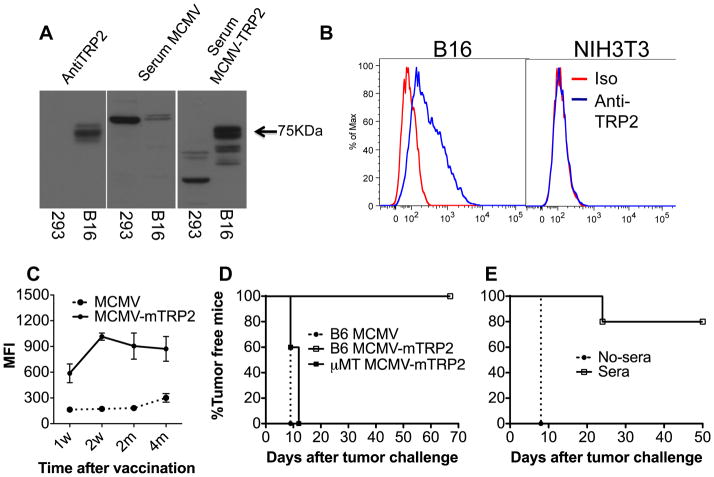
MCMV-TRP2 was constructed and the expression of TRP2 was confirmed in MCMV-TRP2-infected cells by Western blot (see the supplements for details). To see whether MCMV-TRP2 induced anti-tumor immunity, we vaccinated mice i.p with MCMV- TRP2 or control vector (MCMV). Seven days later, mice were challenged with B16-F10 melanoma cells. The vector-vaccinated mice all developed tumor with 2 weeks, while the MCMV-TRP2 vaccinated mice completely rejected the tumor challenge (Figure 1A). These mice have remained tumor free for the entire observation period (more than four months after the tumor challenge).
Fig 1. MCMV-TRP2 induced potent antitumor effect.
(A) Antitumor effect of MCMV-TRP2 in a prophylactic setting. Mice were vaccinated i.p with 4 × 106 pfu of MCMV- TRP2 or control vector (MCMV). Seven days later, mice were challenged with 2 × 105 B16-F10 melanoma cells by s.c. injection into the flank. (B) Antitumor effect of MCMV-TRP2 in a therapeutic setting. Mice were injected with 2 × 105 B16-F10 cells s.c. and then vaccinated with a single injection of MCMV-TRP2 or MCMV 3 days later. * p<0.05.
We next assessed the ability of MCMV-TRP2 to protect mice in a therapeutic model. Mice were injected with 2 × 105 B16-F10 cells s.c. and then vaccinated with a single injection of MCMV-TRP2 3 days later. MCMV-TRP2 significantly delayed the tumor onset in the vaccinated mice compared to that in the vector-vaccinated mice (Figure 1B). Together, these results indicate that TRP2 delivered by MCMV elicited significant anti-tumor effect in both prophylactic and therapeutic settings.
MCMV-TRP2-induced rejection of B16-F10 does not depend on T cells
To understand the mechanism mediating the antitumor effect by the vaccine, we first looked for TRP2 specific CD8 T cells. However, in direct ex vivo assays, we detected only very minimal CD8 T cells specific for the identified immunodominant trp-2 peptide in the peripheral blood either by intracellular cytokine staining for IFN-g (Figure 2A). In the same assay, robust responses to MCMV-peptides were detected (not shown).
Fig 2. T cells are not required during the effector phase.
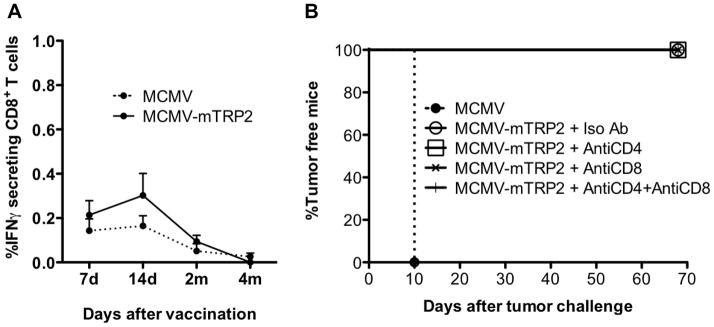
(A) TRP2 specific CD8+ T cell response elicited by MCMV-TRP2. Frequency of IFNγ secreting CD8 T cells was measured by intracellular staining and expressed as percentage of total CD8+ T cells (mean value ± SEM). (B) Groups of mice were immunized with MCMV or MCMV-TRP2. Seven days later, mice were challenged with B16 tumor cells. The mice were treated with antibodies to CD4 (GK1.5), CD8 (2.43) or control antibody (rat IgG) at a dose of 300 μg every 3 days for a total of 5 times. The injection of antibodies was started 2 days before the tumor challenge. Data shown are the representative of two independent experiments.
To determine whether T cells played a role in tumor rejection induced by MCMV-TRP2, we depleted CD4 and CD8 T cells from MCMV-TRP2-vaccinated mice prior to tumor challenge (Figure 2B). In a control experiment, this regimen depleted more than 95% of T cells from the peripheral blood (not shown). Vector-vaccinated mice developed tumors as before. However, MCMV-TRP2 vaccinated mice completely rejected tumor challenge regardless of their T cell depletion status (Figure 2B). The combination of our inability to detect a CD8+ T cell response and the lack of impact on tumor rejection after CD8+ T cell depletion strongly suggests that T cells do not play a critical role during the effector phase of tumor rejection in MCMV-TRP2 vaccinated mice.
The humoral response is important for MCMV-TRP2 induced tumor rejection
We next asked whether humoral immunity plays a role in mediating the antitumor effect by the vaccine. We first determined whether MCMV-TRP2 induced TRP2 specific antibodies. Figure 3A shows a result of western blot using serum from MCMV-TRP2-vaccinated and control mice. Serum from MCMV-TRP2- but not control-vaccinated mice recognized a 75kDa band, the expected MW of TRP2, which was also recognized by a commercial anti-TRP2 antibody. We concluded that MCMV-TRP2 did induce anti-TRP2 antibodies.
Fig 3. Humoral immunity is required for the antitumor effect by MCMV-TRP2.
(A) Vaccination induced TRP2 specific antibodies. TRP2 specific antibodies were measured by Western blot with cell lysate of B16 cells or 293 cells (as a negative control). A commercial TRP2 specific antibody was used as the positive control. (B) TRP2 was expressed on the cell surface of B16 melanoma cells. B16 and NIH3T3 cells were stained with sera containing antiTRP2 antibodies and analyzed by flow cytometry. (C) TRP2 specific antibodies persist at high levels after the vaccination. (D) Wt B6 mice and μMT mice were immunized with MCMV-TRP2. Seven days later, mice were challenged with B16 tumor cells. (E) Naïve B6 mice were transferred with sera from mice immunized with MCMV-TRP2. Mice were challenged with B16 tumor cells on the day when mice receiving the second sera transfer.
However, it was unclear how anti-TRP2 antibody could mediate anti-tumor immunity, since TRP2 is an intracellular protein, residing in melanosomes. Melanosomes are exocytic vesicles, and we considered that some melanosomal proteins may be expressed on the cell surface. To see if B16-F10 expressed any TRP2 on the cell surface, we stained B16 melanoma cells with serum from MCMV-TRP2 vaccinated mice and analyzed them by FACS. Figure 3B shows that B16 cells did express TRP2 on the cell surface.
We also monitored the antibody responses over time after the MCMV-TRP2 vaccination. As shown in Figure 3C, MCMV-TRP2 induced long -TRP2 specific antibodies. The antibody amount remained at high levels for the entire observation period (5 months).
The high level of TRP2 specific antibodies and the lack of effect of CD8 T cell depletion suggested that humoral immunity was responsible for the antitumor effect by the vaccine. Therefore, we studied the antitumor effect by the vaccine in B cell deficient mice (μMT mice). In μMT mice the vaccination did not protect the mice against B16 tumor challenge (Figure 3D), indicating that TRP2 specific humoral immunity plays an essential role in the antitumor effect by the vaccine.
To further confirm the role of the TRP2 specific humoral immunity in the antitumor effect by the vaccine, serum from mice immunized with MCMV-TRP2 was injected intraperitoneally into naïve mice, which were then challenged with B16F10 cells. B16-F10 tumors grew at normal rates in the mice without sera transfer, but tumor growth was significantly inhibited in mice treated with the sera from MCMV-TRP2 vaccinated mice (Figure 3E). Only one mouse that had received serum developed a tumor; and the onset of tumor in that mouse was significantly delayed. This finding supports the conclusion that antiTRP2 antibody elicited by MCMV-TRP-2 vaccination is not only necessary but also sufficient to protect mice against B16 tumor challenge.
Spread-deficient MCMV-TRP2 showed a similar antitumor effect
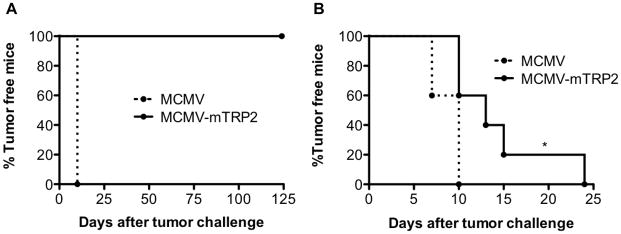
Based on our previous work demonstrating the sustained immunogenicity of a spread-deficient MCMV[11], we deleted the essential glycoprotein L (gL), which is required for the MCMV entry into target cells, from the MCMV-TRP2 vaccine. ΔgL-MCMV-TRP2 was generated as described in the supplement. As shown in Figure 4A, gL deficient MCMV-TRP2 provided a similar protection against B16 tumor challenge as the normal MCMV-TRP2 did. This result indicates that the gL deficient CMV can be used for future development of CMV-based cancer vaccines.
Fig 4. Spread deficient MCMV-TRP2 showed similar antitumor effect.

(A) B6 mice were immunized with MCMV, MCMV-TRP2 or ΔgL-MCMV-TRP2. Seven days later, mice were challenged with B16 tumor cells. (B) Long-term protection by MCMV-TRP2. Mice received a single i.P. injection of MCMV-TRP vaccines 5 months before the subcutaneous B16 tumor challenge. (C) MCMV-TRP2 protected mice against tumor challenge regardless of pre-existing CMV infection. Mice were infected with 2×105 MCMV three months before the vaccination. They were challenged with B16 cells seven days after the vaccination. MCMV group has six mice; and both MCMV-TRP2 and ΔgL-MCMV-TRP2 groups have seven mice.
Long-term antitumor effect and the antitumor effect with the presence of pre-existing MCMV infection
Since MCMV-TRP2 induced long-term immune responses, we thought it might also induce a long-term antitumor effect. To assess the long-term antitumor effect by the vaccines, we vaccinated mice with MCMV-TRP2, ΔgL-MCMV-TRP2 or MCMV and five months later, the mice were challenged with B16F10 cells. We found that a single dose of the vaccines inhibited the tumor growth significantly (Figure 4B), indicating that MCMV-TRP2 induces long-term protection against tumor.
To see if the vaccines were able to induce an antitumor effect in mice with prior immunity to the MCMV vector, we infected mice with MCMV and 3 months later, immunized them with the vaccines or MCMV. As shown in Figure 4C, the vaccines still provided protection against the tumor challenge regardless of the pre-existing CMV immunity.
Discussion
This study shows that CMV expressing a tumor antigen, TRP2, induces a strong antitumor effect in mice. Though CMV has been used as a vaccine vector for infectious diseases [7,8], it has not previously been tested as a cancer vaccine vector. Unlike a vaccine vector for infectious diseases, which delivers foreign antigens targeting the pathogens, a cancer vaccine vector delivers self-antigens. Tolerance to self/tumor antigens makes it harder to generate efficient anticancer immune responses. However, our findings show that a single immunization with CMV expressing an unaltered TRP2 induced an antitumor immune response which was strong enough to reject a B16F10 tumor challenge.
Notably, our CMV-based cancer vaccines conferred a long-term protection. Five months after a single dose of the vaccine, mice were still protected from a B16 challenge. This was particularly striking in the case of the ΔgL-MCMV-TRP2 vaccine, which is unable to spread from the cells it first infects. One of the main goals of cancer vaccine is to prevent cancer recurrence in a long term. Therefore, the long-lasting antitumor effect induced by a CMV-based vaccine would be ideal for the purpose to prevent cancer recurrence. We also found that the vaccination still protected mice regardless of the pre-existing CMV infection, replicating the phenomenon reported in the SIV/CMV-based vaccines in monkeys[7]. This result suggests that, as was described for SIV vaccines, CMV-based cancer vaccines could be administered repeatedly to boost responses, and CMV-based vaccines expressing different tumor antigens could be used in sequence. Given that more than half of the human population is infected with CMV, this result also suggests that CMV-based cancer vaccines could be used in both CMV negative and positive patients. The fact that a completely spread-deficient vaccine is effective, and that CMV-based vaccines are not impaired by prior vector immunity are two highly attractive features for this technology. Furthermore, the fact that immunity is long-lasting suggests that CMV-based vaccines may be developed to play a niche role in multi-pronged immunotherapy: providing ongoing immune surveillance as the immunity elicited by other agents wanes.
Interestingly, in our study, the antitumor effect by CMV-TRP2 during the effector phase was mediated by antibodies, and we found no evidence for a role of CD8 T cells. This is in contrast to the vast majority of melanoma vaccines, including those using TRP-2 as an antigen, in which CD8 T cell mediated immunity is critical for the anti-tumor effect. We think that unique features of the different vectors probably determine the relative importance of the different antitumor mechanisms. These factors could include levels and kinetics of antigen expression, different APCs, the impact of the vector on antigen presentation and also the cytokine environment. It is unclear at present why this vaccine formulation did not induce strong CD8 T cell responses to TRP2; we are currently investigating alternative modes of antigen presentation to overcome this problem.
Although CMV is well known for its ability to induce CMV specific CD8 T cell memory inflation, we did not observe TRP2 specific memory inflation of CD8 T cells after MCMV-TRP2 vaccination. We think that low immunogenicity and peripheral tolerance mechanisms were involved in the poor response to self-antigens. Recently, Klyushnenkova et al. reported that MCMV delivering human prostate antigen induced CD8 T cells responses in a “humanized” mice[12]. Though the antigen specific CD8 T cells were still at low frequency, it was very encouraging. In their study, a m157-deleted MCMV was used to construct the vaccines. It is not clear whether the deletion of m157 helped inducing antigen specific CD8 T cell responses.
We are currently exploring approaches that have been utilized with other vectors to increase immunogenicity for CD8+ T cells, including prime boost strategies and expression of modified peptide epitopes [13,14]. Ultimately, our goal is to elicit both antibody and CD8+ T cell responses for more potent antitumor effects, as has been reported previously [15,16], while taking advantage of CMV’s ability to sustain and increase those responses over time.
In conclusion, our findings demonstrate that a tumor antigen (TRP2) expressed in MCMV induces a strong and long-lasting anti-melanoma effect through an antibody dependent mechanism. We believe that CMV is a promising vector for the development of novel cancer vaccines.
Supplementary Material
Highlights.
MCMV-TRP2 showed prophylactic and therapeutic anti-melanoma effect.
MCMV-TRP2 showed anti-tumor effect regardless of the pre-existing CMV infection.
Antibodies, not T cells, are important in mediating the antitumor effect by the vaccine.
A spread deficient MCMV-TRP2 showed a similar antitumor effect.
CMV might be a promising vector for the development of cancer vaccines.
Acknowledgments
We thank Heather Grey (OHSU) for her help in BAC cloning, and Hongming Hu and Bernard Fox (Earle Chiles Cancer Research Center, Providence Hospital, Portland, OR) for advice and the gift of B16-F10 melanoma cells. This work was supported by the National Cancer Institute (NCI) grant R21 (CA127181).
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 2.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 5.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd ML, Shellam GR, Papadimitriou JM, Lawson MA. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol Reprod. 2003;68:2024–2032. doi: 10.1095/biolreprod.102.012880. [DOI] [PubMed] [Google Scholar]
- 10.Piechocki MP, Pilon SA, Wei WZ. Quantitative measurement of anti-ErbB-2 antibody by flow cytometry and ELISA. J Immunol Methods. 2002;259:33–42. doi: 10.1016/s0022-1759(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 11.Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog. 2011;7:e1002295. doi: 10.1371/journal.ppat.1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, Caposio P, Botto S, Alexander RB, Jarvis MA. A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J Immunother. 2012;35:390–399. doi: 10.1097/CJI.0b013e3182585d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guevara-Patino JA, Engelhorn ME, Turk MJ, Liu C, Duan F, Rizzuto G, Cohen AD, Merghoub T, Wolchok JD, Houghton AN. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 2006;116:1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Stipdonk MJ, Badia-Martinez D, Sluijter M, Offringa R, van Hall T, Achour A. Design of agonistic altered peptides for the robust induction of CTL directed towards H-2Db in complex with the melanoma-associated epitope gp100. Cancer Res. 2009;69:7784–7792. doi: 10.1158/0008-5472.CAN-09-1724. [DOI] [PubMed] [Google Scholar]
- 15.Orlandi F, Guevara-Patino JA, Merghoub T, Wolchok JD, Houghton AN, Gregor PD. Combination of epitope-optimized DNA vaccination and passive infusion of monoclonal antibody against HER2/neu leads to breast tumor regression in mice. Vaccine. 2011;29:3646–3654. doi: 10.1016/j.vaccine.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Orlandi F, Venanzi FM, Concetti A, Yamauchi H, Tiwari S, Norton L, Wolchok JD, Houghton AN, Gregor PD. Antibody and CD8+ T cell responses against HER2/neu required for tumor eradication after DNA immunization with a Flt-3 ligand fusion vaccine. Clin Cancer Res. 2007;13:6195–6203. doi: 10.1158/1078-0432.CCR-07-0258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.