Summary
Anecdotally, middle-aged listeners report difficulty conversing in social settings, even when they have normal audiometric thresholds [1–3]. Moreover, young adult listeners with “normal” hearing vary in their ability to selectively attend to speech amid similar streams of speech. Ignoring age, these individual differences correlate with physiological differences in temporal coding precision present in the auditory brainstem, suggesting that the fidelity of encoding of suprathreshold sound helps explain individual differences [4]. Here, we revisit the conundrum of whether early aging influences an individual’s ability to communicate in everyday settings. Although absolute selective attention ability is not predicted by age, reverberant energy interferes more with selective attention as age increases. Breaking the brainstem response down into components corresponding to coding of stimulus fine structure and envelope, we find that age alters which brainstem component predicts performance. Specifically, middle-aged listeners appear to rely heavily on temporal fine structure, which is more disrupted by reverberant energy than temporal envelope structure is. In contrast, the fidelity of envelope cues predicts performance in younger adults. These results hint that temporal envelope cues influence spatial hearing in reverberant settings more than is commonly appreciated and help explain why middle-aged listeners have particular difficulty communicating in daily life.
Results and Discussion
Selective attention is essential for coping with the bewildering swirl of voices, noises, and reflected sound energy we encounter daily. Acoustic features (onsets, offsets, harmonicity, etc.) enable the auditory system to group related sound components into perceptual objects, each an estimate of one common, physical source [5–7] to which listeners can direct selective attention [7–9]. Although sound segregation cues are sometimes redundant [10, 11], many are degraded by reverberant energy [4, 12–14]. Thus, in everyday settings, listeners may require multiple segregation cues to support selective attention even in cases when a single cue is sufficient in an anechoic setting.
Motivated by these observations, we hypothesized that reverberant energy may reveal perceptual differences not observed in anechoic conditions. Specifically, we reasoned that reverberation might expose effects of early aging, which could help explain why middle-aged listeners report difficulties in challenging listening conditions [2, 15, 16]. In an initial study with young adult to middle-aged normal-hearing listeners (designed to emphasize sensory effects and minimize cognitive factors), we found that reverberant energy interferes with the ability to selectively attend to a speech stream amid similar competing streams [4]. However, we found no effect of age on performance. Instead, even though all listeners had clinically normal hearing thresholds, we found large differences in performance, even among young adult listeners.
Consistent with past studies reporting correlations between the fidelity of brainstem encoding and perceptual ability [17–19], a follow-up study using a subset of the original listeners (recruited from the top and bottom quartiles of the original group) revealed that the overall strength of the frequency following response (FFR, a measure of the sustained brainstem response to a periodic acoustic input measured via scalp electrodes; see [20]) correlates with how well listeners perform in our spatial selective attention task, which requires listeners to use interaural timing differences (ITDs, differences in the timing of the left- and right-ear signals) to focus spatial attention on the center, target speech stream [21]. These results suggest that the fidelity of coding of temporal information in suprathreshold sound impacts the ability to direct attention to understand one sound source amid other sources.
Here, we revisited whether early aging affects selective attention in realistic settings. We recruited five additional middle-aged listeners (total of 22 listeners, age 20.9–54.7 years) and measured selective attention performance and FFR responses for these additional listeners. Previously [21], we analyzed only the overall strength of the FFR at the fundamental frequency (F0) of the input stimulus (the syllable/dah/with F0 = 100 Hz [20]). Here, we broke down the FFR into orthogonal components representing contributions from neural phase locking to the periodic stimulus envelope (FFRENV) and from phase locking to the carrier (FFRCAR; see also [17, 22–24]), analyzing responses both at F0 and at its harmonics.
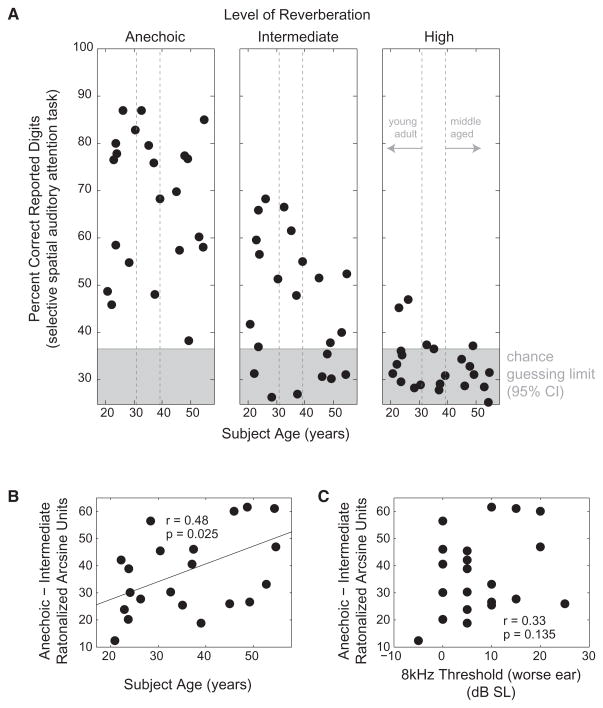
As reported previously [4, 21], performance decreased as reverberant energy increased, reaching chance levels for the majority of listeners in the highest tested reverberation level (Figure 1A; in the high reverberation condition, only five subjects performed significantly above the 33.3% probability of guessing digits correctly from among the three streams [4, 21]). Henceforth, we consider only anechoic and intermediate reverberation results.
Figure 1. The Ability to Direct Spatial Selective Auditory Attention Is Not Related Directly to Age, but the Impact of Reverberation Increases with Age.
(A) Percentage of target digits correctly reported as a function of individual listener age for the three room conditions. Dashed vertical lines denote “young adult” and “middle-aged” cutoffs used in visualizing age interactions (see Figures 3A and 3B). In the high reverberation condition, the majority of listeners performed no better than chance, based on calculations of the 95% confidence interval for a binomial distribution of 600 independent trials.
(B) Change in performance from anechoic to intermediate reverberation conditions (in rationalized arcsine units [RAUs]) as a function of age. The cost of adding reverberant energy increases as age increases.
(C) Change in performance from anechoic to intermediate reverberation conditions (in RAUs) as a function of the threshold of hearing in the worse ear at 8 kHz. The effect of reverberant energy on selective attention performance is unrelated to high-frequency thresholds of audibility.
Different FFR Components Dominate at Different Frequencies
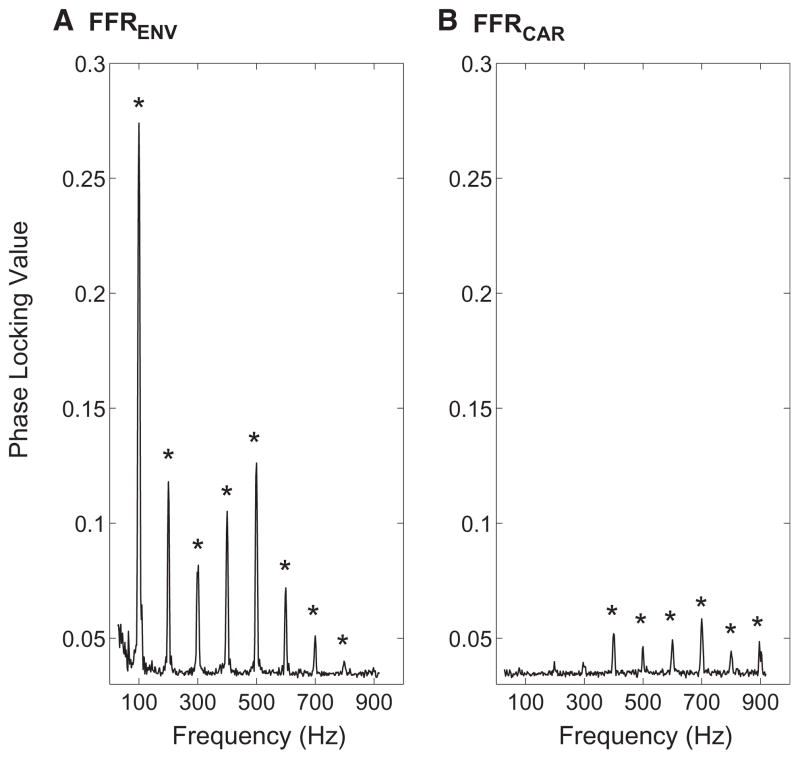
The envelope component dominates FFR responses at F0 and lower harmonics, whereas the carrier component dominates at higher harmonics (Figure 2). Computational models [25, 26] suggest that the envelope component at F0 (FFRENV-100) reflects a sum of responses from peripheral auditory channels tuned to ~1,000 Hz and above, which are excited by multiple harmonics and thus driven by a periodic signal (100 Hz fundamental). Conversely, the strength of FFRCAR at a given frequency likely reflects phase locking to temporal fine structure (TFS) in the narrow range of auditory peripheral channels tuned to that same acoustic frequency. Here, we used FFRENV-100 to quantify individual differences in envelope coding and the average of FFRCAR for four harmonics (600–900 Hz, where FFRCAR dominates the response) to quantify coding of carrier temporal structure (FFRCAR-AVE). Importantly, FFRENV-100 and FFRCAR-AVE were not significantly correlated (r = 0.03, p = 0.905, n = 22), supporting the idea that they reflect different aspects of temporal coding precision driven by different tonotopic portions of the auditory pathway.
Figure 2. Physiological Measures of Brainstem Temporal Coding Correlate with Behavior.
Phase locking in the brainstem FFR envelope (FFRENV) and carrier (FFRCAR) are significant only at stimulus harmonics. Mean phase-locking value (PLV) is shown as a function of frequency, averaged across the 22 subjects. Asterisks mark frequencies for which the mean of the PLV distribution at a given frequency is significantly greater than zero (single-tailed t test: p < 0.05, corrected for multiple comparisons), based on bootstrapping (see Experimental Procedures).
(A) FFRENV is largest at the lowest harmonics and is significant at all stimulus harmonics up to 800 Hz.
(B) FFRCAR is significant for stimulus harmonics from 400 Hz to 900 Hz.
Effects of Reverberation Increase with Age
We performed a multiway, repeated-measure ANOVA on the selective attention behavioral results with factors of reverberation level, age, FFRENV-100, and FFRCAR-AVE (treating reverberation level as categorical and all other factors as continuous). To alleviate floor and ceiling effects, we first transformed the percent correct scores into rationalized arcsine units (RAUs; see Experimental Procedures) [27]. Although we found no statistically significant effect of age on selective attention performance [Figure 1A; F(1,16) = 1.42, p = 0.251], there was a statistically significant interaction between age and reverberation level [F(1,16) = 5.88, p = 0.025] and a significant main effect of reverberation level [F(1,16) = 155.17, p = 7.01 × 10−11]. Although age did not predict how well individual listeners performed, the toll that reverberation took on performance of individual listeners increased with age (Figure 1B; ANOVA result confirmed by regression: r = 0.48, p = 0.025, n = 22).
Age Influences which FFR Component Predicts Selective Attention Ability
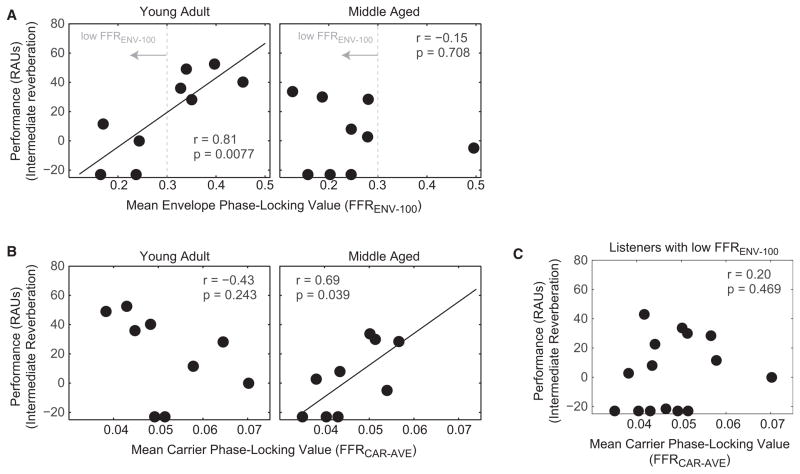
We found previously that the total FFR strength at 100 Hz (a measure dominated by envelope phase locking; see Figure 2) predicted selective attention ability [21]. Consistent with this, the main effect of FFRENV-100 was significant [F(1,16) = 5.03, p = 0.040]; however, the importance of this effect was superseded by a significant interaction between age and FFRENV-100 [F(1,16) = 4.64, p = 0.048]. We also found a significant interaction between age and FFRCAR-AVE [F(1,16) = 4.64, p = 0.047], with no main effect of FFRCAR-AVE [F(1,16) = 0.216, p = 0.649]. The ANOVA regression coefficients revealed that FFRENV-100 was a better predictor of selective attention performance the younger a listener was, whereas FFRCAR-AVE was a better predictor the older the listener was. These results suggest that FFRENV-100 and FFRCAR-AVE reflect different perceptual cues for directing auditory attention that are weighted differently as listeners age.
We visualized the significant interactions between age and the two FFR components by plotting intermediate reverberation performance for a “young adult” group (nine listeners < 31 years) and a “middle-aged” group (nine listeners > 39 years, omitting four listeners in their mid-30s) as a function of FFRENV-100 and of FFRCAR-AVE. (This division into younger and middle-aged groups was arbitrary, undertaken to illustrate the significant interactions uncovered by the ANOVA.) Consistent with the ANOVA, FFRENV-100 (Figure 3A) predicted performance for young adults (r = 0.81, p = 0.008, n = 9), but was low for all but one of the middle-aged listeners and was not predictive of their selective attention ability (r = −0.15, p = 0.708, n = 9). Conversely, FFRCAR-AVE (Figure 3B) was not statistically related to performance in young adults (r = −0.43, p = 0.243, n = 9) but was for our middle-aged listeners (r = 0.69, p = 0.039, n = 9).
Figure 3. The Orthogonal Components of FFR Envelope and FFR Carrier Are Related to Selective Auditory Attention Performance Differently in Young Adult and Middle-Aged Listeners.
(A) FFRENV-100 is significantly correlated with performance in young adult but not middle-aged listeners. Moreover, the envelope component of the FFR at the stimulus fundamental frequency (F0) is low for all but one of the middle-aged listeners. Dashed vertical lines show the cutoff used to define a “low FFRENV-100” subject group (see C).
(B) FFRCAR-AVE is statistically unrelated to performance in young adult listeners; however, in middle-aged listeners, FFRCAR-AVE is positively correlated with selective attention performance.
(C) FFRCAR-AVE is statistically unrelated to performance in all listeners with low FFRENV-100; thus, the interaction of age × FFR component seen in (A) and (B) is not due solely to the fact that most middle-aged listeners have low FFRCAR-AVE.
Weak Envelope Coding Does Not Explain Age Effects
These results raised the possibility that any listeners with weak FFRENV-100 rely on FFRCAR-AVE to direct spatial attention—that is, “age” effects could simply reflect a decrease in FFRENV-100 with age [28]. To test this, we selected all subjects whose FFRENV-100 was similar to the values of the majority of our middle-aged listeners (FFRENV-100 < 0.3; dashed vertical lines in Figure 3A). In contrast to the significant correlation found when considering listeners over age 39 (right panel of Figure 3B), there was no significant correlation between selective attention performance and FFRCAR-AVE for listeners with low FFRENV-100, ignoring age (r = 0.20, p = 0.47, n = 15; see Figure 3C). This result suggests that younger listeners obligatorily rely on mid-to-high-frequency information, so that the fidelity of temporal envelope coding predicts selective attention ability; in contrast, middle-aged listeners seem to rely on lower-frequency components to direct spatial attention.
A weak FFRENV-100 could arise from a generally feeble neural response to mid-to-high-frequency inputs, a robust but temporally imprecise response to those acoustic frequencies, or any combination thereof. We thus wondered whether younger and older listeners had weak FFRENV-100 responses for different reasons. Specifically, we hypothesized that the strength of the auditory response to suprathreshold sound in mid-to-high frequencies decreases by middle age, reducing the influence of envelope ITD and increasing the influence of carrier ITD on spatial perception. In contrast, younger listeners may generally have salient responses to mid-to-high-frequency stimulus content, which therefore have a strong influence on spatial judgments (whether temporally precise, yielding strong FFRENV-100 and good performance, or imprecise, producing weak FFRENV-100 and poor performance).
Elevated High-Frequency Thresholds Do Not Explain Age Effects
The strength of neural responses to suprathreshold mid-to-high-frequency sounds might be related to high-frequency audiometric thresholds. Moreover, aging is known to affect audiometric thresholds. Thus, high-frequency audiometric thresholds might be an even more direct predictor of which FFR cues correlate with selective attention ability than age is. Previously, we found no significant difference in hearing thresholds between younger and older listener groups [4]. However, here, treating age as a continuum, we found a significant correlation between age and the worse ear audiometric thresholds at 8 kHz (8kHzWORSE; r = 0.67, p = 5.9 × 10−4, n = 22).
To determine whether the significant interactions of age with reverberation level, FFRENV-100, and FFRCAR-AVE were related to high-frequency thresholds, we redid the main ANOVA with factors of reverberation level, FFRENV-100, and FFRCAR-AVE, but with 8kHzWORSE as a main factor instead of age. The only significant effect or interaction was the main effect of FFRENV-100 [F(1,16) = 5.210, p = 0.036 (again consistent with [21])]. Importantly, none of the significant interactions found when age was a factor were significant here [8kHzWORSE and reverberation level: F(1,16) = 2.42, p = 0.135; 8kHzWORSE and FFRENV-100: F(1,16) = 0.554, p = 0.467; 8kHzWORSE and FFRCAR-AVE: F(1,16) = 0.938, p = 0.3472]. In contrast to the significant age interaction that we found (Figure 1B), there was no significant correlation between 8kHzWORSE and the decrement in performance caused by reverberation (Figure 1C; r = 0.33, p = 0.135, n = 22). Thus, if our hypothesis is correct and age decreases the overall strength of neural responses to suprathreshold sounds, this effect is not strongly related to age effects on hearing threshold.
Implications
Previous studies found that aging reduces FFR strength [28]; however, not all studies found group age effects [29]. Moreover, even studies that found age-related group differences in brainstem responses have not consistently found corresponding differences in perceptual abilities [28]. The current study helps explain these discrepant findings. Brainstem response strength varies greatly even among young adults; by examining individual subjects and considering different components of the FFR, we find reliable interactions between age, behavioral performance, and specific components of the FFR.
We show that by early middle age, the FFR envelope component at the fundamental frequency of the stimulus tends to be weak, possibly because the neural response to suprathreshold sound in mid-to-high acoustic frequencies is reduced in overall strength. These changes may be an early indicator of hearing disability that is more sensitive than (and not strongly correlated with) age-related changes in hearing threshold. Physiological results hint at this: noise exposure that does not alter hearing threshold can still reduce the magnitude of suprathreshold neural responses [30]. These changes may come about because low-spontaneous-rate auditory nerve fibers, recruited as sound level increases, are particularly vulnerable to damage [31]. Additional experiments are needed to test this explanation for the early aging effects we see.
Many recent studies have addressed the impact of aging and hearing loss on TFS and its importance for understanding speech in noise [32–34]. Our results suggest that temporal coding of envelope information is also important for communicating in everyday environments and is also affected by age, consistent with previous reports that aging alters envelope temporal coding [35–37].
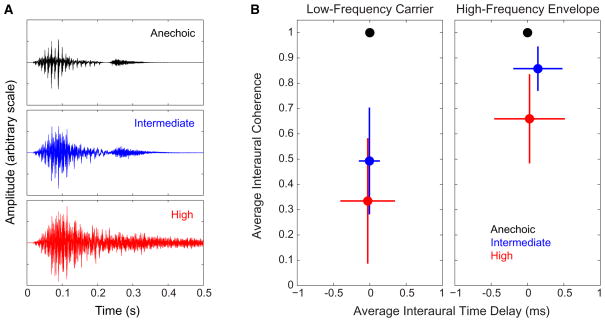
In our task, performance is primarily limited by the ability to extract source location and direct spatial auditory attention, which may help explain why here, performance depends on the fidelity of envelope temporal coding. Envelope ITD cues in high-frequency sounds carry spatial information [38, 39]; however, classic laboratory experiments have established that for wideband, anechoic sounds, low-frequency carrier ITDs perceptually dominate over high-frequency cues [38, 40, 41]. Our results suggest that in reverberant settings, high-frequency ITD cues, encoded in signal envelopes, may be more important for spatial perception than past laboratory studies suggest. In anechoic conditions, TFS and temporal envelope cues both provide reliable information for directing spatial selective auditory attention. Although reverberant energy disrupts both cues, envelope ITD is less affected ( Figure 4 ). Reverberation causes moment-to-moment fluctuations in carrier ITD, decreasing the interaural correlation and interfering with localization; in contrast, although reverberation decreases the depth of envelope modulations, the impact on high-frequency envelope ITDs is relatively modest.
Figure 4. Reverberant Energy Has a Greater Effect on Low-Frequency Carrier ITD Than on High-Frequency Envelope ITD.
(A) Reverberation reduces but does not destroy the temporal envelope structure of a sample digit (a token of the digit “eight”).
(B) Reverberation causes a greater disruption in average interaural coherence when computing low-frequency carrier interaural timing difference (ITD) than when computing high-frequency envelope ITD. Here, we analyzed the left-and right-ear signals generated from the sample token “eight” shown in (A), simulated from straight ahead (see Experimental Procedures). We used the Slaney MATLAB toolbox [42] (functions ERBSpace, MakeERBFilters, and ERBFilterBank) to simulate the signals driving 40 auditory nerve fibers (100–12,000 Hz). Using the Hilbert transform, we extracted the carrier components of the left-and right-ear signals in the 21 lowest-frequency fibers (100–1,840 Hz) and the envelope components of the signals in the 19 highest-frequency fibers (2.04–12 kHz). For each left/right auditory fiber pair, we then computed the normalized cross-correlation function and found the peak falling within the natural ITD range, from −1 to +1 ms. We computed the averages (points) and standard deviations (error bars) of both the peak heights (the interaural coherence) and the ITDs at which these peaks occurred (the primary left/right direction cue) for the population of low-frequency fibers (left, computed from the carrier waveforms in each ear) and the population of high-frequency fibers (right, computed from the envelope waveforms in each ear).
Our results hint that in reverberant settings, relatively reliable high-frequency envelope ITDs play a significant role in sound localization, at least for listeners who have a robust response to suprathreshold mid-to-high-frequency sound. This possibility points to the importance of high-frequency amplification in assistive listening devices [43], which typically consider only frequencies directly affecting speech intelligibility (below 8 kHz) [44]. If envelope ITD cues prove to be more important for spatial hearing in common acoustic settings than is currently appreciated, this also has implications for bilateral cochlear implants, which typically do not provide reliable TFS information. We conclude that middle-aged listeners rely on TFS cues, which are relatively fragile in ordinary listening environments, to direct selective auditory attention; this makes these listeners more vulnerable to the degrading effects of reverberation. In contrast, younger listeners depend more on envelope ITD cues, which are more robust in reverberation, protecting them from some of the communication difficulties that older listeners experience.
Experimental Procedures
Subjects
All subjects had average audiometric hearing thresholds of 20 dB hearing level (HL) or better for frequencies from 250 to 8,000 Hz and relatively symmetric hearing (left/right ear asymmetry of 15 dB or less at all frequencies). (Note that at 8 kHz, one 45-year-old listener had a threshold of 25 dB HL in one ear and 10 dB HL in the other ear; all other thresholds were 20 dB HL or better in both ears of every subject.) Of the 22 subjects, 17 were participants in earlier studies [4, 21]; the remaining 5 were recruited from the over-40 age group specifically for this study. All subjects gave informed consent as overseen by the Charles River Institutional Review Board.
Spatial Attention Task
All subjects performed the spatial selective attention task described in [4], in which they reported a sequence of four spoken digits simulated from directly in front while ignoring two competing digit streams, spoken by the same talker, simulated from 15° to the left and 15° to the right (all at eye level). (Note that the data from the 17 original subjects were previously reported in [4].) Three rectangular rooms were simulated, differing in wall absorption [4]. As discussed previously [4, 21], the primary cues for focusing spatial selective attention are interaural time differences, either in the stimulus carrier (at frequencies below~1,000 Hz) or in the stimulus envelope (for frequencies above ~1,000 Hz). Moreover, listeners rarely guessed a digit that was not physically present in one of the target or masker streams [4], a result showing that performance is not limited by the ability to recall digits but by the ability to isolate and attend to the digits in the target stream coming from straight ahead.
Prior to any statistical analyses, all percent correct scores were transformed into rationalized arcsine units (RAUs) to reduce the impact of floor and ceiling effects on the data [27]. Because chance performance on our selective attention task was determined by the likelihood of correctly guessing the target from among the three spoken digits, raw scores in the range 0.33–1.0 were linearly transformed to the range 0–1.0 (with scores below 0.33 set to 0) prior to applying the RAU transform.
Brainstem Measurement
FFRs were measured in response to a/dah/syllable presented in positive polarity for 2,000 trials and in inverted polarity for 2,000 trials [21]. As in our previous study, noisy trials were removed, leaving at least 1,800 clean trials for each subject, condition, and stimulus polarity. The time series from each trial was windowed with a first-order Slepian taper [45] and the Fourier transform was computed. We generated distributions of phase-locking values (PLVs) for different conditions using a bootstrapping procedure to produce 200 independent PLVs, each computed from a draw (with replacement) of 800 trials for a given subject and condition [21]. Unlike our previous analysis, here we broke the PLV into orthogonal envelope and carrier components (FFRENV and FFRCAR) at every frequency from 30 Hz to 3,000 Hz. FFRENV was calculated with equal draws from responses to each polarity, treating positive-and negative-polarity trials identically. FFRCAR was determined with equal draws from responses to each polarity, but inverting the phase of negative-polarity trials (see also [17, 22–24]).
Acknowledgments
This work was sponsored by the National Institutes of Health (NIDCD R01 DC009477 to B.G.S.-C. and F31 DC011463 to D.R.) and a National Security Science and Engineering Faculty Fellowship (to B.G.S.-C.). We wish to thank Sharon Kujawa for generously taking the time to discuss potential links between our results and the effects of noise exposure and aging on the anatomy and physiology of the auditory pathway.
References
- 1.Humes LE. The contributions of audibility and cognitive factors to the benefit provided by amplified speech to older adults. J Am Acad Audiol. 2007;18:590–603. doi: 10.3766/jaaa.18.7.6. [DOI] [PubMed] [Google Scholar]
- 2.Leigh-Paffenroth ED, Elangovan S. Temporal processing in low-frequency channels: effects of age and hearing loss in middle-aged listeners. J Am Acad Audiol. 2011;22:393–404. doi: 10.3766/jaaa.22.7.2. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Frisina RD, Mapes FM, Hickman ED, Frisina DR. Effect of age on binaural speech intelligibility in normal hearing adults. Speech Commun. 2006;48:591–597. [Google Scholar]
- 4.Ruggles D, Shinn-Cunningham B. Spatial selective auditory attention in the presence of reverberant energy: individual differences in normal-hearing listeners. J Assoc Res Otolaryngol. 2011;12:395–405. doi: 10.1007/s10162-010-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darwin CJ. Auditory grouping. Trends Cogn Sci. 1997;1:327–333. doi: 10.1016/S1364-6613(97)01097-8. [DOI] [PubMed] [Google Scholar]
- 6.Shamma SA, Elhilali M, Micheyl C. Temporal coherence and attention in auditory scene analysis. Trends Neurosci. 2011;34:114–123. doi: 10.1016/j.tins.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamma SA, Micheyl C. Behind the scenes of auditory perception. Curr Opin Neurobiol. 2010;20:361–366. doi: 10.1016/j.conb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinn-Cunningham BG. Object-based auditory and visual attention. Trends Cogn Sci. 2008;12:182–186. doi: 10.1016/j.tics.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinn-Cunningham BG, Best V. Selective attention in normal and impaired hearing. Trends Amplif. 2008;12:283–299. doi: 10.1177/1084713808325306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brungart DS. Informational and energetic masking effects in the perception of two simultaneous talkers. J Acoust Soc Am. 2001;109:1101–1109. doi: 10.1121/1.1345696. [DOI] [PubMed] [Google Scholar]
- 11.Freyman RL, Balakrishnan U, Helfer KS. Effect of number of masking talkers and auditory priming on informational masking in speech recognition. J Acoust Soc Am. 2004;115:2246–2256. doi: 10.1121/1.1689343. [DOI] [PubMed] [Google Scholar]
- 12.Culling JF, Hodder KI, Toh CY. Effects of reverberation on perceptual segregation of competing voices. J Acoust Soc Am. 2003;114:2871–2876. doi: 10.1121/1.1616922. [DOI] [PubMed] [Google Scholar]
- 13.Lavandier M, Culling JF. Speech segregation in rooms: monaural, binaural, and interacting effects of reverberation on target and interferer. J Acoust Soc Am. 2008;123:2237–2248. doi: 10.1121/1.2871943. [DOI] [PubMed] [Google Scholar]
- 14.Lee AK, Shinn-Cunningham BG. Effects of reverberant spatial cues on attention-dependent object formation. J Assoc Res Otolaryngol. 2008;9:150–160. doi: 10.1007/s10162-007-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon-Salant S, Yeni-Komshian GH, Fitzgibbons PJ, Barrett J. Age-related differences in identification and discrimination of temporal cues in speech segments. J Acoust Soc Am. 2006;119:2455–2466. doi: 10.1121/1.2171527. [DOI] [PubMed] [Google Scholar]
- 16.Noble W, Naylor G, Bhullar N, Akeroyd MA. Self-assessed hearing abilities in middle- and older-age adults: a stratified sampling approach. Int J Audiol. 2012;51:174–180. doi: 10.3109/14992027.2011.621899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornickel J, Anderson S, Skoe E, Yi HG, Kraus N. Subcortical representation of speech fine structure relates to reading ability. Neuroreport. 2012;23:6–9. doi: 10.1097/WNR.0b013e32834d2ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song JH, Skoe E, Banai K, Kraus N. Perception of speech in noise: neural correlates. J Cogn Neurosci. 2011;23:2268–2279. doi: 10.1162/jocn.2010.21556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parbery-Clark A, Marmel F, Bair J, Kraus N. What subcortical-cortical relationships tell us about processing speech in noise. Eur J Neurosci. 2011;33:549–557. doi: 10.1111/j.1460-9568.2010.07546.x. [DOI] [PubMed] [Google Scholar]
- 20.Skoe E, Kraus N. Auditory brain stem response to complex sounds: a tutorial. Ear Hear. 2010;31:302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. Proc Natl Acad Sci USA. 2011;108:15516–15521. doi: 10.1073/pnas.1108912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goblick TJ, Jr, Pfeiffer RR. Time-domain measurements of cochlear nonlinearities using combination click stimuli. J Acoust Soc Am. 1969;46:924–938. doi: 10.1121/1.1911812. [DOI] [PubMed] [Google Scholar]
- 23.Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear Res. 2008;245:35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Gockel HE, Carlyon RP, Mehta A, Plack CJ. The frequency following response (FFR) may reflect pitch-bearing information but is not a direct representation of pitch. J Assoc Res Otolaryngol. 2011;12:767–782. doi: 10.1007/s10162-011-0284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dau T. The importance of cochlear processing for the formation of auditory brainstem and frequency following responses. J Acoust Soc Am. 2003;113:936–950. doi: 10.1121/1.1534833. [DOI] [PubMed] [Google Scholar]
- 26.Harte JM, Rønne F, Dau T. Modeling human auditory evoked brainstem responses based on nonlinear cochlear processing. In: Burgess M, Davey J, Don C, McMinn T, editors. Proceedings of the 20th International Congress on Acoustics. Sydney, Australia: Australian Acoustical Society; 2010. pp. 422–431. [Google Scholar]
- 27.Studebaker GA. A “rationalized” arcsine transform. J Speech Hear Res. 1985;28:455–462. doi: 10.1044/jshr.2803.455. [DOI] [PubMed] [Google Scholar]
- 28.Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear Res. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vander Werff KR, Burns KS. Brain stem responses to speech in younger and older adults. Ear Hear. 2011;32:168–180. doi: 10.1097/AUD.0b013e3181f534b5. [DOI] [PubMed] [Google Scholar]
- 30.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76:2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- 32.Grose JH, Mamo SK. Processing of temporal fine structure as a function of age. Ear Hear. 2010;31:755–760. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore BC, Glasberg BR, Stoev M, Füllgrabe C, Hopkins K. The influence of age and high-frequency hearing loss on sensitivity to temporal fine structure at low frequencies (L) J Acoust Soc Am. 2012;131:1003–1006. doi: 10.1121/1.3672808. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BC. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proc Natl Acad Sci USA. 2006;103:18866–18869. doi: 10.1073/pnas.0607364103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He NJ, Mills JH, Ahlstrom JB, Dubno JR. Age-related differences in the temporal modulation transfer function with pure-tone carriers. J Acoust Soc Am. 2008;124:3841–3849. doi: 10.1121/1.2998779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helfer KS, Vargo M. Speech recognition and temporal processing in middle-aged women. J Am Acad Audiol. 2009;20:264–271. doi: 10.3766/jaaa.20.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grose JH, Mamo SK, Hall JW., 3rd Age effects in temporal envelope processing: speech unmasking and auditory steady state responses. Ear Hear. 2009;30:568–575. doi: 10.1097/AUD.0b013e3181ac128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macpherson EA, Middlebrooks JC. Listener weighting of cues for lateral angle: the duplex theory of sound localization revisited. J Acoust Soc Am. 2002;111:2219–2236. doi: 10.1121/1.1471898. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein LR, Trahiotis C. Why do transposed stimuli enhance binaural processing?: Interaural envelope correlation vs envelope normalized fourth moment. J Acoust Soc Am. 2007;121:EL23–EL28. doi: 10.1121/1.2401225. [DOI] [PubMed] [Google Scholar]
- 40.Wightman FL, Kistler DJ. The dominant role of low-frequency interaural time differences in sound localization. J Acoust Soc Am. 1992;91:1648–1661. doi: 10.1121/1.402445. [DOI] [PubMed] [Google Scholar]
- 41.Heller LM, Trahiotis C. Extents of laterality and binaural interference effects. J Acoust Soc Am. 1996;99:3632–3637. doi: 10.1121/1.414961. [DOI] [PubMed] [Google Scholar]
- 42.Slaney M. Auditory Toolbox Version 2. Interval Research Corporation; Palo Alto, CA: 1998. https://engineering.purdue.edu/~malcolm/interval/1998-010/ [Google Scholar]
- 43.Best V, Carlile S, Jin C, van Schaik A. The role of high frequencies in speech localization. J Acoust Soc Am. 2005;118:353–363. doi: 10.1121/1.1926107. [DOI] [PubMed] [Google Scholar]
- 44.Moore BC, Füllgrabe C, Stone MA. Effect of spatial separation, extended bandwidth, and compression speed on intelligibility in a competing-speech task. J Acoust Soc Am. 2010;128:360–371. doi: 10.1121/1.3436533. [DOI] [PubMed] [Google Scholar]
- 45.Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70:1055–1096. [Google Scholar]