Abstract
Despite advances in chemo- and immunotherapeutic agents for B chronic lymphocytic leukemia (B-CLL), the undesirable adverse side effects due to non-specific cellular uptake remain to be addressed. We identified anti-CD37 monoclonal antibody immunoliposomes (ILs) as vehicles for targeted delivery to B chronic lymphocytic leukemia cells. To achieve maximal benefits for all patients, a new strategy of dual-ligand immunoliposomes (dILs) was developed. A combinatorial antibody microarray technology was adapted to quickly identify optimal antibody combinations for individual patient cells. For proof-of-concept, a B-cell specific antibody, either anti-CD19 or anti-CD20, was combined with anti-CD37 to construct dILs with enhanced selectivity and efficacy. Consistent with data from the antibody microarray, these dILs provided highly specific targeting to both leukemia cell lines and B-CLL patient cells. Compared with the single antibody ILs, the anti-CD19/CD37 dILs clearly demonstrated superior delivery efficiency and apoptosis induction to B-CLL patient cells, whereas the anti-CD20/anti-CD37 dILs were found to be the most efficient for delivery to leukemia cell lines. In addition, it was observed that anti-CD37 ILs without payload drug mediated effective CD37 cross-linking and induced potent apoptosis induction. The anti-CD19/CD20 dILs showed the improved cell apoptosis induction compared to either anti-CD19 ILs or anti-CD20 ILs. Our findings suggest that the dual-ligand ILs may provide a preferred strategy of personalized nanomedicine for the treatment of B-cell malignancies.
1. Introduction
B-CLL is a common type of adult leukemia for which current treatments are not curative. Alkylating agents and purine nucleoside analogs have been considered the drugs of choice for treatment of CLL for many years. The chemotherapeutic agent fludarabine used by itself or in combination with alkylator-based agents is effective in a subset of patients but non-specific effects of these drugs on bystander cells are problematic [1]. Undesirable side effects associated with these therapies include prolonged immune suppression resulting from direct apoptosis induction to normal immune effector cells [1–3].
The introduction of the anti-CD20 monoclonal antibody rituximab (RIT) [4–6] has substantially impacted CLL therapy [4, 7, 8]. RIT, when given in combination with fludarabine and cyclophosphamide, has been shown to extend survival in symptomatic CLL [4, 7, 9]. In addition to rituximab, alemtuzumab that targets CD52, an antigen expressed on normal lymphocytes as well as many T- and B-cell neoplasms has been used for first-line treatment for CLL [5, 6]. The immunosuppressive effects of alemtuzumab caused by T and NK cell depletion, however, impose limit to its use in aged patients. New antibodies against CD19, CD40, CD23, CD37, and CD74 are in early clinical trials for the treatment of CLL [10–13]. Recently, CD37 antigen has been identified as a potential target for therapy in B-cell malignancies [13–15]. CD37, a 40~52kDa glycoprotein, is highly expressed on B cells and has limited or no expression on other hematopoietic cells such as T cells and NK cells [16, 17]. In particular, CD37 on B-CLL cells is uniformly present and relatively elevated [13, 15].
B-cell lymphomas and leukemias often involve multiple, different pathological factors and pathways. Therapeutic efficacy of most of the antibodies in clinical use is attributed to their interaction with a single target. Simultaneous blockade of multiple targets either via the combination of two antibodies (Abs) or by a bispecific antibody (BsAb) may provide better clinical efficacy and/or reach a broader patient population [18–20]. In fact, improved therapeutic efficacy of combining milatuzumab and RIT monoclonal antibodies (mAbs) has already been demonstrated in the preclinical model of mantle cell lymphoma (MCL) [21]. In addition, the bispecific anti-CD20/CD22 and anti-CD20/CD74 antibodies have demonstrated enhanced efficacy for B-cell lymphomas and leukemias [18, 22].
Specific and efficient in vivo delivery of therapeutic agents to target B-CLL cells remains a major challenge in the clinic. To address these issues, monoclonal antibody conjugated nanocarriers such as immunoliposomes (IL) have been increasingly recognized as a promising strategy for selective delivery of anti-cancer drugs to B-CLL cells [11, 23, 24]. In addition, recent efforts on dual-ligand mediated delivery approaches offer the potential to improve selectivity and efficiency over single-ligand approaches [25–29]. Dual Ab targeted ILs have shown improved therapeutic effects of anti-cancer drugs in B-cell malignancies [30, 31]. However, dual-ligand ILs against antigens co-expressed on the same cells have not been investigated in CLL.
Creation of multivalent antibody constructs using liposomes or gold nanoparticles have recently been shown to have enhanced efficacy compared to free, bivalent antibody [32–36]. Because of the extensive cross-linking of the target/antibody complex via the multivalent antibody constructs, various cellular responses such as inhibition of cell growth, induction of apoptosis, or internalization of the surface molecules, can be significantly enhanced. For example, RIT-coated liposomes (devoid of encapsulated drug) have displayed much higher efficacies than equal amounts of free monomeric RIT [34, 35]. Our recent work also indicates that anti-CD74 ILs mediate potent cell killing of B-CLL cells even without an anti-cancer drug payload [33]. Nevertheless, the multivalency of nanoparticle-based antibody constructs has only been focused so far on single therapeutic antibodies.
Based on the above rationale, we sought to achieve high selectivity and targeting efficacy to B-CLL cells through ILs. In this work, we developed liposomal nanoconstructs that are simultaneously surface modified with two types of antibody ligands having specificity and high affinity to B-CLL cells. To fulfill the purposes of screening for the proper Ab combination for individual CLL patient cells, a combinatorial antibody microarray technology was used to quantitatively characterize binding efficiencies of single and dual antibodies in a systematic and high throughput fashion. Anti-CD37 ILs was first demonstrated to provide highly specific targeting to both leukemia cell lines and B-CLL patient cells. Combination of anti-CD37 Ab with either anti-CD19 or anti-CD20 Ab on the same ILs was used to further improve the targeting of B-CLL cells. The multivalent effects of dual antibody immunoliposomes (dILs) on B-CLL patient cells were examined by in vitro apoptosis induction assay. Finally, a small molecule drug, FTY720, as a model of a therapeutic payload, was encapsulated in the dILs and evaluated for therapy efficacy in B-CLL cells.
2. Material and methods
2.1. Reagents
Egg phosphatidylcholine (Egg PC) and methoxy-polyethylene glycol (MW ~ 2,000 Da)-distearoyl phosphatidylethanolamine (PEG2000-DSPE) were obtained from Lipoid (Newark, NJ). Maleimide-PEG2000-DSPE (Mal-PEG2000–DSPE) was purchased from Avanti Polar Lipids, Inc (Alabaster, AL). Cholesterol (Chol), calcein, 2-Iminothiolane (Traut’s reagent) and other chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Rituxan (Rituximab) was obtained from Genentech, Inc (South San Francisco, CA). Purified anti-human CD37 and anti-human CD19 monoclonal murine antibodies were purchased from BD Biosciences (San Diego, CA). Alexa 488 labeled anti–mouse secondary antibody was from Invitrogen (Carlsbad, CA). FTY720 was purchased from ChemieTek (Indianapolis, IN).
2.2. Cell culture
Raji Burkitt’s lymphoma, Daudi, Romas, RS11846 and Jurkat cell lines were obtained from American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS, Hyclone Laboratories, Logan, UT), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), and penicillin (100 U/mL)/streptomycin (100 μg/ml; Sigma-Aldrich, St. Louis) at 37°C in an atmosphere of 5% CO2. Blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the institutional review board (IRB) of The Ohio State University (Columbus, OH). All patients examined in this series had immunophenotypically defined CLL as outlined by the modified 96 National Cancer Institute criteria. CLL B cells were isolated from freshly donated blood using Ficoll density gradient centrifugation (Ficoll-Paque Plus, Amersham Biosciences, Piscataway, NJ). Enriched CLL fractions were prepared by using the “Rosette-Sep” kit from Stem Cell Technologies (Vancouver, British Columbia, Canada) according to the manufacturer’s instructions.
2.3. Immunostaining and flow cytometry
Cell lines (0.5×105/ml) or B-CLL cells (1.0×105/ml) were incubated with PE-labeled anti-CD20, anti-CD19, and anti-CD37 as well as mouse IgG1 isotype control antibody (BD Biosciences, San Diego, CA), at 4°C for 30 min. The cells were then spun down at 300 g for 10 min and rinsed twice with cold phosphate-buffered saline (PBS, pH=7.4) and then analyzed by flow cytometry on a Beckman Coulter EPICS XL (Beckman Coulter) to determine antigen expression levels. A minimum of 10,000 events were collected under the LIST mode for each assay. The data was analyzed using WinMDI software.
For surface staining, PBMC cells were incubated with PE labeled anti-CD19 (as a B cell marker) or PE labeled anti-CD3 (as a T cell marker) on ice for 30 min. The cells were washed twice with cold PBS (pH=7.4) and analyzed by flow cytometry. To study B-cell selectivity, PBMC cells were incubated with FITC labeled anti-CD37 on ice for 30 min. The cells were then spun down and rinsed twice with cold phosphate-buffered saline (PBS, pH=7.4). The treated cells were further stained with PE-labeled anti-CD19 or anti-CD3 antibodies to identify B and T cell populations respectively. After another washing and spin-down, the cells were analyzed by flow cytometry. The data was analyzed using WinMDI 2.8 software.
2.4. Antibody internalization assay
Cells were incubated with PE-labeled antibodies (PE-anti-CD20, PE-anti-CD19 and PE-anti-CD37) at 37°C for 30, 60, 120 and 240 min. The antibody control was added at 0 min on ice to ensure that internalization did not occur till temperature was raised to 37°C. After incubation, cell surface bound antibodies were removed with stripping buffer (100 mM glycine, 100 mM NaCl (pH=2.5) thus allowing detection of only internalized fluorochlorome labeled Ab by flow cytometry. Appropriate IgG isotypes were used as negative controls. Internalization is defined as time-dependent increase in the Mean Fluorescent Intensity (MFI) after acidic washing by stripping buffer.
2.5. Combinatorial antibody microarray
The Ab microarrays were constructed by printing antibodies against CD19, CD20, CD37 and their dual combinations onto a surface-modified glass slide. Three pure (anti-CD19, anti-CD20, and anti-CD37) and dual antibody combinations were printed onto Nexterion® slide H using a non-contact piezoelectric arrayer (Perkin Elmer, Waltham MA), at the same total antibody concentration (0.5 mg/ml). Each sample was arrayed in triplicate with a spot center-to-center distance of 400 μm into a subarray of 6.8 mm × 6.8 mm. Cells, from B-CLL patients, were labeled with CFSE (carboxy-fluorescein diacetate, succinimidyl ester; Invitrogen, Carlsbad CA) according to manufacturer’s protocol. After incubation of B-CLL cells (1.5×106/ml) with the Ab microarray for 1 hr at room temperature, the microarray slide was carefully dip-washed in PBS solution to remove unbound cells and then was imaged. Microarray images were acquired by ProScanArray fluorescence scanner (Perkin Elmer, Waltham MA). Slides were scanned at 5 μm resolution. Image quantification and analysis were performed by using ScanArray Express 3.0 (Perkin Elmer, Waltham MA).
2.6. Preparation of ILs
A lipid mixture of EggPC/Chol/PEG2000-DSPE at 65/34/1.0 (molar ratio) was dissolved in ethanol. The lipids formed a homogeneous thin lipid layer in rotavapor.. The thin film was re-hydrated with PBS buffer (pH=7.4) to obtain liposomes and the particle size was reduced to ~ 100 nm range by high pressure extrusion with track-etched polycarbonate membranes (pore sizes: 0.2 and 0.1 mm, Northern Lipids).
A post-insertion method was adopted to incorporate antibody ligands into preformed liposomes carrying calcein [37, 38]. Antibody was reacted with 10× Traut’s reagent (2 hr, room temperature) to yield sulfhydryl modified antibodies (Ab-SH). The unreacted Traut’s reagent was removed by chromatography on a Sephadex G-50 column with degassed HBS (pH 7.4). Ab-SH was coupled to micelles of Mal-PEG2000-DSPE at a protein-to-lipid molar ratio of 1:10. The resulting Ab-PEG2000-DSPE micelles were then incubated with calcein loaded liposomes for 1 hr at 37°C at an Ab to total lipid molar ratio of 1: 4000 (0.025 mol%). Keeping the total Ab amount constant, two individual Ab-PEG2000-DSPE micelles were mixed according to the indicated ratios and then incubated with calcein loaded liposomes to prepare the dILs. The efficiency of post-insertion method can reach almost 100% at low Ab to total lipid ratio [37, 38]. Thus, equal amounts of Abs are kept for both dual- and single targeted ILs, which was essential for the comparison between the study groups.
For the binding study, calcein (50 μM, pH=8.0) was incorporated into the liposomes during re-hydration as a green fluorescent marker and the free calcein was removed by Sepharose CL-4B column. Various green fluorescent ILs or dILs were prepared by the post-insertion of Ab-PEG200-DSPE micelles with the calcein loaded liposomes. For the proof-of-concept of drug delivery to B-CLL cells, FTY720 was used as a model therapeutic payload. FTY720 encapsulated liposomes were prepared by an ethanol injection method, as described by us previously[39]. The single or dual Ab targeted liposomal FTY720 were then obtained by the post-insertion method.
The particle sizes of ILs were analyzed using a NICOMP Particle Sizer Model 370 (Particle Sizing Systems, Santa Barbara, CA). The volume-weighted Gaussian distribution analysis was used. The zeta potential (ξ) was determined on a ZetaPALS (Brookhaven Instruments Corp., Worcestershire, NY).
2.7. Cellular uptake of ILs and dILs
Binding and internalization of the ILs and dILs in Raji cells were examined by fluorescence microscopy. Cells were incubated with anti-CD20 ILs, anti-CD37 ILs and anti-CD20/anti-CD37 dILs for 4 hrs at 37°C and wash ed twice with PBS, followed by fixation with 2% paraformaldehyde for 30 min. The cells were mounted on a poly-D-lysine coated cover glass slide (Sigma-Aldrich, St. Louis, MO). Green fluorescence of FAM-ODN was analyzed, and images were produced by using a Zeiss 510 META Laser Scanning Confocal Imaging Systems at 600X magnification (Carl Zeiss MicroImaging, Inc., NY, USA).
2.8. Apoptosis assay
Apoptosis activity was determined in primary CLL cells after 24-hr incubation with ILs or dILs by annexin V/PI staining. Samples from the same patients were treated with mAbs in the presence or absence of an anti-IgG Fc cross-linker as controls. The degree of apoptosis induction is displayed as the total percentage of annexin V and PI double negative cells normalized to untreated condition.
2.9. Statistical analyses
Statistical analyses were performed by Center for Biostatisitics at The Ohio State University. Linear mixed effect models were used to estimate unrestricted covariance structures and produce robust hypothesis tests. P-values were adjusted by Holm’s method. A significance level of α = 0.05 was used for all tests.
3. Results
3.1. Design of dual-antibody immunoliposomes (dILs)
Figure 1 illustrates the concept of dILs designed in this work. By simultaneously bearing two B-cell specific antibodies, dILs have been hypothesized to more efficiently target B cells expressing multiple antigens than single-antibody ILs. Previous studies have revealed that the activity of therapeutic Abs can be enhanced by creating multivalent constructs such as ILs [33, 35]. Combination of multiple monoclonal antibodies has shown synergistic therapeutic effects in B cells leukemia [40, 41]. Therefore, the dual mAbs on the same ILs may potentially enhance the cross-linking induced apoptosis of CLL B cells. Compared to single ILs or mixed single ILs, dILs may have the advantages of increased binding, uptake and apoptosis induction to the target cells, and synergistic killing via signaling.
Fig. 1. Schematic of dual-antibody immunoliposomes.
Simultaneous use of two different antibodies allow dual-Ab immunoliposomes to efficiently bind to and target B cells expressing variable levels of each of the target antigens.
3.2. Specificity for B-CLL cells and internalization
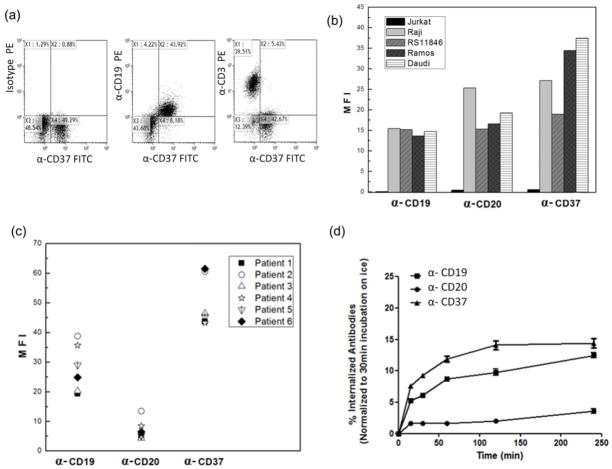
CD37 has been recently identified as a good target for the treatment of B cell malignancies [13, 14, 16, 42, 43]. It is predominantly expressed on B cells, and has minimal to no surface expression on other types of blood cells, including T and NK cells [16, 17]. To confirm its specificity to B but not T cells, peripheral blood mononuclear cells (PBMCs) isolated from patients with CLL were stained with anti-CD37-FITC, followed by anti-CD19-APC or anti-CD3-APC. As shown in (Figure 2a), two-color flow cytometry confirmed that anti-CD37-FITC was selectively bound to B cells that were CD19 (+) but not to other cell populations, including T cells identified by anti-CD3-APC. Thus, anti-CD37 can be selected as a good targeting ligand for drug delivery to B cell malignancies.
Fig. 2. CD37 is an optimal candidate for targeted delivery to B-CLL cells.
(a) Selective binding of anti-CD37 to CD19+ B cells but not CD3+ T cells in human PBMC. For surface staining, the PBMC cells were incubated with or without anti-CD37-FITC on ice for 30 min and washed twice with cold PBS. Then, the treated cells were further stained with PE labeled anti-CD19 (B-Cell marker) or PE labeled anti-CD3 (T-Cell marker) on ice for another 30 min and rinsed twice with cold PBS. (b) Mean fluorescent intensity of antigen expression levels on cell lines and (c) mean fluorescent intensity of antigen expression levels on CLL patients (n=6). (d) Determination on internalization rates of various mAbs. Cells were incubated with fluorochrome labeled antibodies (anti-CD20-PE, anti-CD19-PE and anti-CD37-PE) at 37°C for 15, 30, 60, 120 and 240 min and extracellular bound antibodies were removed with stripping buffer thus allowing detection of only internalized fluorochrome labeled antibody by flow cytometry. Appropriate IgG isotypes were used as negative controls. Internalization is defined as time-dependent increase in the Mean Fluorescent Intensity (MFI) after acidic washing by stripping buffer, which removed any surface bound antibody (n=3, mean ± SD).
We further evaluated the levels of expression of CD37, CD19 and CD20 antigens in B cell lines and primary CLL B cells using fluorescence-labeled antibodies by FACS (Figure 2b, c). CD37 levels are comparable with CD19 levels and their variations of expression were small in both B cell lines and B-CLL cells. In addition, the average expression of CD19 or CD37 on Raji cells is comparable to that seen on B-CLL cells.
The internalization rate of antibody is an important consideration factor for choosing appropriate candidates for antibody-mediated drug delivery[44, 45]. Antibodies for the antigens of CD37, CD19 and CD20 were examined for internalization in Raji cell line (Figure 2d). In contrast to anti-CD20, anti-CD37 was found to be consistently internalized by Raji cells, which is comparable to anti-CD19. This supports our selection of anti-CD37 as the primary ligand to mediate B-cell specific targeting delivery of drug loaded ILs.
3.3. Screening of optimal Ab combination through combinatorial antibody microarray
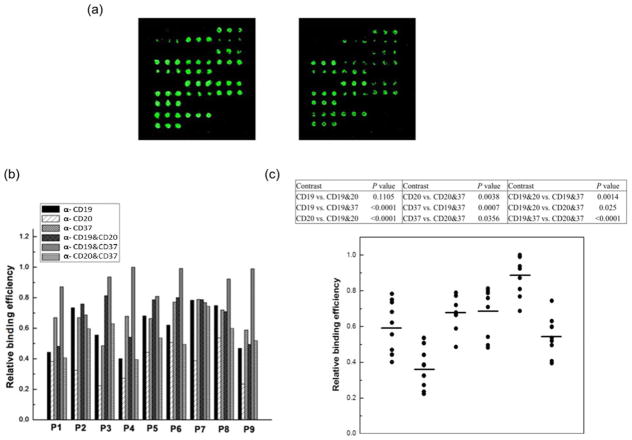
Antibody microarray assays can be used for quantitative immunophenotyping of leukemia cells in a high throughput manner [46–48]. In contrast to its typical application for detection of certain CD antigens on cells, we intended to develop this technology for evaluation of the binding efficiency of single or mixtures of antibodies, screening for the optimal single or combined antibodies that are optimal for ILs based targeted delivery. The Ab microarrays were constructed by printing antibodies against CD19, CD20, CD37 and their dual combinations onto a surface-modified glass slide. The highest binding fluorescence intensity in the Ab microarray was set as 1 (Figure 3a) and quantitative binding profiles of cells from nine B-CLL patients on the combinatorial antibody microarrays are shown (Figure 3b). The levels of cell binding across the spots printed with a single antibody (anti-CD19, anti-CD20, anti-CD37) varied from patient to patient. For all nine patients the highest binding occurred on spots printed with mixtures of two antibodies. The greater binding efficiencies in all nine tested primary CLL cells were validated when dual combinations of antibodies such as anti-CD20/anti-CD37 and anti-CD19/anti-CD37 were used (n=9, p < 0.05 between all single antibody groups compared to anti-CD20/anti-CD37 or anti-CD19/anti-CD37 group).
Fig. 3. Binding efficiency of B-CLL cells on combinatorial antibody microarrays.
A library of antibody mixtures containing three antibodies (anti-CD19, anti-CD20 and anti-CD37) at equal total concentrations with all possible combinations was used for microarray assay. Total antibody concentration was maintained at 0.5 mg/ml. The CFSE (carboxy-fluorescein diacetate, succinimidyl ester; Invitrogen, Carlsbad CA) fluorescence labeled B-CLL patient cells (1.5×106/ml) were incubated with the Ab microarray. After removal of unbound cells, the quantified mean spot intensities were acquired and data (n=3, mean ± SD) was presented as relative binding efficiency. (A) Representative of Ab microarrays constructed by printing the anti-CD19, anti-CD20, anti-CD37 antibodies and their dual combinations at different concentrations. The highest binding fluorescence intensity in the Ab microarray was set as 1. (B and C) Quantitative binding profiles of cells from nine B-CLL patients on the combinatorial antibody microarrays (n=9). Linear mixed effect models were used to estimate unrestricted covariance structures and produce robust hypothesis tests. Holm’s method was used to adjust for multiplicity.
3.4. Synthesis and optimization of anti-CD37 based dILs
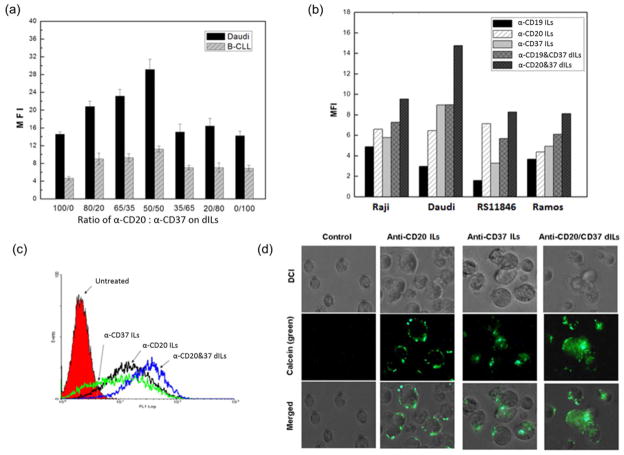
Based on results from the antibody microarray, we designed and synthesized a series of ILs. The “post-insertion” method was used to construct ILs with different compositions [11, 30]. The internalization rate of an antibody is an important factor for its selection [44, 45]. To determine the optimal mAb ratio for dILs, we evaluated various combinations of anti-CD37 and anti-CD20 antibody on ILs through flow cytometry analysis in both Daudi and B-CLL cells. The binding efficiencies of anti-CD20/anti-CD37 dILs reached the highest value (n=3, p-value < 0.0001) at the 50/50 composition for both Daudi and B-CLL cells (Figure 4a). Furthermore, the dual-mAb ILs worked better than single-mAb ILs. Keeping the 50/50 composition as a constant, we examined the delivery efficiency of two dILs (anti-CD19/anti-CD37 and anti-CD20/anti-CD37) in other transformed B cell lines (Figure 4b, c). The MFI of anti-CD20/anti-CD37 dILs reached 14.8 while the corresponding single ILs, anti-CD20-ILs and anti-CD37-ILs had the mean fluorescent intensity (MFI) of 6.9 and 8.5., respectively. Consistent with data from Daudi cells, dual anti-CD20/anti-CD37 dILs showed the strongest delivery efficiency compared to individual mAb ILs and anti-CD19/anti-CD37 dILs in Raji, RS11846 and Ramos cell lines as well (Figure 4b).
Fig. 4. Optimization of Ab ratio in dILs for efficient delivery to leukemia cell lines and B-CLL cells.
(a) Effect of mAb ratio in dILs on Daudi and B-CLL cells. Indicated ratios of anti-CD20 and anti-CD37 antibodies were immobilized onto liposomes with the post insertion method previously described. The binding efficiency was determined by MFI via flow cytometry detection (n=3, P < 0.0001 for all 100/0 vs. 50/50 or 50/50 vs. 0/100 in Daudi or B-CLL cells). Linear mixed effect models were used to estimate unrestricted covariance structures and produce robust hypothesis tests. Holm’s method was used to adjust for multiplicity. (b) Comparison of delivery efficiency of ILs on B cell lines. Fluorescent labeled ILs were incubated with different cell lines for 30 min followed by twice wash, and MFI were determined by flow cytometry. (c) Histogram comparison of anti-CD20 ILs, anti-CD37 ILs and anti-CD20/anti-CD37 (50/50) dILs. MFI for anti-CD20-ILs, anti-CD37-ILs and anti-CD20/anti-CD37-ILs were 12.3, 13.5 and 18.9, respectively. (d) Confocal microscopy analysis on the enhanced cellular uptake by anti-CD20/anti-CD37 (50/50) dILs compared with the two single-Ab ILs. Cells were incubated with anti-CD20 ILs, anti-CD37 ILs and anti-CD20/anti-CD37 dILs for 4 hrs at 37°C and washed twice with PBS, followed by fixation with 2% paraformaldehyde for 30 min. Anti-CD20/anti-CD37 dILs demonstrated the highest cellular uptake.
Fluorescence microscopy was used to compare the uptake and cellular localization of anti-CD20 ILs, anti-CD37 ILs and anti-CD20/anti-CD37 (50/50) dILs encapsulated calcein in Raji cells (Figure 4d). Compared to anti-CD20 ILs, anti-CD37 ILs exhibited greater uptake in Raji cells while the anti-CD20/anti-CD37 dILs demonstrated the highest cellular uptake (Figure 4D).
3.5. Anti-CD37 based dILs for delivery to B-CLL cells
Anti-CD37 based dILs were xamined on B-CLL cells (Figure 5a). Among all tested B-CLL cells, the anti-CD19/anti-CD37 dILs demonstrated superior delivery efficiency compared to all individual mAb ILs and the anti-CD20/anti-CD37 dILs. The targeting improvement of anti-CD19/anti-CD37 combination was significant compared to anti-CD20/anti-CD37 dILs (n=11, P-value = 0.002) with 43.3% increase in binding to B-CLL cells. And anti-CD20/anti-CD37 dILs enhanced the delivery and binding efficiency compared to anti-CD20 ILs with over 2.36 fold change in MFI in B-CLL cells. The anti-CD37 ILs have much better delivery efficiency than anti-CD19 and anti-CD20 ILs, further confirming the benefits of using anti-CD37 as a targeting ligand (Figure 5b). It should be noted that the delivery efficiency of anti-CD20 ILs varied greatly on patient cells because of the variability in CD20 antigen expression [3, 12, 49]..
Fig. 5. Delivery efficiency evaluation of various ILs on B-CLL cells.
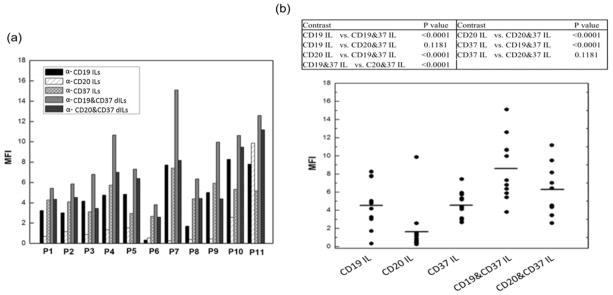
(a and b) 1×106 B-CLL cells were incubated with indicated calcein encapsulated ILs and dILs for 1.0 hr at 37°C. Following washing aw ay free ILs, the fluorescence intensity was determined by flow cytometry (n=11). Linear mixed effect models were used to estimate unrestricted covariance structures and produce robust hypothesis tests. Holm’s method was used to adjust for multiplicity.
3.6. Apoptosis induction of anti-CD37-based dILs in B-CLL cells
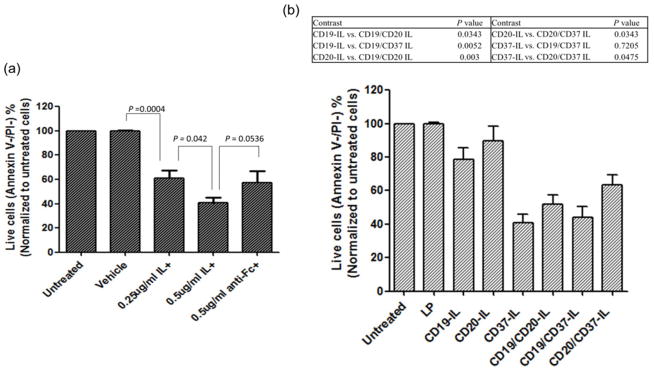
Previous studies have revealed that ILs may cause a cross-linking effect similar to that from anti-hFc receptor [33, 35]; the same phenomenon was observed with anti-CD37 in our study (Figure 6a). B-CLL cells isolated form six patients showed equivalent apoptosis induction responding to 0.25 μg/ml of anti-CD37 in the ILs as that of 0.5 μg/ml of anti-CD37 with the existence of anti-hFc linker. By increasing the concentration of anti-CD37-ILs to 0.5 μg/ml of anti-CD37, there were more cells undergoing apoptosis though it is not significantly different compared to the anti-Fc cross-linking effect (n=6, p-value=0.0536). To prove our hypothesis that dILs not only increase the binding efficiency of dILs to the targets, B-CLL cells, but also induce more apoptosis induction, combinations of dILs were tested with B-CLL cells with investigation of the cross-linking effect of anti-CD19-ILs and anti-CD20-ILs. All three mAb ILs more or less represented the cross-linking effect caused by ILs (Figure 6b). Furthermore, compared to their single ILs form, both anti-CD19 and anti-CD20 exhibited greater effect on inducing apoptosis of B-CLL cells whether combined with each other or with anti-CD37. While anti-CD37 dILs did not show enhanced apoptosis induction compared to its single ILs form, an enhancing effect of anti-CD37 dILs was observed when combined with anti-CD19 or anti-CD20 without payload.
Fig. 6. Cytotoxic effect on B-CLL cells with single anti-human CD37 ILs in comparison with cross-linking effect.
(a and b) B-CLL cells (1×106) were co-cultured with indicated reagents for 24 hr and cytotoxicity was determined via annexin V FITC/PI analysis by flow cytometry (mean ± SD). Data was analyzed by mixed effect model and p-values were adjusted by Holm’s method.
3.7. The therapeutic efficacy of dILs loaded with FTY720
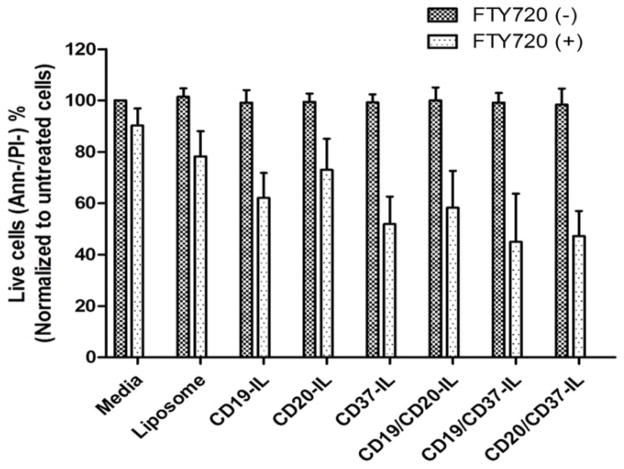
To determine if the dILs will increase the efficacy of payload mediated apoptosis induction, we evaluated FTY720, a sphingosine analogue that was previously reported to mediate potent apoptosis induction in B cells [50, 51]. Predetermined concentrations of mAbs that were optimized to mediate efficient targeting but not apoptosis induction were used for the dIL-FTY720 formulations. As shown in Figure 7, encapsulation of FTY720 into ILs resulted in significant apoptosis of target cells compared to the corresponding control IL or dIL groups without FTY720 (n=6, p-value<0.0001) (Figure. 7). Both anti-CD19/anti-CD37-dIL-FTY720 and anti-CD20/anti-CD37-dIL-FTY720 exhibited significantly improved apoptosis induction compared to anti-CD19-IL FTY720 (n=6, p-value=0.0018) and anti-CD20-IL FTY720 (n=6, p-value<0.0001), respectively.
Fig. 7. Improved efficiency in delivering of liposomal FTY720 with dILs and enhanced cytotoxicity on B-CLL cells.
B-CLL cells were incubated with indicated formulations of IL or dIL FTY720 at 4 μM of FTY720 and 0.1 μg/ml mAbs in total for 24 hr. The viability of the cells were then assessed by annexin V/PI analysis by flow cytometry (mean ± SD). Encapsulating FTY720 into liposomes and targeting it with mAbs can induce potent target cell apoptosis compared to the corresponding IL or dIL groups without FTY720 is shown (n= 6, p-value<0.0001).
4. Discussion
Monoclonal antibodies such as RIT are widely used for the treatment of B-CLL and other B- cell malignancies. However, the variability in the expression of the target antigens among patient populations warrants innovative approaches for enhancing the antibody therapy response. Consequently, a combination of antibodies simultaneously targeting multiple antigens, which could trigger multiple pathways, may result in improved therapeutic efficacy [19, 20]. In this context, combinations of multiple drugs and antibodies that target different pathways and bispecific antibodies (BsAb) are promising. The dual targeting strategy including the combination of two Abs and a BsAb is emerging as an attractive strategy in both preclinical and clinical settings [18, 21, 22].
One of the major challenges in targeted drug delivery for leukemia treatment is associated with limitations in the choice of diverse potential targeting molecules for individualized therapy based upon the genetic or immuno-phenotype of the leukemic cell. Antibody-targeted nanoparticles such as ILs are a promising strategy for the treatment of B-malignancies including CLL [33, 52]. In this study, we described a strategy to select optimal target antigens in B cell malignancies using an antibody microarray and demonstrated anti-CD37 antibody as a candidate target ligand for efficient B cell specific delivery. Anti-CD37 based dILs showed a synergistic effect on targeted delivery to both transformed B cell lines and B-CLL cells, which may translate into better therapeutic efficacy for the treatment of B-CLL.
The appropriate selection of mAb or mAb fragments is important for effective targeted delivery of ILs. The receptor expression and internalization rate of mAbs are two key factors in mAb selection. Generally, the higher the antigen density, the greater the therapeutic effects of ILs. In addition, an antibody with a higher internalizing rate can often provide better delivery efficiency of ILs. For B-cell malignancies, CD19, CD20 and CD37 have been recognized as good targets for immunotherapy[10, 14, 53]. It was found that doxorubicin loaded anti-CD19 ILs caused higher apoptosis induction than anti-CD20 ILs in B-lymphoma Namalwa cells and the corresponding xerograft mouse model. Anti-CD37 is a highly B-cell specific antibody (Figure 2). CD37 is highly expressed on mature B cells but not on other hematopoetic cells [13, 16, 17]. Furthermore, anti-CD37 provides a much higher internalizing rate when compared to anti-CD19 and anti-CD20. Hence, anti-CD37 is an excellent candidate for targeting delivery to B-CLL cells.
The targeting efficiency of ILs is also well correlated with the antigen expression level (or antigen density) on the target cells [11, 44]. ILs with antibody against a high antigen density can significantly improve the therapeutic responses. Given the difficulties and unpredictability associated with the desired optimal antigen density on the target cells for in vivo applications, we resorted to dual targeting approach using two antibodies against antigens expressed on the same cell. This has been demonstrated previously by the combination of immunotoxins showing significantly improved therapeutic responses compared with single immunotoxin therapy[44]. In B-CLL cells, the single antigen expression level on the cell surface generally varies from patient to patient. Thus, it is useful to combine several antibodies (e.g. anti-CD37, anti-CD19 and anti-CD20) together to achieve higher binding and delivery efficiency of ILs for individual patients. Additionally, the anti-CD37 antibody based dual targeting may increase the B-cell selectivity of ILs because anti-CD19, anti-CD20 and anti-CD37 antibodies are very B-cell type specific. To enhance the delivery efficiency of anti-CD37 mediated ILs, two anti-CD37 based dILs, anti-CD20/anti-CD37 dILs and anti-CD19/anti-CD37 dILs, were synthesized. We found that the composition of 50/50 on dILs provided the best results. Enhanced binding efficiencies of anti-CD19/anti-CD37 and anti-CD20/anti-CD37 dILs over the single-mAb ILs were clearly demonstrated (Figures 4 and 5). The combination of anti-CD20/anti-CD37 on dILs showed the greatest delivery efficiency for transformed leukemia lines, whereas the combination of anti-CD19/anti-CD37 was the best for delivery of ILs to B-CLL cells.
The anti-CD19 and anti-CD37 antibodies used in this study are commercial reagents which are not designed for clinical use in humans. They potentially could be replaced by antibodies or peptide directed SMIPS approved for use in human clinical trials such as Xmab-5574 for anti-CD19 and TRU-016 for anti-CD37. Such replacement may facilitate faster translation of dILs into the clinic.
Our recent work indicates that ILs can provide a therapeutic strategy even without anti-cancer drug payload [33, 34]. Owing to the strong cross-linking effect caused by mAb on ILs, the anti-CD74 ILs showed the potent cell killing on B-CLL cells, although the underlying mechanisms remain unknown. Combination of monoclonal antibody therapy has shown synergistic effects in B cells leukemia [21], therefore, the dual Ab ligands on the same ILs may potentially show the synergistic cell killing on B-CLL cells, which was partly confirmed in our study with enhanced apoptosis induction of anti-CD19/CD20 dILS comparing to the original single anti-CD19 or anti-CD20 ILs (Figure 6b). However, the cross-linking induced apoptosis of anti-CD37 ILs is the best among all study groups, which could be attributed to the recently described SHP-1 dependent death of CLL cells caused by anti-CD37 [14].
Finally, we used FTY720 as the model drug to validate the efficiency of dILs in targeted delivery (Figure 7). The enhanced targeting of CD37 and CD19 by dILs over the single-targeted ILs suggested that the anti-CD37 dual targeting strategy is likely to improve the clinical efficacy of dILs and reach a broader patient population for B-CLL. Since liposomes are capable of carrying a variety of drugs, it is possible to encapsulate chemotherapy drugs for CLL such as fludarabine, flavopiridol and dexamethasone in ILs and dILs.
5. Conclusions
We rationalized that liposome surface-modified with dual Ab ligands that simultaneously target to B-CLL cells will have higher selectivity as well as higher binding affinity, ensuring enhanced targeted delivery of therapeutics. The combinatorial antibody microarray technology was developed to quickly identify the optimal Ab combinations for cells from individual B-CLL patient. Consistent with Ab microarray screening, anti-CD37 based dILs with either anti-CD19 or anti-CD20 showed higher specific targeting to both leukemia cell lines and B-CLL patient cells. Meanwhile, the anti-CD19/CD20 dILs without payload drug displayed the improved cross-linking induced cellular apoptosis induction compared to either anti-CD19 ILs or anti-CD20 ILs. Overall, our results presented in this study validated the utility of a dual ligand delivery approach. The application of such an approach may ultimately provide the ability to tailor ligand-targeted nanocarriers to fit the profile of individual CLL patient allowing for patient-specific treatments.
Acknowledgments
This work was supported by NSF Nanoscale Science and Engineering Center (NSEC) grant EEC-0914790, NIH (R01 CA159296, R01 CA135332, R01 CA135243 and P50-CA140158), Leukemia Lymphoma Society and D. Warren Brown Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tam CS, Keating MJ. Chemoimmunotherapy of chronic lymphocytic leukemia. Nat Rev Clin Oncol. 2010;7(9):521–532. doi: 10.1038/nrclinonc.2010.101. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 3.Pleyer L, Egle A, Hartmann TN, Greil R. Molecular and cellular mechanisms of CLL: novel therapeutic approaches. Nat Rev Clin Oncol. 2009;6(7):405–418. doi: 10.1038/nrclinonc.2009.72. [DOI] [PubMed] [Google Scholar]
- 4.Jaglowski SM, Byrd JC. Rituximab in chronic lymphocytic leukemia. Semin Hematol. 2010;47(2):156–169. doi: 10.1053/j.seminhematol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H. Humanized CD52 monoclonal antibody Campath-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol. 1996;93(1):151–153. doi: 10.1046/j.1365-2141.1996.450989.x. [DOI] [PubMed] [Google Scholar]
- 6.Hale G. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy. 2001;3(3):137–143. doi: 10.1080/146532401753174098. [DOI] [PubMed] [Google Scholar]
- 7.Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 8.Lin TS, Moran M, Lucas M, Waymer S, Jefferson S, Fischer DB, et al. Antibody therapy for chronic lymphocytic leukemia: a promising new modality. Hematol Oncol Clin North Am. 2004;18(4):895–913. doi: 10.1016/j.hoc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359(6):613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 11.Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, et al. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood. 2010;116(14):2554–2558. doi: 10.1182/blood-2009-11-253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaglowski SM, Alinari L, Lapalombella R, Muthusamy N, Byrd JC. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood. 2010;11;116(19):3705–3714. doi: 10.1182/blood-2010-04-001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Lapalombella R, Joshi T, Cheney C, Gowda A, Hayden-Ledbetter MS, et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110(7):2569–2577. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapalombella R, Yeh YY, Wang L, Ramanunni A, Rafiq S, Jha S, et al. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21(5):694–708. doi: 10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robak T, Robak P, Smolewski P. TRU-016, a humanized anti-CD37 IgG fusion protein for the potential treatment of B-cell malignancies. Curr Opin Investig Drugs. 2009;10(12):1383–1390. [PubMed] [Google Scholar]
- 16.Moldenhauer G. Cd37. J Biol Regul Homeost Agents. 2000;14(4):281–283. [PubMed] [Google Scholar]
- 17.Schwartz-Albiez R, Dorken B, Hofmann W, Moldenhauer G. The B cell-associated CD37 antigen (gp40-52). Structure and subcellular expression of an extensively glycosylated glycoprotein. J Immunol. 1988;140(3):905–914. [PubMed] [Google Scholar]
- 18.Gupta P, Goldenberg DM, Rossi EA, Cardillo TM, Byrd JC, Muthusamy N, et al. Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas. Blood. 2012;119(16):3767–3778. doi: 10.1182/blood-2011-09-381988. [DOI] [PubMed] [Google Scholar]
- 19.Kontermann R. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4(2) doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parren PW, Burton DR. Immunology. Two-in-one designer antibodies. Science. 2009;323(5921):1567–1568. doi: 10.1126/science.1172253. [DOI] [PubMed] [Google Scholar]
- 21.Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, et al. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. 2011;117(17):4530–4541. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta P, Goldenberg DM, Rossi EA, Chang CH. Multiple signaling pathways induced by hexavalent, monospecific, anti-CD20 and hexavalent, bispecific, anti-CD20/CD22 humanized antibodies correlate with enhanced toxicity to B-cell lymphomas and leukemias. Blood. 2010;116(17):3258–3267. doi: 10.1182/blood-2010-03-276857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapalombella R, Yu B, Triantafillou G, Liu Q, Butchar JP, Lozanski G, et al. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood. 2008;112(13):5180–5189. doi: 10.1182/blood-2008-01-133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapalombella R, Zhao X, Triantafillou G, Yu B, Jin Y, Lozanski G, et al. A novel Raji-Burkitt’s lymphoma model for preclinical and mechanistic evaluation of CD52-targeted immunotherapeutic agents. Clin Cancer Res. 2008;14(2):569–578. doi: 10.1158/1078-0432.CCR-07-1006. [DOI] [PubMed] [Google Scholar]
- 25.Saul JM, Annapragada AV, Bellamkonda RV. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release. 2006;114(3):277–287. doi: 10.1016/j.jconrel.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release. 2011;153(2):141–148. doi: 10.1016/j.jconrel.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Kluza E, Jacobs I, Hectors SJ, Mayo KH, Griffioen AW, Strijkers GJ, et al. Dual-targeting of alphavbeta3 and galectin-1 improves the specificity of paramagnetic/fluorescent liposomes to tumor endothelium in vivo. J Control Release. 2012;158(2):207–214. doi: 10.1016/j.jconrel.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Modery CL, Ravikumar M, Wong TL, Dzuricky MJ, Durongkaveroj N, Sen Gupta A. Heteromultivalent liposomal nanoconstructs for enhanced targeting and shear-stable binding to active platelets for site-selective vascular drug delivery. Biomaterials. 2011;32(35):9504–9514. doi: 10.1016/j.biomaterials.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 29.Chen CH, Liu DZ, Fang HW, Liang HJ, Yang TS, Lin SY. Evaluation of multi-target and single-target liposomal drugs for the treatment of gastric cancer. Biosci Biotechnol Biochem. 2008;72(6):1586–1594. doi: 10.1271/bbb.80096. [DOI] [PubMed] [Google Scholar]
- 30.Laginha K, Mumbengegwi D, Allen T. Liposomes targeted via two different antibodies: Assay, B-cell binding and cytotoxicity. Bba-Biomembranes. 2005;1711(1):25–32. doi: 10.1016/j.bbamem.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Sapra P, Allen TM. Improved outcome when B-cell lymphoma is treated with combinations of immunoliposomal anticancer drugs targeted to both the CD19 and CD20 epitopes. Clin Cancer Res. 2004;10(7):2530–2537. doi: 10.1158/1078-0432.ccr-03-0376. [DOI] [PubMed] [Google Scholar]
- 32.Chu TW, Yang J, Kopecek J. Anti-CD20 multivalent HPMA copolymer-Fab′ conjugates for the direct induction of apoptosis. Biomaterials. 2012;33(29):7174–7181. doi: 10.1016/j.biomaterials.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, et al. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood. 2010;116(14):2554–2558. doi: 10.1182/blood-2009-11-253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu B, Mao Y, Bai LY, Herman SE, Wang X, Ramanunni A, et al. Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood. 2013;121(1):136–147. doi: 10.1182/blood-2012-01-407742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu GN, Edwards LA, Kapanen AI, Malinen MM, Dragowska WH, Warburton C, et al. Modulation of cancer cell survival pathways using multivalent liposomal therapeutic antibody constructs. Mol Cancer Ther. 2007;6(3):844–855. doi: 10.1158/1535-7163.MCT-06-0159. [DOI] [PubMed] [Google Scholar]
- 36.Jesse Popov AIK, Turner Christopher, Ng Rebecca, Tucker Catherine, Chiu Gigi, Klasa Richard, Bally Marcel B, Chikh Ghania. Multivalent rituximab lipid nanoparticles as improved lymphoma therapies: indirect mechanisms of action and in vivo activity. Nanomedicine. 2011;6(9):1575–1591. doi: 10.2217/nnm.11.50. [DOI] [PubMed] [Google Scholar]
- 37.Iden DL, Allen TM. In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochim Biophys Acta. 2001;1513(2):207–216. doi: 10.1016/s0005-2736(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 38.Allen TM, Sapra P, Moase E. Use of the post-insertion method for the formation of ligand-coupled liposomes. Cell Mol Biol Lett. 2002;7(3):889–894. [PubMed] [Google Scholar]
- 39.Yicheng Mao JW, Wu Yun, Chen Ching-Shih, Phelps Mitch A, Lee Robert J, James Lee L, Muthusamy Nataranjan. A Novel Liposome Formulation of FTY720. Nanomedicine. 2013 doi: 10.1016/j.nano.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loisel S, Andre PA, Golay J, Buchegger F, Kadouche J, Cerutti M, et al. Antitumour effects of single or combined monoclonal antibodies directed against membrane antigens expressed by human B cells leukaemia. Molecular cancer. 2011;10:42. doi: 10.1186/1476-4598-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heider KH, Kiefer K, Zenz T, Volden M, Stilgenbauer S, Ostermann E, et al. A novel Fc-engineered monoclonal antibody to CD37 with enhanced ADCC and high proapoptotic activity for treatment of B-cell malignancies. Blood. 2011;118(15):4159–4168. doi: 10.1182/blood-2011-04-351932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krause G, Patz M, Isaeva P, Wigger M, Baki I, Vondey V, et al. Action of novel CD37 antibodies on chronic lymphocytic leukemia cells. Leukemia. 2012;26(3):546–549. doi: 10.1038/leu.2011.233. [DOI] [PubMed] [Google Scholar]
- 44.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 45.Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62(24):7190–7194. [PubMed] [Google Scholar]
- 46.Belov L, Huang P, Barber N, Mulligan SP, Christopherson RI. Identification of repertoires of surface antigens on leukemias using an antibody microarray. Proteomics. 2003;3(11):2147–2154. doi: 10.1002/pmic.200300599. [DOI] [PubMed] [Google Scholar]
- 47.Belov L, Mulligan SP, Barber N, Woolfson A, Scott M, Stoner K, et al. Analysis of human leukaemias and lymphomas using extensive immunophenotypes from an antibody microarray. Br J Haematol. 2006;135(2):184–197. doi: 10.1111/j.1365-2141.2006.06266.x. [DOI] [PubMed] [Google Scholar]
- 48.Kato K, Toda M, Iwata H. Antibody arrays for quantitative immunophenotyping. Biomaterials. 2007;28(6):1289–1297. doi: 10.1016/j.biomaterials.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Grillo-Lopez AJ, White CA, Dallaire BK, Varns CL, Shen CD, Wei A, et al. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr Pharm Biotechnol. 2000;1(1):1–9. doi: 10.2174/1389201003379059. [DOI] [PubMed] [Google Scholar]
- 50.Liu Q, Alinari L, Chen CS, Yan F, Dalton JT, Lapalombella R, et al. FTY720 shows promising in vitro and in vivo preclinical activity by downmodulating Cyclin D1 and phospho-Akt in mantle cell lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(12):3182–3192. doi: 10.1158/1078-0432.CCR-09-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111(1):275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kontermann RE. Immunoliposomes for cancer therapy. Curr Opin Mol Ther. 2006;8(1):39–45. [PubMed] [Google Scholar]
- 53.White CA, Weaver RL, Grillo-Lopez AJ. Antibody-targeted immunotherapy for treatment of malignancy. Annu Rev Med. 2001;52:125–145. doi: 10.1146/annurev.med.52.1.125. [DOI] [PubMed] [Google Scholar]