Abstract
Although aspirin-exacerbated respiratory disease (AERD) has attracted a great deal of attention because of its association with severe asthma, it remains widely under-diagnosed in the asthmatic population. Oral aspirin challenge is the best method of diagnosing AERD, but this is a time-consuming procedure with serious complications in some cases. Thus, development of non-invasive methods for easy diagnosis is necessary to prevent unexpected complications of aspirin use in susceptible patients. For the past decade, many studies have attempted to elucidate the genetic variants responsible for risk of AERD. Several approaches have been applied in these genetic studies. To date, a limited number of biologically plausible candidate genes in the arachidonate and immune and inflammatory pathways have been studied. Recently, a genome-wide association study was performed. In this review, the results of these studies are summarized, and their limitations discussed. In addition to the genetic variants, changes in methylation patterns on CpG sites have recently been identified in a target tissue of aspirin hypersensitivity. Finally, perspectives on application of new genomic technologies are introduced; these will aid our understanding of the genetic pathogenesis of aspirin hypersensitivity in asthma.
Keywords: Aspirin, hypersensitivity, asthma, single nucleotide polymorphism, genome-wide association study, methylation
INTRODUCTION
Since its introduction to medicine more than 110 years ago, aspirin has been the most commonly prescribed medication for control of pain and prevention of various vascular diseases. Aspirin (acetylsalicylic acid, ASA)-hypersensitivity refers to development of bronchoconstriction, nasal symptoms (aspirin-exacerbated respiratory diseases, AERD), and ocular and skin manifestations in asthmatics following ingestion of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs).1 Recently, aspirin hypersensitivity has attracted a great deal of attention because of its association with increased asthma severity, such as refractory asthma, and possible remodeling of both the upper and lower airways.2 The prevalence of aspirin hypersensitivity in adult asthmatics varies widely depending on whether it is identified by clinical history alone or by challenge with ASA.3 Based on patients' histories alone, the incidence of aspirin hypersensitivity in asthmatic adults is 3-5%, but this percentage doubles or triples when diagnosis is by challenge with ASA via the oral or bronchial route.4,5 Of note, more than 15% of patients are entirely unaware of suffering from aspirin intolerance; only provocation tests ultimately revealed patients' hypersensitivity in a European study.3 Thus, identification of aspirin hypersensitivity, especially in hidden cases, is essential to avoid occurrence of serious complications.
Diagnosis of AERD can be established with certainty only by provocation tests using increasing doses of ASA. Oral aspirin challenge (OAC) is the gold standard for confirmation of a diagnosis. However, OAC is a time-consuming procedure, and in some cases, serious complications can occur. Thus, the development of non-invasive diagnostic methods is necessary to prevent the unexpected complications of aspirin use in susceptible patients. Fewer than 100 association studies of genetic variants have attempted to discover the genetic variants associated with development of AERD. Some results have not been replicated due to small sample sizes or ethnic differences between study populations. In the present review, the genetic variants showing association with AERD are discussed.
HERITABILITY OF AERD AND A WHOLE-GENOME LINKAGE STUDY
Asthma is a genetically complex disease that is associated with the family syndrome of atopy and increased levels of total serum IgE, bronchial hyperreactivity (BHR), and elevated blood eosinophil count. These intermediate phenotypes are themselves highly heritable and are the subject of much research into the genetics of asthma. They cluster in families, indicating that a genetic component is likely to be operating. Linkage-based methods have been used in individual families where members are affected by the disease in an attempt to demonstrate linkage between the occurrence of disease and genetic markers in a chromosomal region. This approach has been successfully used to map and clone genes causing monogenic disorders with simple Mendelian inheritance such as cystic fibrosis.6 Using this approach, at least 5 asthma genes including a disintegrin and metalloprotease 33 (ADAM33) on 20p13, dipeptidylpeptidase 10 on 2q14.1, plant homeodomain zinc finger protein 11 on 13q14.2, G protein-coupled receptor for asthma susceptibility on 7p15-p14, and prostaglandin 2 receptor on 14q 24 have been identified as being associated with a high risk of asthma.
An intermediate genetic background may be present in aspirin hypersensitivity. The European Network on Aspirin-induced Asthma found that 6% of AERD patients had a family history of aspirin hypersensitivity.3 However, the low incidence of familial aggregation is a serious limitation to application of the whole-genome linkage study approach in AERD. Besides, the growing recognition of the limitations of linkage analysis in complex human diseases has shifted emphasis toward single nucleotide polymorphisms (SNPs) genotyping and association and haplotype analyses. Thus, further application of linkage analysis may not be expected.
CANDIDATE GENE ASSOCIATION STUDY USING SNPS OF ARACHIDONATE PATHWAY GENES
Association studies with AERD began with biologically plausible genes responsible for over- or under-production of critical modulators in the metabolism of arachidonic acids. Of these, a two-compartment model has been proposed in which both augmentation of cysteinyl leukotriene (CysLT) production and overexpression of CysLT receptors on inflammatory cells occur within the respiratory tract.7 In addition, lipoxins, thromboxanes, and prostaglandins contribute to adverse reactions to aspirin.
Cysteinyl leukotrienes and their receptors
CysLTs are important inflammatory mediators in the development of asthma, as they mediate bronchoconstriction, mucus oversecretion, and vascular permeability, as well as cell trafficking and innate immune responses. CysLTs are overproduced in the airways and circulation of asthmatics who are intolerant to aspirin.8 Aspirin challenge induces increased concentrations of leukotriene E4 in the urine and airways of those with aspirin-intolerant asthma (AIA) compared with patients with aspirin-tolerant asthma (ATA). The CysLTs are synthesized by the 5-lipoxygenase (ALOX5) from arachidonic acid. The ALOX5 pathway contains several distinct enzymes, including cytosolic phospholipase A2, ALOX5, ALOX5-activating protein, and leukotriene C4 synthase (LTC4S), which is the terminal enzyme for CysLTs production. LTC4S is highly expressed in the bronchial mucosa of AIA compared with ATA patients, and this increase is significantly correlated with bronchial hyperresponsiveness to inhaled lysine aspirin.9
LEUKOTRIENE C4 SYNTHASE (LTC4S on 5q35, MIM#246530): LTC4S rs730012 (-444A>C) on the promoter was associated with the risk of AIA in a Polish population (Table 1).10 This allele is a transcription-factor-binding site for histone H4 transcription factor-2, binding of which results in increased transcription. However, other studies have found no significant association between LTC4S polymorphism and AIA in other ethnic groups.11,12 In a study of the Korean population,13 the frequency of the LTC4S -444C allele in AIA was similar to that in a Japanese population, which was one-half the frequency of that in Polish and American populations, suggesting that ethnic differences in LTC4S gene polymorphism contribute to AIA (Table 1 and Fig. 1C).
Table 1.
AERD associated single nucleotide polymorphisms in the genes of cysteinyl leukotrienes synthesis and leukotriene receptors
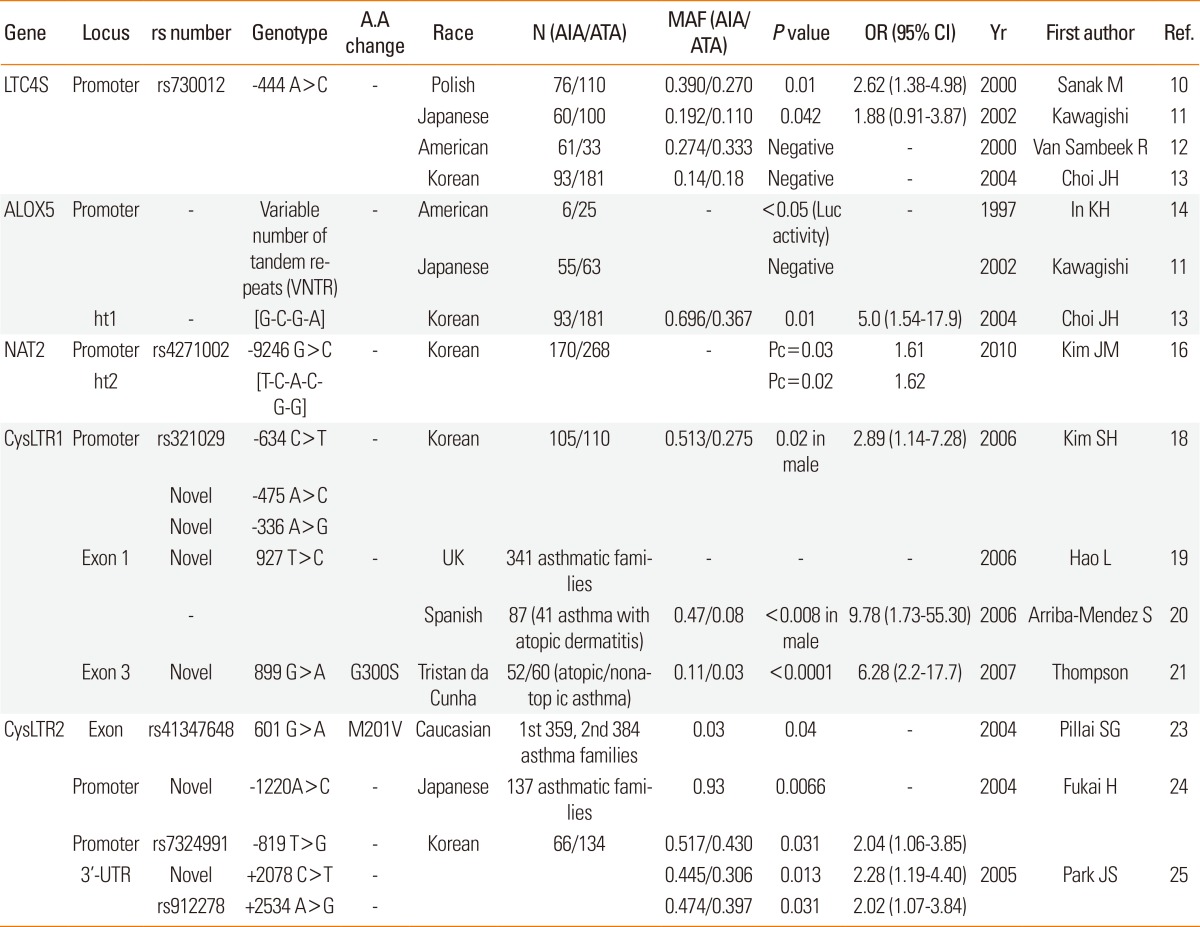
N, Number of study subjects; A.A, amino acid; AIA, Aspirin-induced asthma; ATA, Aspirin-tolerance asthma; MAF, Minor allele frequency; OR, odds ratio; CI, confidence interval; Yr, year; Ref, reference; LTC4S, Leukotriene C4 syntrhase; ALOX5, Arachidonate 5-lipoxygenase; NAT2, N-Acytyltransferase 2; CysLTR1, Cysteinyl leukotriene receptor 1; CysLTR2, Cysteinyl leukotriene receptor 2.
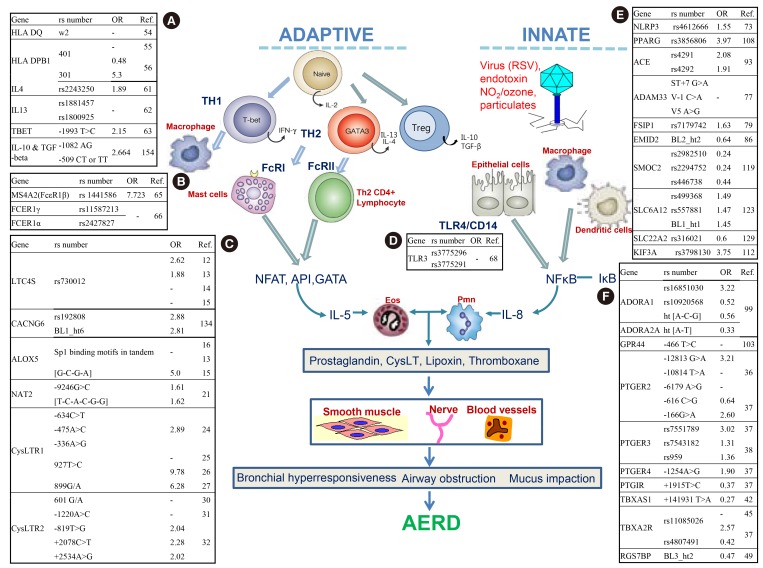
Fig. 1.
Summary of AERD associated single nucleotide polymorphisms in the genes of immune response and arachidonate pathway. OR, odds ratio; Ref, reference. (A) HLA DQ, Major histocompatibility complex, class II, DQ; HLA-DPB1, Major histocompatibility complex, class II, DP beta 1; IL4, INTERLEUKIN 4; IL 13, INTERLEUKIN 13; TBET, T-BOX EXPRESSED IN T CELLS; IL-10, Interleukin 10; TGF-beta 1, transforming growth factor, beta 1. (B) MS4A2, MEMBRANE-SPANNING 4-DOMAINS, SUBFAMILY A, MEMBER 7; FCER1γ, Fc fragment of IgE, high affinity I, receptor for; gamma polypeptide; FCER1α, Fc fragment of IgE, high affinity I, receptor for; alpha polypeptide. (C) LTC4S, Leukotriene C4 syntrhase; CACNG6, Calcium channel, voltage-dependent gamma-6 subunit; ALOX5, Arachidonate 5-lipoxygenase; NAT2, N-Acytyltransferase 2; CysLTR1, Cysteinyl leukotriene receptor 1; CysLTR2, Cysteinyl leukotriene receptor 2. (D) TLR3, TOLL-LIKE RECEPTOR 3. (E) NLRP3, NLR FAMILY, PYRIN DOMAIN-CONTAINING 3; PPARG, Peroxisome proliferator-activated receptor-gamma; ACE, Angiotensin 1-converting enzyme; ADAM33, A distegrin and metalloproteinase domain 33; FSIP1, Fibrous sheath interacting protein 1; EMID2, Emilin/multimerin domain-containing protein 2; SMOC2, Sparc-related modular calcium-binding protein 2; SLC6A12, Solute carrier family 6 (neurotransmitter transporter, betaine/gaba), member 12; SLC22A2, Solute carreier familyY 22 (organic cation transporter), member 2; KIF3A, Kinesin family member 3A. (F) ADORA1, adenosine A1 receptor; ADORA2A, adenosine A2a receptor; GPR44, G protein-coupled receptor 44; PTGER2, Prostaglandin E Receptor 2; PTGER3, Prostaglandin E Receptor 3; PTGER4, Prostaglandin E Receptor 4; PTGIR, Prostaglandin I2 receptor; TBXAS1, Thromboxane A synthase 1; TBXA2R, Thromboxane A2 receptor; RGS7BP, Regulator of G protein signaling 7-binding protein.
ARACHIDONATE 5-LIPOXYGENASE (ALOX5 on 10q11.2, MIM#152390): The initial enzymatic step in leukotriene (LT) production is the oxidation of arachidonic acid by ALOX5 to LTA4. A variable number of tandem repeats (VNTR), other than 5 in the Sp1-binding motif GGGCGG in the promoter region, diminishes ALOX5 gene expression.14 However, VNTR was not related to the AIA phenotype in a study of a Japanese population.11 In a Korean population,13 the frequency of the ALOX5-ht1[G-C-G-A] haplotype was significantly higher in the AIA than in the ATA group (Table 1 and Fig. 1), suggesting possible involvement of ALOX5 gene polymorphisms in AIA.
N-ACETYLTRANSFERASE 2 (NAT2 on 8p22, MIM#612182): The CysLTs, comprising LTC4, LTD4, and LTE4, are eliminated from the bloodstream by the liver and kidneys. The CysLTs can be inactivated by N-acetylation, and their ώ-backbone is subject to carboxylation and β-elimination. The o-carboxy-N-acetyl-LTE4 is degraded exclusively in peroxisomes. The CysLTs are inactivated by acetyl coenzyme A-dependent NAT2.15 Thus, functional alterations in the NAT gene may contribute to the risk of AIA. Of six common SNPs of the NAT2 gene in a Korean population, minor allele frequencies of NAT2 -9246G>C and haplotype 2 (TCACGG) were significantly higher in the AIA than in the ATA group (Table 1 and Fig. 1C),16 suggesting that the genetic variant of NAT2 gene may be associated with aspirin hypersensitivity via the different degradation of CystLTs.
CYSTEINYL LEUKOTRIENE RECEPTOR 1 (CYSLT1R on Xq13.2-q21.1, MIM#30020) and CYSLT2R (on 13q14.2-21.1, MIM#605666): CysLTs exert their biological actions by binding two types of G-protein-coupled seven-transmembrane receptors: CysLTR 1and CysLTR2. CysLTs bind to CYSLTR1, which is antagonized selectively by leukotriene modifiers such as montelukast, pranlukast, and zafirlukast. The main role of CysLTR2 in the lung is mediated through effects on macrophages and smooth muscle.17 Genetic variants -634C>T, -475A>C, and -336A>G in the promoter region of the CYSLT1R gene were associated with AERD in a Korean population via modulation of CysLTR1 m-RNA expression.18 Allele frequencies of the three SNPs were significantly different in male subjects only. A three-SNP haplotype, ht2 [T-C-G], is associated with increased risk of AERD. A variant of the CYSLT1R gene (+927T>C) was associated with atopy severity in British and Spanish populations.19,20 The CYSLT1R +899G>A variant in the coding region was detected at a significantly higher frequency in atopics and asthmatics in a Tristan da Cunha population (Table 1 and Fig. 1C).21
The CYSLT2R gene is located close to a chromosomal locus for increased risk of asthma in various populations.22 Because CysLTR2 mRNA is abundantly expressed in eosinophils, the main effector cells in asthma and AERD, CysLTR2 may have an important physiological role in CYSLT2R gene polymorphisms are associated with the risk of asthma development in Caucasian and Japanese populations.23,24 In a Korean population, asthmatics having minor alleles for -819G>T in the promoter and 2078C>T or 2534A>G in the 3'-UTR exhibited a more pronounced bronchospasm by aspirin provocation than did those who carried common alleles (Table 1 and Fig. 1C).25 This suggests that CYSLT2R polymorphisms may induce AERD by increasing mRNA levels via affecting their stability.26
Prostaglandins and their receptors
Prostaglandin E receptors (PTGERs): PGE2 modulates airway tone by inhibiting acetylcholine release by cholinergic nerve endings and histamine release from mast cells, and by directly suppressing aspirin-induced CysLTs synthesis from eosinophils and mast cells infiltrating the bronchial mucosa. These effects may be exerted via prostaglandin E receptors on airway leukocytes. At least four subtypes of PTGERs have been identified to date.27 They differ in their tissue distribution, ligand-binding affinity, and coupling to intracellular signaling pathways. An extensive association study of the 370 SNPs mainly on the arachidonate pathway demonstrated that SNPs in the promoter region of the PTGER2 gene (-12813G>A, -10814T>C, and -6179A>G) were significantly associated with aspirin hypersensitivity in asthma in a Japanese population (Table 2 and Fig. 1F).28 The most significantly associated SNP, -12813 G/A, is located in the regulatory region of the PTGER2 gene in which a STATs binding consensus sequence is present. In Korean populations (Table 2 and Fig. 1F),29 several SNPs on PTGER2, 3, and 4 showed a good association with aspirin hypersensitivity in asthma. Of seven SNPs on PTGER2, the minor allele frequency of -616C>G was significantly lower in AIA than in ATA patients, whereas AIA patients had a significantly higher frequency of GG homozygotes of -166G>A (Table 2 and Fig. 1F).29 One PTGER3 polymorphism in the promoter region (-1709T>A) exhibited a different genotype distribution in Korean AIA and ATA patients. The minor allele frequency was higher in AIA than in ATA patients (Table 2 and Fig. 1F).29 Another Korean study revealed that rs7543182 and rs959 in PTGER3 retained their susceptibility to aspirin intolerance (Table 2 and Fig. 1F).30 In the case of PTGER4, the frequencies of GG homozygotes and heterozygotes of -1254A>G in the promoter region were significantly higher in AIA than in ATA patients (Table 2 and Fig. 1F).29 In the case of prostaglandin I receptors (PTGIR), patients with AIA had one-half of the frequency of the +1915T>C CC/CT genotype compared to ATA patients (Table 2 and Fig. 1F).29 These data suggest that SNPs on the PTGERs genes might exert their genetic effect through coordination of each gene.
Table 2.
AERD associated single nucleotide polymorphisms in the genes of prostaglandin and thromboxane synthease and their receptors
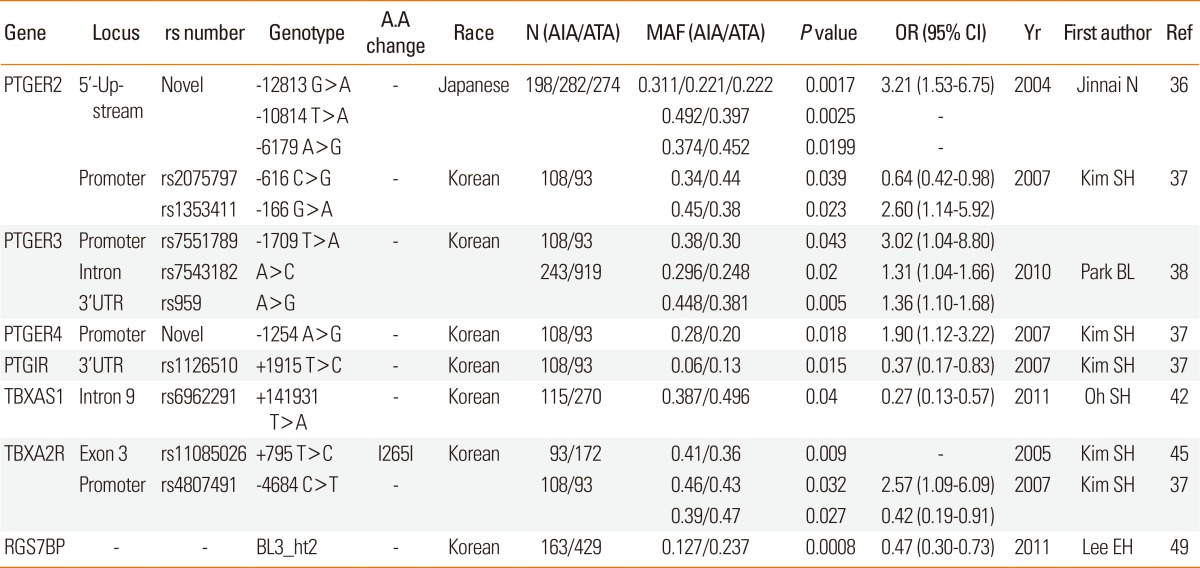
A.A, amino acid; AIA, Aspirin-induced asthma; ATA, Aspirin-tolerance asthma; MAF, Minor allele frequency; OR, odds ratio; CI, confidence interval; Yr, year; Ref, reference; PTGER2, Prostaglandin E Receptor 2; PTGER3, Prostaglandin E Receptor 3; PTGER4, Prostaglandin E Receptor 4; PTGIR, Prostaglandin I2 receptor; TBXAS1, Thromboxane A synthase 1; TBXA2R, Thromboxane A2 receptor; RGS7BP, Regulator of G protein signaling 7-binding protein.
THROMBOXANE A SYNTHASE 1 (TBXAS1 on 7q34-q35, MIM#274180) and THROMBOXANE A2 RECEPTOR (TBXA2R on 19p13.3, MIM#188070): Thromboxane A2 (TXA2) induces bronchoconstriction and bronchial hyper-responsiveness and stimulates proliferation of human airway smooth muscle cells and immune cells.31 Metabolites of thromboxane increase in the urine and airways of AIA compared to ATA in the basal condition before aspirin challenge.32 Aspirin challenge decreases concentrations of thromboxane B2 in asthmatics. Thromboxane synthase catalyzes the conversion of prostaglandin H to thromboxane A2. A genetic variant study of a Korean population demonstrated that the frequency of the minor allele +141931T>A (rs6962291) in intron 9 was significantly lower in the AIA than the ATA groups. AA carriers of +141931T>A had significantly lower plasma TXB2 levels than TT carriers. The mRNA levels of the full-length wild-type and an exon-12-deleted splice variant were significantly higher in the TT than in the AA homozygotes of +141931T>A. Based on these data, it appears that the minor allele of rs6962291 may protect against aspirin hypersensitivity via a lower catalytic activity of the TBXAS1 gene, itself attributable to an increased nonfunctioning isoform of TBXAS1 (Table 2 and Fig. 1F).33
TXA2 exerts its effects by interacting with the G protein-coupled TBXA2R. Genetic association studies have identified a positive association between TBXA2R polymorphisms and the risk of asthma, atopy, and atopic dermatitis.34,35 The minor C allele frequency of TBXA2R rs11085026 (+795T>C) on exon 3 was significantly higher in AIA than in ATA (Table 2 and Fig. 1F).36 The association was validated in another Korean AIA population. Of the three SNPs rs4807491 (-4684C>T), +795T>C, and R343Q of TBXA2R, one SNP in the promoter area (-4684C>T) and one non-synonymous coding SNP in exon 3 (+795T>C) were significantly associated with the AIA phenotype. The frequency of CC homozygote in -4684C>T on the promoter in the AIA patients was one-half and that of CC homozygote in 795T>C was two times higher in the AIA patients compared to those in the ATA patients (Table 2 and Fig. 1F).29
REGULATOR OF G PROTEIN SIGNALING 7-BINDING PROTEIN (RGS7BP on 5q12.3, MIM#610890): G protein coupled receptors (GPCRs) mediate cellular responses to diverse signals, including neurotransmitters, hormones, and sensory stimuli. Many arachidonic acid metabolites signal via GPCRs, including CysLTR1 and 2, prostaglandin D2 receptor, PTGERs, TBXA2R, and M2 muscarinic receptor. These receptors have seven membrane-spanning regions and interact with intracellular, heterotrimeric G proteins, which comprise α, β, and γ subunits and play a critical role in signal transduction by coupling extracellular receptors to intracellular signaling pathways.37 Signal-regulated palmitoylation of RGS7BP initiates the activation of GPCRs via regulation of RGS-binding proteins.38 Thus, genetic alterations in the RGS7BP gene may induce functional changes in GPCRs, including the M2 muscarinic receptor, as well as others, including those through which CysLTs, prostaglandins, lipoxins, and thromboxanes signal. In a Korean population, a haplotype of block 3, consisting of the minor alleles +98092C>G, +98853C>T in intron 5, and +104450T>G in the 3'UTR of the RGS7BP gene, was associated with AERD. The log-transformed provocation concentration that caused a decrease in forced expiratory volume in 1 second (FEV1) of 20% for methacholine was significantly dependent on the BL3-ht2 haplotype, suggesting these genetic variants protect against aspirin hypersensitivity in asthma, perhaps by altering muscarinic responsiveness (Table 2 and Fig. 1F).39
Genes outside the arachidonate pathway
Th1 and Th2 immune responses
Development of the disease is controlled by both host genetic and a variety of environmental factors. Although environmental influences such as improvements in hygiene may have increased the prevalence of allergic diseases, at least several dozen polymorphic genes regulate development of asthma. These control the inflammatory and immune responses and mesenchymal or epithelial function leading to airway remodeling. A shift from T helper type 1 (Th1) to a Th2 immune response is the main mechanism of asthma, resulting in the overproduction of cytokines such as interleukin 4 (IL-4), IL-5, and IL-13 and underproduction of the Th1-type cytokine interferon-gamma (IFN-γ). As in other asthmatics, the airways of AIA patients show persistent inflammation, with marked eosinophilia, cytokine production, and upregulation of inflammatory molecules.40 Thus, the Th1/Th2 imbalance may contribute to development of AERD.
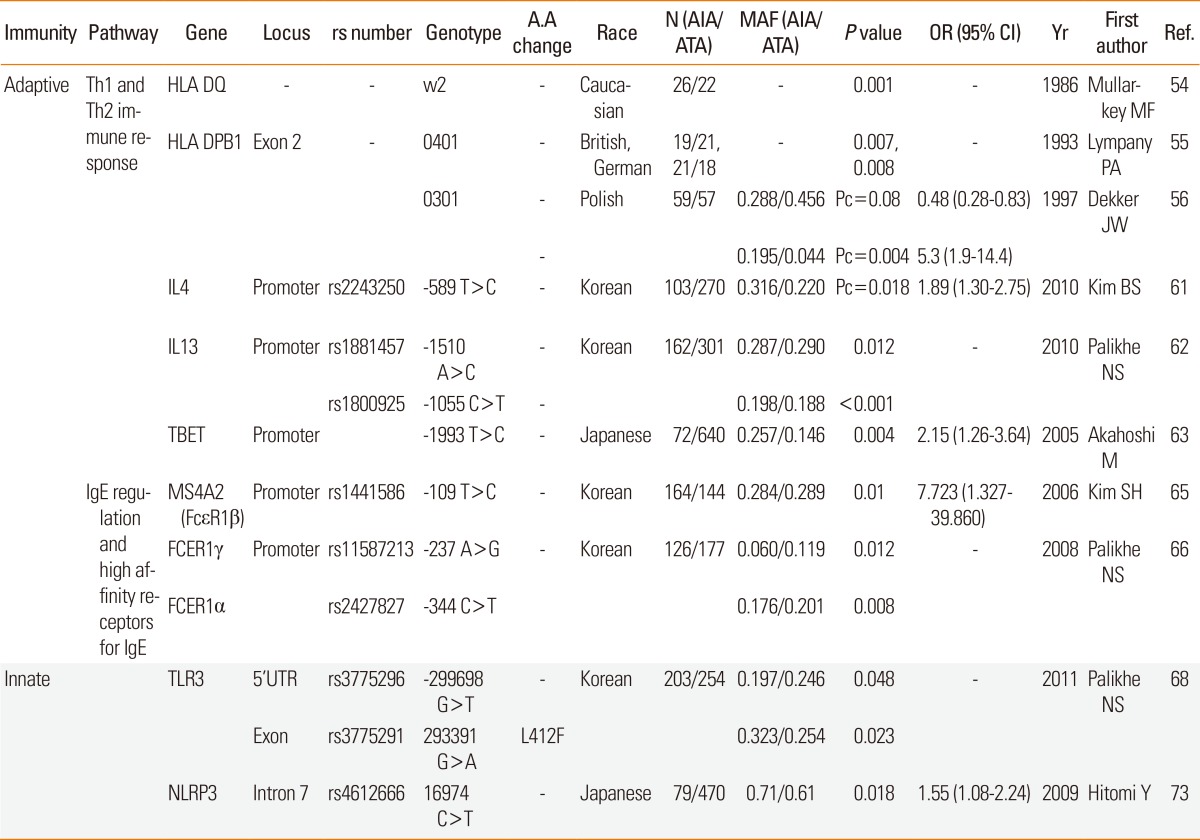
MAJOR HISTOCOMPATIBILITY COMPLEX, CLASS II (MHC on chromosome 6p21.3): HLA-DQ (DQ) is a cell-surface receptor found on antigen-presenting cells. DQ is a αβ heterodimer of the MHC Class II type. The α and β chains are encoded by HLA-DQA1 and HLA-DQB1, respectively, and vary greatly. Different DQ isoforms bind to and present different antigens to T-cells. T-cells are then stimulated to grow and can signal B-cells to produce antibodies. In addition to foreign antigens, DQ is involved in recognizing common self-antigens and presenting those antigens to the immune system to develop tolerance. HLA-DP is a protein/peptide-antigen receptor composed of two subunits, DPα and DPβ, which are encoded by two loci, HLA-DPA1 and HLA-DPB1. Amino acids located at key positions along the α-helical portions of these HLA heterodimers dictate which peptide antigens can bind. Even single amino acid substitutions in these regions may alter the shape of the HLA-peptide binding pocket sufficiently to change its specificity.41 In a limited number of Caucasian patients, a significant increase in HLA-DQw2 was associated with AIA (Table 3 and Fig. 1A).42 In British and German populations, the incidence of DPB1*0401 was lower in ATA compared with AIA (Table 3 and Fig. 1A).43 DPB1*0401 was more frequently present in a polish population, where the DPB1*0301 frequency was increased fourfold in AIA compared with ATA patients (Table 3 and Fig. 1A).44 These findings suggest that AIA represents a disease entity that involves different immune responses than other forms of asthma.
Table 3.
AERD associated single nucleotide polymorphisms in the genes of immune response
A.A, amino acid; AIA, Aspirin-induced asthma; ATA, Aspirin-tolerance asthma; MAF, Minor allele frequency; OR, odds ratio; CI, confidence interval; Yr, year; Ref, reference; HLA DQ, Major histocompatibility complex, class II, DQ; HLA-DPB1, Major histocompatibility complex; class II, DP beta 1; IL4, INTERLEUKIN 4; IL 13, INTERLEUKIN 13; T-bet, T-BOX EXPRESSED IN T CELLS; MS4A2, MEMBRANE-SPANNING 4-DOMAINS; SUBFAMILY A, MEMBER 7; FCER1γ, Fc fragment of IgE, high affinity I, receptor for; gamma polypeptide; FCER1α, Fc fragment of IgE, high affinity I, receptor for; alpha polypeptide; TLR3, TOLL-LIKE RECEPTOR 3; NLRP3, NLR FAMILY, PYRIN DOMAIN-CONTAINING 3.
INTERLEUKIN 4 (IL4 on 5q31.1, MIM#147780): Given that IL-4 plays a central role in the development of allergic asthma and atopy, genetic variation of IL-4 may alter its transcription and translation and influence the pathogenesis of allergic diseases. The possible associations of -589C>T (rs2243250) with asthma and airway obstruction in asthmatics have been investigated.45,46 As one of the important biochemical pathways regulating inflammatory cells, ASA inhibits nuclear factor k-light-chain-enhancer of activated B cells activation, and interleukin-4 (IL-4) and IL-13-induced STAT6 activation.47 In a Korean population, of 15 SNPs tested, the frequency of rare allele rs2243250 (-589T>C) was higher in the AIA than the ATA group (Table 3 and Fig. 1A).48 Functional characterization of this SNP indicates that CCAAT-enhancer-binding proteins β and nuclear factor of activated T-cells are its transcription factors and that their binding is augmented by aspirin. These data indicate that aspirin may regulate IL4 expression in an allele-specific manner by altering the availability of transcription factors to the key regulatory elements in the IL4 promoter, thus leading to aspirin hypersensitivity.
INTERLEUKIN 13 (IL13 on 5q31, MIM#147683): IL-13 is a key cytokine involved in allergic inflammation by inducing airway eosinophilia and bronchial hyper-reactivity. AIA is characterized by chronic rhinosinusitis and nasal polyposis due to persistent upper and lower airway inflammation with marked eosinophilia. In a Korean population, AIA patients with the AA genotype rs1881457 (-1510A>C) and CC genotype rs1800925 (-1055C>T) on the promoter had a significantly higher frequency of rhinosinusitis compared with those with the minor alleles of these two SNPs (Table 3 and Fig. 1A),49 suggesting participation of IL-13 gene polymorphisms in AERD upper airway remodeling.
T-BOX EXPRESSED IN T CELLS (TBET or T-BOX 21 on 17q21.32, MIM#604895t): A Th1-specific transcription factor of the T-box family controls INF-γ production by T helper cells. Of the 24 known SNPs, a promoter -1993T>C SNP was significantly associated with a risk of AIA. This allele is in linkage disequilibrium with a synonymous coding +390A>G SNP in exon 1. This association has also been confirmed in additional independent samples of asthma with nasal polyposis. On functional characterization, the -1993T>C substitution increased the affinity of a particular nuclear protein to the binding site of TBX21 covering the -1993 position (Table 3 and Fig. 1A).50 These data indicate that increased T-beta and subsequent change of IFN-γ production in human airways of individuals with the -1993T>C polymorphism may contribute to the development of AIA.
IgE regulation and high affinity receptors
MEMBRANE-SPANNING 4-DOMAINS, SUBFAMILY A, MEMBER 7 (MS4A7 on 11q13, CD20/FCER1B FAMILY MEMBER 4, MIM#606502): The beta chain of the high-affinity receptor for IgE is linked to atopy and asthma. The β-chain of FcepsilonR1 enhances receptor maturation and signal transduction capacity, leading to the release of pro-inflammatory mediators such as prostaglandins, leukotrienes, and cytokines such as IL-4, IL-5, IL-13, TNF-α and chemokines including IL-8, and monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β from mast cells, and basophils. In a Korean population, two genetic polymorphisms of the FcεR1β gene [FcεR1 β rs1441586 (-109T>C) and FcεR1β E237G] were associated with AIA. FcεR1β -109T>C polymorphism was significantly associated with the presence of IgE to staphylococcal enterotoxin B (SEB), with higher MS4A2 promoter activity in cell lines (Table 3 and Fig. 1B).51
Of the two SNPs of FcεR1α [ rs2427827 (-344C>T) and rs2251746 (-95T>C) ], two SNPs of FcεR1β [MS4A2 rs1441586 (-109T>C) and MS4A2 E237G], and two SNPs of F FcεR1γ [FcεR1γ rs11587213 (-237A>G) and F FcεR1γ-54G>T], the genotype frequencies of FcεR1γ-237A>G differed significantly between AIA and ATA patients. Additionally, AIA patients carrying the homozygous AA genotype of FcεR1γ-237A>G had significantly higher total serum IgE levels than did those with the GG/AG genotype. AIA patients expressing the CT/TT genotype at FcεR1α-344C>T had a higher prevalence of serum IgE specific to staphylococcal enterotoxin A than did those with the CC genotype (Table 3 and Fig. 1B),52 suggesting that the FcεR1γ-237A>G and FcεR1α-344C>T polymorphisms may contribute to development of AIA via regulation of IgE production.
TOLL-LIKE RECEPTOR 3 (TLR3 on 4q35, MIM#603029): Because eosinophils activated via TLR3, one of the virus-recognizing TLRs, recruit leukocytes to sites of inflammation, eosinophils may function as a link between viral infection and exacerbation of allergic disease. Viral respiratory infections contribute to allergic sensitization and the development of asthma and exacerbation in subjects with already established asthma. Aspirin hypersensitivity is diminished in some AERD patients during acyclovir treatment of herpes simplex infection.53 In a Korean population, AERD patients had a significantly higher frequency of missense variants "A" allele of rs3775291 (+293391G>A, Leu412Phe) than did the ATA group (Table 3 and Fig. 1D).54 The amino acid change from Leu to Phe results in functional deterioration of TLR3 and predisposes individuals to increased susceptibility to the innate immune response, which may be a cause of viral-induced AERD.
NLR FAMILY, PYRIN DOMAIN-CONTAINING 3(NLRP3 on 1q44, MIM#606416): A member of the nucleotide-binding domain, leucine-rich repeat-containing (NLR) family controls the activity of inflammatory caspase-1 by forming inflammasomes. Tight collaboration between pathogen-associated molecular patterns and their receptors initiates an innate immune response, and NLRP3 inflammasomes are activated by pathogen-associated molecular patterns including microbial toxins, live bacteria, and viruses.55 After being activated, NALP3 recruits apoptosis-associated speck-like proteins containing procaspase-1, leading to activation of caspase-1. Activated caspase-1 cleaves the procytokines IL-1b and IL-18 into their active forms. Of 15 tag SNPs of NLRP in a large population, one (rs4612666) was significantly associated with AIA. The risk allele of rs4612666 increases the enhancer activity of NLRP3 expression and NLRP3 mRNA stability (Table 3 and Fig. 1E),56 indicating that the NLRP3 SNP might play an important role in the development of AIA in a gain-of-function manner.
Airway remodeling and fibrosis genes
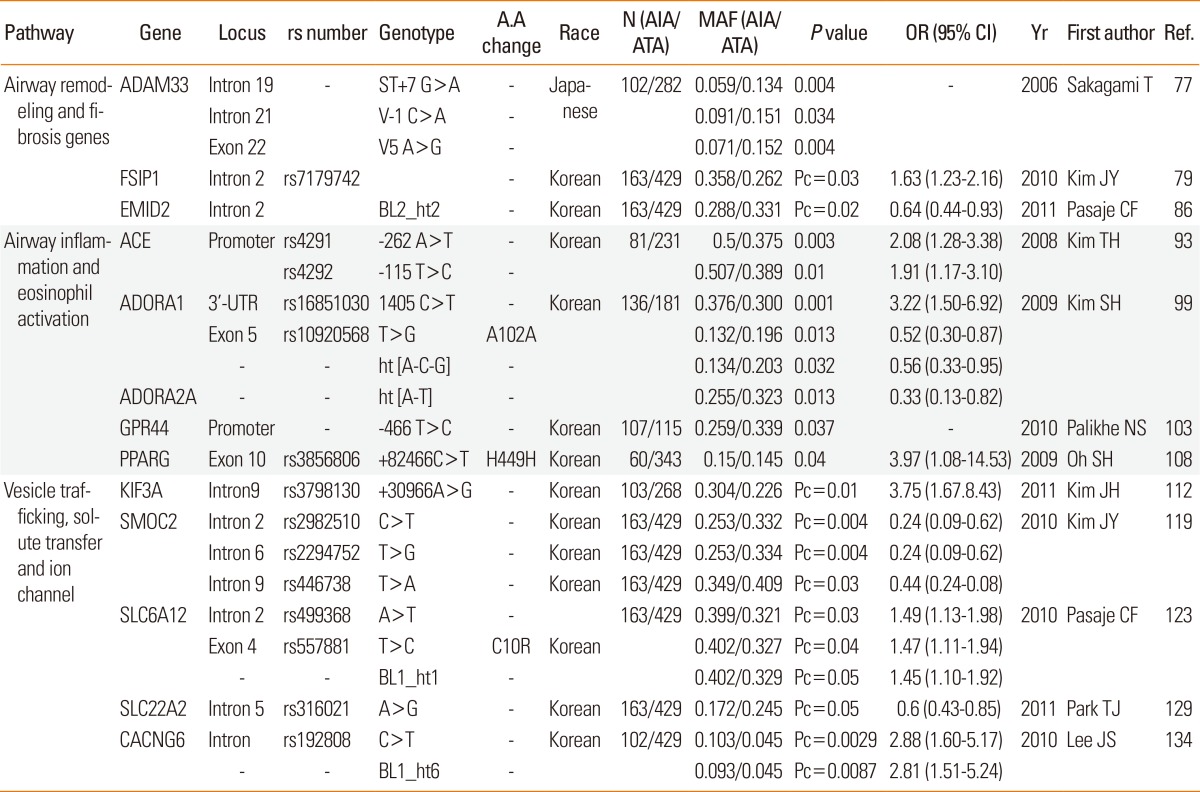
A DISINTEGRIN AND METALLOPROTEINASE DOMAIN 33 (ADAM33 on 20q13, MIM#607114): ADAM33 is expressed strongly in smooth muscle layers and basement membrane in more than 80% of subjects with asthma but not in normal control subjects, indicating that ADAM33 may be involved in airway remodeling in asthma.57 A genome-wide screen revealed ADAM33 to be a novel asthma-susceptibility gene that plays a role in AHR.58 ADAM33 polymorphisms are associated with asthma susceptibility and airway hyperreactivity in several ethnicities, including the Korean population.59 In a Japanese population, of 10 polymorphic sites (ST+4, ST+7, T1, T2, T+1, V-3, V-2, V-1, V4, V5), the ST+7, V-1, and V5 sites in the AIA group were significantly different from those in the ATA group. Haplotypes of three sites (ST+7, V-1, and V5) were significantly different in frequency between the AIA and ATA groups, indicating that the ADAM33 sequence variations are likely to correlate with susceptibility to AIA (Table 4 and Fig. 1E).60
Table 4.
AERD associated single nucleotide polymorphisms in the genes of airway inflammation and remodeling
A.A, amino acid; AIA, Aspirin-induced asthma; ATA, Aspirin-tolerance asthma; MAF, Minor allele frequency; OR, odds ratio; CI, confidence interval; Yr, year; Ref, reference; ADAM33, A distegrin and metalloproteinase domain 33; FSIP1, Fibrous sheath interacting protein 1; EMID2, Emilin/multimerin domain-containing protein 2 ; ACE, Angiotensin 1-converting enzyme; ADORA1, adenosine A1 receptor; ADORA2A, adenosine A2a receptor; GPR44, G protein-coupled receptor 44; PPARG, Peroxisome proliferator-activated receptor-gamma; KIF3A, Kinesin family member 3A; SMOC2, Sparcrelated modular calcium-binding protein 2; SLC6A12, Solute carrier family 6 (neurotransmitter transporter, betaine/gaba), member 12; SLC22A2, Solute carreier familyY 22 (organic cation transporter), member 2; CACNG6, Calcium channel, voltage-dependent gamma-6 subunit.
FIBROUS SHEATH-INTERACTIONG PROTEIN 1 (FSIP1, HSD10): With its primary function in protein binding, the FSIP1 gene is expressed in the airway epithelium. FSIP1 is regulated by amyloid beta precursor protein (APP).61 APP is an integral membrane protein expressed in many tissues, particularly in the synapses of neurons. APP is cleaved by ADAM33. Of 66 SNPs in the FSIP1 gene, one (rs7179742) was associated with AIA in a Korean population (Table 4 and Fig. 1E).62 In Asian populations from the International HapMap Project (http://hapmap.ncbi.nlm.nih.gov/), the FSIP1 gene is in LD with the thrombospondin-1 (THBS1 or TSP-1) gene. The THBS1 gene has been implicated in a network underlying the pulmonary response to oxidative stress in asthma.63 Aspirin leads to a reduction in THBS1 levels.64 These data suggest that FSIP1 affects aspirin hypersensitivity in asthma associated with the nearby THBS1 gene.
EMILIN/MULTIMERIN DOMAIN-CONTAINING PROTEIN 2 (EMID2 on 7q22.1, MIM#608927): Airway remodeling is the dominant physiological event that leads to the reversible clinical symptoms in asthma such as airway hyperresponsiveness and airflow obstruction. Airway remodeling may not be entirely reversible, and subepithelial fibrosis has been highlighted as an integral component of the remodeling response. Accumulation of extracellular matrix (ECM) components such as fibronectin and types I, III, and V collagens in the basement membrane results in thickening of the subepithelial space.65 Emilin is a component of elastic fibers located at the elastin-microfibril interface. Pathological studies have observed an abnormal deposition of elastic fibers in both large and small asthmatic airways. Additionally, EMID2 is a glycosylated protein that is secreted and deposited in the ECM, serving a variety of structural and functional roles.66 In Korean subjects, five of 49 SNPs and the BL2 ht2 haplotype (unique to the minor alleles of rs4727494 and rs13233066) were significantly associated with AIA, indicating that EMID2 polymorphisms could cause meaningful deficits in the upper and lower airways of AIA patients (Table 4 and Fig. 1E).67
Airway inflammation and eosinophil activation
Angiotensin 1-converting enzyme (ACE on 17q23, MIM# 106180) is a membrane-bound peptidase expressed by epithelial and endothelial cells. ACE inactivates a wide range of peptides, including kinins and substance P, which are overproduced in the lungs of asthmatics.68 The inhibition of ACE is linked to suppression of kinase II activity, resulting in the accumulation of kinins, substance P, and prostaglandins in the airways and consequent stimulation of vagal afferents, which in turn leads to bronchial hyper-reactivity and airway inflammation, especially eosinophilic inflammation, in asthmatics.69 Of four SNPs [rs4291 (-262A>T) and rs4292 (-115T>C) in the 5'-flanking region and +5467T>C [Pro450Pro] and +11860A>G [Thr776Thr] in the coding region] and one ins/del (+21288 CT), the frequency of the minor alleles of -262A>T and -115T>C was higher in subjects with AIA than in subjects with ATA in a Korean asthma cohort. ACE promoters containing the minor -262A>T allele possessed lower promoter activity than did the common allele, indicating that the minor allele may confer aspirin hypersensitivity via down-regulation of ACE expression (Table 4 and Fig. 1E).70
ADENINE DEAMINASE (ADA on 20q13, MIM#608958): Adenosine is endogenous in human tissues at low concentrations but increases markedly in the extracellular space during inflammation. Inhalation of adenosine induces acute bronchoconstriction in those with asthma through interactions with four GPCRs for adenosine.71 In subjects with asthma, lysine aspirin inhalation attenuates the bronchoconstrictor response induced by AMP inhalation, which is linked to cyclooxygenase (COX) inhibition, causing reduced production of prostaglandins and thromboxanes. Additionally, adenosine receptor agonist-attenuated airway inflammation in an allergen-induced animal model of asthma72 and adenosine receptor-deficient mice exhibited enhanced AHR and airway inflammation. Of 13 SNPs in adenosine deaminase (ADA) and the four adenosine receptors (ADORA1, ADORA2A, ADORA2B, and ADORA3), significant differences were detected between patients with AIA and those with ATA. ADORA1 SNPs [rs16851030 (1405C>T), rs10920568 (A102A)] and haplotypes (ht[A-C-G] in ADORA1 and ht[A-T] in ADORA2) are significantly associated with AIA, suggesting that adenosine might play a crucial role in the development of AIA through interactions with the A(1) and A(2A) receptors (Table 4 and Fig. 1F).73
G PROTEIN-COUPLED RECEPTOR 44 (GPR44, CRTH2, on 11q12-q13.3, MIM#604837): PGD2, a major prostanoid produced by allergen-activated mast cells, is an important mediator in the pathogenesis of eosinophilic airway inflammation via its receptor, a chemoattractant receptor molecule expressed on Th2 cells (CRTH2). The human CRTH2 gene is also expressed in other allergy-related cells such as eosinophils, basophils, and monocytes and mediates chemotaxis of these cells toward PGD2.74 Genetic alteration of CRTH2 is related to allergic asthma in African-Americans.75 In Korean subjects, two polymorphisms (-466T>C and -129C>A) were significantly different between AIA and ATA patients. The -466T allele exhibits higher eotaxin-2 production in human lung epithelial cells, indicating that this polymorphism increases serum and cellular eotaxin-2 production in AERD patients through lowered GRP44 expression, leading to eosinophilic infiltration (Table 4 and Fig. 1F).76
PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR-GAMMA (PPARG on 3p25, MIM#601487). PPARs are transcription factors activated by ligands of the nuclear hormone receptor superfamily. Three different PPAR subtypes have been identified: PPARα (PPARA), PPARγ (PPARG), and PPARð (PPARD), which is also called PPARβ. A variety of natural substances, including arachidonate pathway metabolites such as 15-hydroxyeicosatetranoic acid (15-HETE), strongly promote PPARG expression. Stimulation of the PPARG ligand significantly inhibited the downregulation of eosinophil function.77 PPARG expression is associated with the inflammatory and remodeling responses in the asthmatic airway. Additionally, levels of proinflammatory, immune cytokines and chemokines, including IL-2, IL-3, IL-4, IL-5, IL-13, GM-CSF, and eotaxin are increased in the airways and systemic circulation in AIA.78 The production of these molecules is regulated by various transcription factors, including PPARG. PPARG gene polymorphisms are associated with risk of asthma exacerbation in Caucasian populations.79 In Korean subjects, rs3856806 (+82466C>T, His449His) and haplotype 1 (CC) (+34C>G [Pro12Ala] were associated with development of aspirin hypersensitivity in asthmatics. The frequency of the minor allele of +82466C>T was two times higher in AIA than in ATA patients. Additionally, the frequency of PPARG haplotype 1 was significantly lower in AIA than in ATA patients, indicating that the +82466C>T polymorphism and haplotype 1 of the PPARG gene may be linked to an increased risk of aspirin hypersensitivity in asthma (Table 4 and Fig. 1E).80
Vesicle trafficking, solute transfer, and ion channels
KINESIN FAMILY MEMBER 3A (KIF3A on 5q31-33, MIM#604683): KIF3A encodes a motor subunit of kinesin-2, an important component for cilia formation, and plays a crucial role in the generation and functioning of cilia. In the case of Bardet-Biedl syndrome, which includes an increased incidence of asthma (25%), abnormal functions of cilia have been discovered.81 Differential expression of KIF3A is present in nasal epithelial cells of exacerbated asthmatics and normal controls, and KIF3A polymorphisms are significantly associated with asthma.82 KIF3A mRNA level is increased in aspirin-induced bronchial epithelial cells and protein expression is also up-regulated in nasal polyp epithelia in AIA patients.83 CysLTD4, a key mediator in AIA, can affect ciliary activity and orientation. Interestingly, the IL-4 gene has a strong LD with KIF3A in the same LD block.83 Aspirin also regulates IL4 expression in an allele-specific manner by altering the availability of transcription factors to the key regulatory elements in the IL4 promoter, leading to aspirin hypersensitivity.48 Another explanation for the association between KIF3A and aspirin hypersensitivity in asthma may be over-production of CysLTs by cyclooxygenase (COX) inhibition through constrained β-catenin-dependent Wnt signaling derived from a KIF3A abnormality.84 This stabilization of β-catenin may lead to NF-κB activation, which also plays a crucial role in immune responses, resulting in the activation of target effectors, such as COX-2. Of a total of 22 SNPs in introns and five haplotypes, several SNPs were significantly associated with AIA. The KIF3A ht3 haplotype, which is unique to most of the minor alleles, showed the most significant association with AIA. Several KIF3A isoforms derived from alternative splicing events in intron 9 have been observed; thus, it is possible that the rs3798130 in intron 9 may produce dysfunctional products in respiratory epithelia, resulting in increased expression of KIF3A (Table 4 and Fig. 1E).83
SPARC-RELATED MODULAR CALCIUM-BINDING PROTEIN 2 (SMOC2 on 6q27, MIM#607223): A number of intracellular signaling proteins are implicated in various aspects of the inflammatory processes that regulate asthma pathogenesis. Pulmonary surfactant synthesis is decreased in experimentally induced asthma when the intracellular storage capacity and its physical activity are hindered.85 Alveolar type II cells synthesize pulmonary surfactant, which is a surface-active lipoprotein complex. SMOC2 is involved in the recycling of lamellar bodies-associated proteins or in surfactant uptake from the extracellular space.86 SMOC2 exhibits GTPase activity and interacts with clathrin and EpsinR on the early endosome/trans-Golgi network, thus affecting vesicle trafficking.87 In Korean subjects, of 19 SNPs, rs2982510, rs2294752, and rs446738 were associated with the increased susceptibility to AIA (Table 4 and Fig. 1E).88
SOLUTE CARRIER FAMILY 6 (NEUROTRANSMITTER TRANSPORTER, BETAINE/GABA), MEMBER 12 (SLC6A12 on 12p13, MIM#603080): Although the SLC6A12 gene, also referred to as sodium and chloride-dependent betaine/GABA transporter-1, is widely expressed in the proximal tubules of the kidney and cells of the central nervous system,89 an excitatory GABAergic system was described recently in the airway epithelium. γ-aminobutyric acid (GABA) signaling in the airway epithelium plays a critical role in asthma development through its ability to enhance mucus production.90 Aspirin is involved in the detoxification of GABA-lytic picrotoxin (PT), an antagonist of GABA-receptor chloride channels.91 The inhibition of picrotoxin by aspirin restores GABA activity. In Korean subjects, of eight SNPs, two polymorphisms (rs499368 and rs557881 [non-synonymous C10R]) and the BL1 ht1 haplotype were significantly associated with AIA (Table 4 and Fig. 1E).92 A non-synonymous variant translates to an amino acid (C10R) change from T to C in the rs557881 polymorphism. Modification of cysteine by introduction of either a methyl or t-butyl group on the free sulfhydryl group and replacement of the guanidine group with a urea linkage in the side chain of arginine may be a risk factor for AIA.
SOLUTE CARRIER FAMILY 22 (ORGANIC CATION TRANSPORTER), MEMBER 2 (SLC22A2 on 6q26, MIM#602608): SLC22A2 is a member of the solute carrier family 22 superfamily of organic cation transporters and mediates the transport of prostaglandins in the basolateral membrane of the proximal tubule.93 Polyspecific organic cation transporters are involved in transportation of acetylcholine as a novel regulator of airway remodeling.94 Release of acetylcholine from bronchial epithelial cells mediates bronchoconstriction following the release of serotonin from mast cells. Additionally, airway epithelial cells possess various muscarinic receptors that serve as potential targets of locally released acetylcholine, and dysregulation of these receptors in airway epithelial cells is a major cause of asthma. Overexpression of SLC22A2 variants (Thr199Ileu, Thr201Met, and Ala270Ser) decreases uptake of [3H]methyl-4-phenylpyridinium acetate and [14C]tetraethylammonium (TEA) compared with wild-type SLC22A2-expressing cells.95 These data suggest that the three SLC22A2 SNPs are involved in development of asthma via a reduced vital capacity, itself caused by a decrease in the transport of TEA because TEA plays a role in increased vital capacity among asthmatic patients. In Korean subjects, of 18 variants, a SNP in intron 5 (rs316021) was significantly associated with susceptibility to AIA. The minor allele frequency of rs316021 in the AIA group was significantly lower than that in the ATA controls, suggesting that SLC22A2 may be associated with susceptibility to aspirin intolerance in asthmatics (Table 4 and Fig. 1E).96
CALCIUM CHANNEL, VOLTAGE-DEPENDENT, GAMMA-6 SUBUNIT (CACNG6 on 19q13.4, MIM#606898): Among the remarkable signaling molecules released by mast cells, LTC4 is secreted following Ca2+ influx through store-operated calcium-release-activated calcium (CRAC) channels. Airway smooth muscle cell contraction is regulated by changes in intracellular Ca2+ concentration and airway bronchodilation. Novel effects of NSAIDs on vascular ion channels, including L-type calcium channels, have also been suggested.97 L-type calcium channels are composed of five subunits. The CACNG6 gene encodes one of these, specifically the gamma subunit protein, which was first identified in muscle cells. Recent studies have revealed negative associations of CACNG6 gene expression with chronic obstructive pulmonary disease and responses of the human airway epithelium to injury.98 In Korean subjects, of eight variants, a SNP (rs192808C>T) in the intron and a haplotype unique to this variant (CACNG6 BL1 ht6) were significantly associated with risk of AIA, suggesting an association with risk of AIA (Table 4 and Fig. 1C).99
GENOME-WIDE ASSOCIATION STUDY
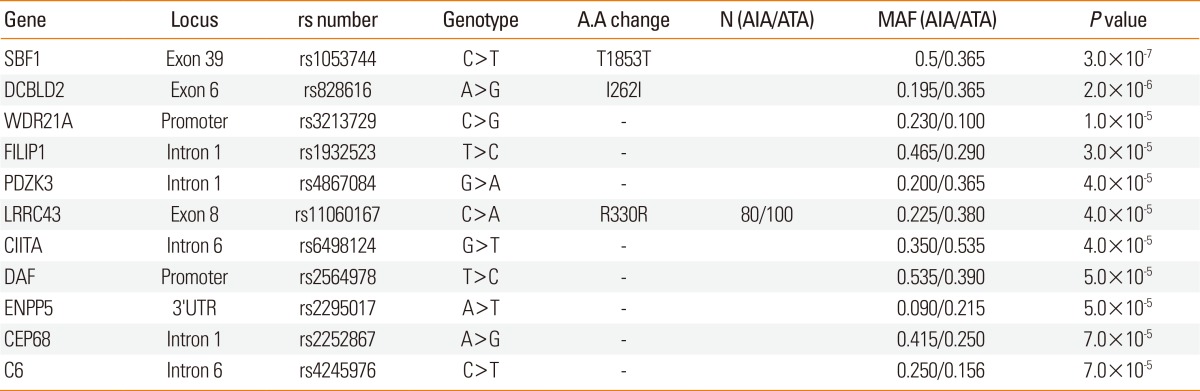
In addition to the genes of the arachidonate pathway, variants of several genes in the immune response and inflammatory pathways are also associated with aspirin hypersensitivity in asthmatics. These data suggest that genetic variations in other pathways may be more relevant to the development of aspirin hypersensitivity in asthmatics than has previously been thought. The International Hap-Map Consortium has revealed nearly 4,000,000 SNPs and demonstrated that individual SNPs predict adjacent SNPs, suggesting that genotyping of 500,000 SNPs may allow a nearly complete survey of all common genetic variations. Based on this concept, whole-genome SNP genotyping arrays were developed and have for the past 5 years been applied to studies of the genetic background underlying multifactorial complex diseases. Recently, genome-wide association studies (GWAS) of asthma and related phenotypes have reported several susceptibility-associated genes, including ORMDL3, PDE4D, and IL1RL1.100-102 A GWAS of 109,365 SNPs was undertaken in a Korean AIA cohort using aspirin-tolerant asthma subjects as controls. Results suggested 11 candidate genes with the greatest differences in frequency between AIA and ATA (Table 5). The second stage of fine mapping of 150 common SNPs from the 11 candidate genes showed that SNPs of CEP68 had the most significant association with aspirin intolerance. Seven such SNPs and a CEP68 ht4 haplotype (T-G-A-A-A-C-G) exhibited a highly significant association with aspirin intolerance. Moreover, the non-synonymous CEP68 rs7572857G>A variant, in which glycine is replaced with serine, shows greater aspirin-provocation-induced bronchospasm than other variants, implying that CEP68 may be associated with susceptibility to aspirin intolerance in asthmatics.103 This study also confirmed the association of several other genes (TNF, TGF, HLA-DPB1, ALOX5, and IL-10) with AIA. There has been a debate concerning whether GWAS can successfully detect variants that are associated with diseases. However, GWAS has not only made possible prediction of risk factors associated with diseases but has also led to discovery of additional disease-associated variants.104,105 Dense genome-wide genotyping chips containing over 1 M SNPs have recently been developed and will reveal further genes associated with AERD.
Table 5.
AERD associated single nucleotide polymorphisms in the genes of Korean Genome-Wide Association Study (modified from Ref. 103)
A.A, amino acid; AIA, Aspirin-induced asthma; ATA, Aspirin-tolerance asthma; MAF, Minor allele frequency; Ref, reference; SBF1, SET binding factor 1; DCBLD2, Discoidin, CUB and LCCL domain containing 2; WDR21A, DDB1 and CUL4 associated factor 4; FILIP1, Filamin A interacting protein 1; PDZK3, PDZ domain containing 2; LRRC43, Leucine rich repeat containing 43; CIITA, Class II, major histocompatibility complex, transactivator; DAF, CD55 molecule, decay accelerating factor for complement; ENPP5, Ectonucleotide pyrophosphatase/phosphodiesterase 5; CEP68, Centrosomal protein 68 kDa; C6, Complement component 6.
GENE-GENE INTERACTIONS
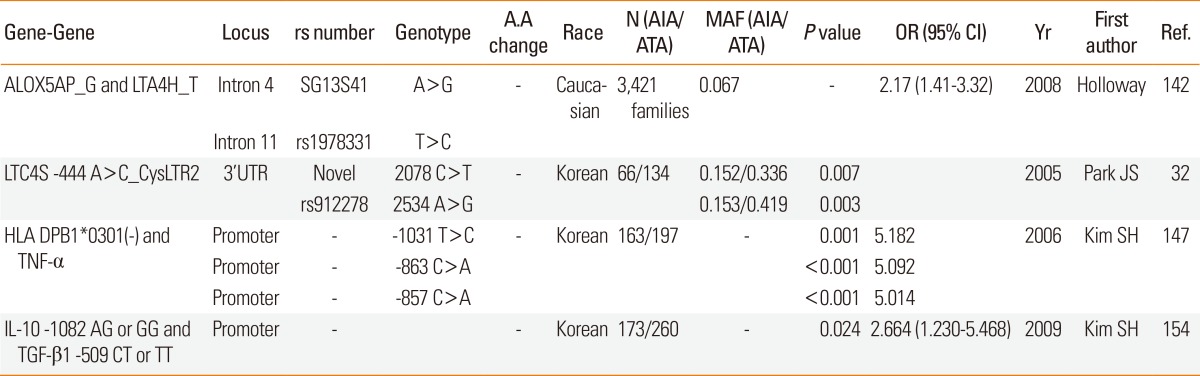
ALOX5AP and LTA4H genes: In a Korean population study,13 the frequency of the ALOX5-ht1[G-C-G-A]-containing genotype in the AIA group was significantly higher than in the ATA group. Individual odds ratios for risk alleles were 1.78 for SG13S41(G) on ALOX5AP and 1.22 for rs1978331(T) on LTA4H. Gene-gene interactions between these two demonstrated that asthmatics carrying both risk alleles of ALOX5AP and LTA4H (G allele in SG13S41/Intron4 and a T allele in rs1978331/Intron11, respectively) had a twofold increased risk of aspirin hypersensitivity (Table 6).106
Table 6.
AERD associated single nucleotide polymorphisms in gene to gene interaction
A.A, amino acid; AIA, Aspirin-induced asthma; ATA, Aspirin-tolerance asthma; MAF, Minor allele frequency; OR, odds ratio; CI, confidence interval; Yr, year; Ref, reference; ALOX5AP, arachidonate 5-lipoxygenase-activating protein; LTA4H, leukotriene A4 hydrolase; LTC4S, leukotriene C4 synthase; CysLTR2, cysteinyl leukotriene receptor 2; TNF-α, tumor necrosis factor α; HLA-DPB1, major histocompatibility complex, class II, DP β 1; IL-10, Interleukin 10; TGF-β 1, transforming growth factor, β 1.
CYSLTR2 and LTC4S genes: The association of CYSLTR2 2078 C>T and CYSLTR2 rs912278 (2534 A>G) with decreased FEV1 following aspirin challenge became more pronounced when paired analysis was performed with LTC4S -444 A>C. Asthmatics homozygotic for the rare allele CYSLTR2 2078 C>T and the minor allele LTC4S -444 A>C showed the greatest FEV1 decrease following aspirin challenge in recessive models. Additionally, asthmatics homozygotic for the rare allele CYSLTR2 2534 A>G and the minor allele LTC4S -444 A>C showed the greatest bronchospam following aspirin challenge (Table 6).25
TNF-α and HLA DPB1: TNF-α is associated with airway hyper-responsiveness (AHR) and the phenotype of severe asthma. The TNF-α gene and HLA DPB1 lie adjacent to each other within the class III region of the MHC on chromosome 6p21, so gene-to-gene interaction between TNF-α and the HLA subtype may contribute to the development of AIA. Three SNPs of TNF-α gene (-1031T>C, -863C>A, and -857C>T) polymorphisms are in significant LD with HLA B and HLA DRB1 allele.107 TNF-α -308G>A was significantly associated with atopic asthma in a Korean adult and child asthma cohort.108,109 Gene-to-gene interaction between TNF-α -1031T>C (or -863C>A or -857C>A) and HLA DPB1*0301 significantly increased susceptibility to AIA (Table 6).110
IL-10 and TGF-β1: AIA is characterized by excessive proliferation of target tissues such as eosinophilic rhinosinusitis and nasal polyposis. The TGF-β1 -509 C>T polymorphism on the promoter is associated with rhinosinusitis in AIA patients.111 The regulatory function of TGF-β1 is interrelated with IL-10.112 A gene-gene interaction between TGF-β1 and IL-10 polymorphisms has also been implicated in allergy and asthma.113 The IL-10 haplotype (-1082A, -819T, and -592A) is considered to be a risk haplotype in atopic asthmatics in North India.114 The IL-10 ATA haplotype was associated with reduced IL-10 production observed in severe asthma.115 Furthermore, the -1082A>G of IL-10 was significantly associated with the AIA phenotype. Moreover, a synergistic effect between TGF-β1 -509 C>T and IL-10 -1082 A>G has been observed in the AIA phenotype. The frequency of minor alleles (the CT or TT genotype of TGF-β1-509 C>T and AG or GG genotype of IL-10-1082 A>G) was three times higher in patients with AIA than in those with ATA (Table 6 and Fig. 1A).116
PHARMACOGENETICS
Both augmentation of CysLTs production and overexpression of CysLT receptors on inflammatory cells occur within the respiratory tract in patients with AIA.7,117 The CysLT receptor is selectively antagonized by several currently available leukotriene modifiers, including montelukast, pranlukast, and zafirlukast. However, clinical studies have demonstrated that the response to these medications is incomplete.118,119 Individual differences in the response to leukotriene antagonists may be genetically determined by genes involved in either arachidonate metabolism or extra-arachidonate pathways. The HLA allele DPB1*0301 may represent the AERD phenotype. Furthermore, higher doses of leukotriene receptor antagonists are required to control asthma symptoms in patients with AERD.120 Of the arachidonate pathway genes, the CysLTR1 promoter polymorphism -634C>T is strongly associated with the mean montelukast dose required to maintain asthma control.121 Of the extra-arachidonate pathway genes, angiotensinogen is associated with individual differences in the effect of montelukast on bronchospasm protection following aspirin challenge.122 The angiotensinogen gene enhances the effect of several bronchoconstrictors and produces angiotensin in the airways of asthmatics. Of 38 SNPs in the AGT gene, two (+2401C>G and +2476C>T) in the AGT intron were significantly associated with modification of a long-term leukotriene-receptor antagonist effect. However, these data are preliminary, and a larger-scale study is warranted.
STRUCTURAL VARIATION AND EPIGENESIS IN AERD GENETICS
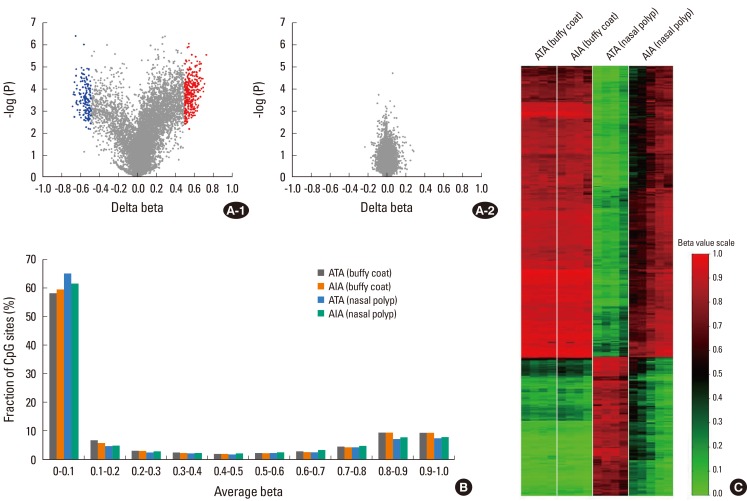
Previous epidemiologic studies have demonstrated that SNPs are not responsible for all differences in phenotype. Because monozygotic twins are genetically identical, asthma develops almost concurrently. However, a twin cohort study showed one fifth of concordance rate of self-reported asthma in monozygotic twin pairs.123 A possible explanation for this is epigenesis. Epigenetics is the study of heritable changes in DNA structure that do not alter the underlying sequence; well-known examples are DNA methylation and histone modification. These changes may remain through cell divisions for the remainder of the cell's life and may also last for multiple generations. For example, human epidemiologic studies have shown that the mother's diet affects her offspring's risk of allergic asthma.124 It is not known whether exposure to environmental agents induces epigenetic changes in aspirin hypersensitivity. In nasal polyps from subjects with AERD and ATAs, the methylation pattern of 27,168 DNA CpG sites was assessed using a whole-genome methylation analysis.125 Methylation patterns were significantly different in nasal polyps, but not so different in buffy coats. A volcano plot showed differential methylation levels in AERD and ATA: 332 CpG sites on 296 genes were hypomethylated, and 158 sites on 141 genes were hypermethylated (Fig. 2). CpG-site methylations in nasal polyps were not correlated with those of buffy coats, indicating that the difference in methylation pattern is nasal-tissue specific. Of the genes in the arachidonate pathway, prostaglandin E synthase was hypermethylated, whereas prostaglandin D synthase, arachidonate 5-lipooxygenase activating protein, leukotriene B4 receptor, and lipoxygenase homology domain1 were hypomethylated, indicating that this may be responsible for the existence of specific phenotypes, such as AERD, in asthma.
Fig. 2.
Summary of DNA methylation data. (A) Volcano plot of differential methylation level between AIA and ATA in nasal polyp tissues (A-1) and buffy coat samples (A-2). Red dots: Deltabeta≥0.5 and P value≤0.01, blue dots: Deltabeta ≤-0.5 and P value≤0.01, grey dots: -0.5≤Deltabeta≤0.5 and P value>0.01. Delta-Beta: difference of DNA methylation level (subtracting the DNA methylation level of ATA from AIA). -log (P): log-transformed t-test P values. (B) Distribution of the DNA methylation level of AIA and ATA in buffy coat and nasal polyp. Average Beta: DNA methylation level (0 to 1). (C) Heatmap of 490 differentially methylated CpGs between AIA and ATA in buffy coat and nasal polyp (modified from reference 125).
PERSPECTIVES
The GWAS and fine-mapping studies will increase the number of candidate genes associated with a well-defined sub-phenotype AERD. However, the genetic impact will improve only when sample sizes are more increased and the role of environmental contributors, such as the extent of aspirin exposure, are considered. Furthermore, much of the speculation about missing heritability from GWAS has focused on the possible contribution of rare variants (low minor-allele frequency [MAF] <0.5%). Because GWAS chips to date have been capable of analyzing common variants (>5% MAF), identification of rare variants is problematic, even though these rare alleles confer a substantial risk of disease.126 Sequencing of individual genomes has begun and should provide more information on these rare variants.127
In addition to SNPs, genomic variability can take many other forms, including variable numbers of tandem repeats (VNTRs), presence/absence of transposable elements (e.g., alu elements), and structural alterations (e.g., deletions, duplications, and inversions). Until recently, SNPs were thought to be the predominant form of genomic variation and to account for much normal phenotypic variation. However, the widespread presence of copy-number variation was recently reported in normal individuals.128 Contrary to our previous beliefs, identical twins are not genetically identical. A study of 19 pairs of monozygotic, identical twins found differences in copy-number variation and found that this was associated with Parkinson's disease.129 A genome-wide association study of CNV in 16,000 cases of eight common diseases, including types 1 and 2 diabetes, rheumatoid arthritis, and Crohn's disease, revealed that some CNV are strongly associated with the risk of disease development. It seems much more likely, however, that most genetic control is due to rarer variants, either single-site or structural, that are not represented in current studies and that have considerably larger effects than common variants. Therefore, integration of several-omics may be the solution to the search for the genetic background and mechanism underlying aspirin hypersensitivity in asthma.
ACKNOWLEDGMENTS
This study was supported by a grant from the Soonchunhyang University.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Lee RU, Stevenson DD. Aspirin-exacerbated respiratory disease: evlauation and management. Allergy Asthma Immunol Res. 2011;3:3–10. doi: 10.4168/aair.2011.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascia K, Haselkorn T, Deniz YM, Miller DP, Bleecker ER, Borish L TENOR Study Group. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2005;116:970–975. doi: 10.1016/j.jaci.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. 2000;16:432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 4.Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717–722. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- 5.Kalyoncu AF, Karakaya G, Sahin AA, Bariş YI. Occurrence of allergic conditions in asthmatics with analgesic intolerance. Allergy. 1999;54:428–435. doi: 10.1034/j.1398-9995.1999.00963.x. [DOI] [PubMed] [Google Scholar]
- 6.Zielenski J, Tsui LC. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet. 1995;29:777–807. doi: 10.1146/annurev.ge.29.120195.004021. [DOI] [PubMed] [Google Scholar]
- 7.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 8.Picado C. Aspirin-intolerant asthma: role of cyclo-oxygenase enzymes. Allergy. 2002;57(Suppl 72):58–60. doi: 10.1034/j.1398-9995.57.s72.14.x. [DOI] [PubMed] [Google Scholar]
- 9.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, Lam BK, Penrose JF, Austen FK, Holgate ST, Sampson AP. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–846. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanak M, Pierzchalska M, Bazan-Socha S, Szczeklik A. Enhanced expression of the leukotriene C(4) synthase due to overactive transcription of an allelic variant associated with aspirin-intolerant asthma. Am J Respir Cell Mol Biol. 2000;23:290–296. doi: 10.1165/ajrcmb.23.3.4051. [DOI] [PubMed] [Google Scholar]
- 11.Kawagishi Y, Mita H, Taniguchi M, Maruyama M, Oosaki R, Higashi N, Kashii T, Kobayashi M, Akiyama K. Leukotriene C4 synthase promoter polymorphism in Japanese patients with aspirin-induced asthma. J Allergy Clin Immunol. 2002;109:936–942. doi: 10.1067/mai.2002.124466. [DOI] [PubMed] [Google Scholar]
- 12.Van Sambeek R, Stevenson DD, Baldasaro M, Lam BK, Zhao J, Yoshida S, Yandora C, Drazen JM, Penrose JF. 5' flanking region polymorphism of the gene encoding leukotriene C4 synthase does not correlate with the aspirin-intolerant asthma phenotype in the United States. J Allergy Clin Immunol. 2000;106:72–76. doi: 10.1067/mai.2000.107603. [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, Park HS, Oh HB, Lee JH, Suh YJ, Park CS, Shin HD. Leukotriene-related gene polymorphisms in ASA-intolerant asthma: an association with a haplotype of 5-lipoxygenase. Hum Genet. 2004;114:337–344. doi: 10.1007/s00439-004-1082-1. [DOI] [PubMed] [Google Scholar]
- 14.In KH, Asano K, Beier D, Grobholz J, Finn PW, Silverman EK, Silverman ES, Collins T, Fischer AR, Keith TP, Serino K, Kim SW, De Sanctis GT, Yandava C, Pillari A, Rubin P, Kemp J, Israel E, Busse W, Ledford D, Murray JJ, Segal A, Tinkleman D, Drazen JM. Naturally occurring mutations in the human 5-lipoxygenase gene promoter that modify transcription factor binding and reporter gene transcription. J Clin Invest. 1997;99:1130–1137. doi: 10.1172/JCI119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orning L. Omega-oxidation of cysteine-containing leukotrienes by rat-liver microsomes. Isolation and characterization of omega-hydroxy and omega-carboxy metabolites of leukotriene E4 and N-acetylleukotriene E4. Eur J Biochem. 1987;170:77–85. doi: 10.1111/j.1432-1033.1987.tb13669.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim JM, Park BL, Park SM, Lee SH, Kim MO, Jung S, Lee EH, Uh ST, Park JS, Choi JS, Kim YH, Kim MK, Choi IS, Cho SH, Choi BW, Park HS, Chang HS, Shin HD, Park CS. Association analysis of N-acetyl transferase-2 polymorphisms with aspirin intolerance among asthmatics. Pharmacogenomics. 2010;11:951–958. doi: 10.2217/pgs.10.65. [DOI] [PubMed] [Google Scholar]
- 17.Metters KM. Leukotriene receptors. J Lipid Mediat Cell Signal. 1995;12:413–427. doi: 10.1016/0929-7855(95)00027-n. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Oh JM, Kim YS, Palmer LJ, Suh CH, Nahm DH, Park HS. Cysteinyl leukotriene receptor 1 promoter polymorphism is associated with aspirin-intolerant asthma in males. Clin Exp Allergy. 2006;36:433–439. doi: 10.1111/j.1365-2222.2006.02457.x. [DOI] [PubMed] [Google Scholar]
- 19.Hao L, Sayers I, Cakebread JA, Barton SJ, Beghé B, Holgate ST, Sampson AP, Holloway JW. The cysteinyl-leukotriene type 1 receptor polymorphism 927T/C is associated with atopy severity but not with asthma. Clin Exp Allergy. 2006;36:735–741. doi: 10.1111/j.1365-2222.2006.02511.x. [DOI] [PubMed] [Google Scholar]
- 20.Arriba-Mendez S, Sanz C, Isidoro-Garcia M, Davild I, Laffond E, Horeno E, Avila C, Lorente F. 927T>C polymorphism of the cysteinyl-leukotriene type-1 receptor (CYSLTR1) gene in children with asthma and atopic dermatitis. Pediatr Allergy Immunol. 2006;17:323–328. doi: 10.1111/j.1399-3038.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 21.Thompson MD, Capra V, Takasaki J, Maresca G, Rovati GE, Slutsky AS, Lilly C, Zamel N, McIntyre Burnham W, Cole DE, Siminovitch KA. A functional G300S variant of the cysteinyl leukotriene 1 receptor is associated with atopy in a Tristan da Cunha isolate. Pharmacogenet Genomics. 2007;17:539–549. doi: 10.1097/FPC.0b013e328012d0bf. [DOI] [PubMed] [Google Scholar]
- 22.Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, Faux JA, Ryan GF, le Söuef PN, Lathrop GM, Musk AW, Cookson WO. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996;383:247–250. doi: 10.1038/383247a0. [DOI] [PubMed] [Google Scholar]
- 23.Pillai SG, Cousens DJ, Barnes AA, Buckley PT, Chiano MN, Hosking LK, Cameron LA, Fling ME, Foley JJ, Green A, Sarau HM, Schmidt DB, Sprankle CS, Blumenthal MN, Vestbo J, Kennedy-Wilson K, Wixted WE, Wagner MJ, Anderson WH, Ignar DM Investigators of the GAIN Network. A coding polymorphism in the CYSLT2 receptor with reduced affinity to LTD4 is associated with asthma. Pharmacogenetics. 2004;14:627–633. doi: 10.1097/00008571-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Fukai H, Ogasawara Y, Migita O, Koga M, Ichikawa K, Shibasaki M, Arinami T, Noguchi E. Association between a polymorphism in cysteinyl leukotriene receptor 2 on chromosome 13q14 and atopic asthma. Pharmacogenetics. 2004;14:683–690. doi: 10.1097/00008571-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Park JS, Chang HS, Park CS, Lee JH, Lee YM, Choi JH, Park HS, Kim LH, Park BL, Choi YH, Shin HD. Association analysis of cysteinyl-leukotriene receptor 2 (CYSLTR2) polymorphisms with aspirin intolerance in asthmatics. Pharmacogenet Genomics. 2005;15:483–492. doi: 10.1097/01.fpc.0000166456.84905.a0. [DOI] [PubMed] [Google Scholar]
- 26.Shin JA, Chang HS, Park SM, Jang AS, Park SW, Park JS, Uh ST, Lim GI, Rhim T, Kim MK, Choi IS, Chung IY, Park BL, Shin HD, Park CS. Genetic effect of CysLTR2 polymorphisms on its mRNA synthesis and stabilization. BMC Med Genet. 2009;10:106. doi: 10.1186/1471-2350-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 28.Jinnai N, Sakagami T, Sekigawa T, Kakihara M, Nakajima T, Yoshida K, Goto S, Hasegawa T, Koshino T, Hasegawa Y, Inoue H, Suzuki N, Sano Y, Inoue I. Polymorphisms in the prostaglandin E2 receptor subtype 2 gene confer susceptibility to aspirin-intolerant asthma: a candidate gene approach. Hum Mol Genet. 2004;13:3203–3217. doi: 10.1093/hmg/ddh332. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Kim YK, Park HW, Jee YK, Kim SH, Bahn JW, Chang YS, Kim SH, Ye YM, Shin ES, Lee JE, Park HS, Min KU. Association between polymorphisms in prostanoid receptor genes and aspirin-intolerant asthma. Pharmacogenet Genomics. 2007;17:295–304. doi: 10.1097/01.fpc.0000239977.61841.fe. [DOI] [PubMed] [Google Scholar]
- 30.Park BL, Park SM, Park JS, Uh ST, Choi JS, Kim YH, Kim MK, Choi IS, Choi BW, Cho SH, Hong CS, Lee YW, Lee JY, Park CS, Shin HD. Association of PTGER gene family polymorphisms with aspirin intolerant asthma in Korean asthmatics. BMB Rep. 2010;43:445–449. doi: 10.5483/bmbrep.2010.43.6.445. [DOI] [PubMed] [Google Scholar]
- 31.Capra V, Habib A, Accomazzo MR, Ravasi S, Citro S, Levy-Toledano S, Nicosia S, Rovati GE. Thromboxane prostanoid receptor in human airway smooth muscle cells: a relevant role in proliferation. Eur J Pharmacol. 2003;474:149–159. doi: 10.1016/s0014-2999(03)02014-4. [DOI] [PubMed] [Google Scholar]
- 32.Kumlin M, Dahlén B, Björck T, Zetterström O, Granström E, Dahlén SE. Urinary excretion of leukotriene E4 and 11-dehydro-thromboxane B2 in response to bronchial provocations with allergen, aspirin, leukotriene D4, and histamine in asthmatics. Am Rev Respir Dis. 1992;146:96–103. doi: 10.1164/ajrccm/146.1.96. [DOI] [PubMed] [Google Scholar]
- 33.Oh SH, Kim YH, Park SM, Cho SH, Park JS, Jang AS, Park SW, Uh ST, Lee YM, Kim MK, Choi IS, Cho SH, Hong CS, Lee YW, Lee JY, Choi BW, Park BL, Shin HD, Park CS. Association analysis of thromboxane A synthase 1 gene polymorphisms with aspirin intolerance in asthmatic patients. Pharmacogenomics. 2011;12:351–363. doi: 10.2217/pgs.10.181. [DOI] [PubMed] [Google Scholar]
- 34.Leung TF, Tang NL, Lam CW, Li AM, Chan IH, Ha G. Thromboxane A2 receptor gene polymorphism is associated with the serum concentration of cat-specific immunoglobulin E as well as the development and severity of asthma in Chinese children. Pediatr Allergy Immunol. 2002;13:10–17. doi: 10.1034/j.1399-3038.2002.01033.x. [DOI] [PubMed] [Google Scholar]
- 35.Shin HD, Park BL, Jung JH, Wang HJ, Park HS, Choi BW, Hong SJ, Lee YM, Kim YH, Park CS. Association of thromboxane A2 receptor (TBXA2R) with atopy and asthma. J Allergy Clin Immunol. 2003;112:454–457. doi: 10.1067/mai.2003.1641. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Choi JH, Park HS, Holloway JW, Lee SK, Park CS, Shin HD. Association of thromboxane A2 receptor gene polymorphism with the phenotype of acetyl salicylic acid-intolerant asthma. Clin Exp Allergy. 2005;35:585–590. doi: 10.1111/j.1365-2222.2005.02220.x. [DOI] [PubMed] [Google Scholar]
- 37.De Vries L, Gist Farquhar M. RGS proteins: more than just GAPs for heterotrimeric G proteins. Trends Cell Biol. 1999;9:138–144. doi: 10.1016/s0962-8924(99)01515-9. [DOI] [PubMed] [Google Scholar]
- 38.Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY. R7BP, a novel neuronal protein interacting with RGS proteins of the R7 family. J Biol Chem. 2005;280:5133–5136. doi: 10.1074/jbc.C400596200. [DOI] [PubMed] [Google Scholar]
- 39.Lee EH, Park BL, Park SM, Lee SH, Park SW, Park JS, Uh ST, Kim MK, Choi IS, Cho SH, Hong CS, Lee JY, Choi BW, Shin HD, Park CS. Association analysis of RGS7BP gene polymorphisms with aspirin intolerance in asthmatic patients. Ann Allergy Asthma Immunol. 2011;106:292–300.e6. doi: 10.1016/j.anai.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111:913–921. doi: 10.1067/mai.2003.1487. quiz 922. [DOI] [PubMed] [Google Scholar]
- 41.Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Fernández-Viña M, Geraghty DE, Holdsworth R, Hurley CK, Lau M, Lee KW, Mach B, Maiers M, Mayr WR, Müller CR, Parham P, Petersdorf EW, Sasazuki T, Strominger JL, Svejgaard A, Terasaki PI, Tiercy JM, Trowsdale J. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullarkey MF, Thomas PS, Hansen JA, Webb DR, Nisperos B. Association of aspirin-sensitive asthma with HLA-DQw2. Am Rev Respir Dis. 1986;133:261–263. doi: 10.1164/arrd.1986.133.2.261. [DOI] [PubMed] [Google Scholar]
- 43.Lympany PA, Welsh KI, Christie PE, Schmitz-Schumann M, Kemeny DM, Lee TH. An analysis with sequence-specific oligonucleotide probes of the association between aspirin-induced asthma and antigens of the HLA system. J Allergy Clin Immunol. 1993;92:114–123. doi: 10.1016/0091-6749(93)90045-h. [DOI] [PubMed] [Google Scholar]
- 44.Dekker JW, Nizankowska E, Schmitz-Schumann M, Pile K, Bochenek G, Dyczek A, Cookson WO, Szczeklik A. Aspirin-induced asthma and HLA-DRB1 and HLA-DPB1 genotypes. Clin Exp Allergy. 1997;27:574–577. [PubMed] [Google Scholar]
- 45.Adjers K, Karjalainen J, Pessi T, Eklund C, Hurme M. Epistatic effect of TLR4 and IL4 genes on the risk of asthma in females. Int Arch Allergy Immunol. 2005;138:251–256. doi: 10.1159/000088726. [DOI] [PubMed] [Google Scholar]
- 46.Burchard EG, Silverman EK, Rosenwasser LJ, Borish L, Yandava C, Pillari A, Weiss ST, Hasday J, Lilly CM, Ford JG, Drazen JM. Association between a sequence variant in the IL-4 gene promoter and FEV(1) in asthma. Am J Respir Crit Care Med. 1999;160:919–922. doi: 10.1164/ajrccm.160.3.9812024. [DOI] [PubMed] [Google Scholar]
- 47.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 48.Kim BS, Park SM, Uhm TG, Kang JH, Park JS, Jang AS, Uh ST, Kim MK, Choi IS, Cho SH, Hong CS, Lee YW, Lee JY, Choi BW, Park HS, Park BL, Shin HD, Chung IY, Park CS. Effect of single nucleotide polymorphisms within the interleukin-4 promoter on aspirin intolerance in asthmatics and interleukin-4 promoter activity. Pharmacogenet Genomics. 2010;20:748–758. doi: 10.1097/FPC.0b013e3283402155. [DOI] [PubMed] [Google Scholar]
- 49.Palikhe NS, Kim SH, Cho BY, Choi GS, Kim JH, Ye YM, Park HS. IL-13 Gene Polymorphisms are Associated With Rhinosinusitis and Eosinophilic Inflammation in Aspirin Intolerant Asthma. Allergy Asthma Immunol Res. 2010;2:134–140. doi: 10.4168/aair.2010.2.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akahoshi M, Obara K, Hirota T, Matsuda A, Hasegawa K, Takahashi N, Shimizu M, Nakashima K, Cheng L, Doi S, Fujiwara H, Miyatake A, Fujita K, Higashi N, Taniguchi M, Enomoto T, Mao XQ, Nakashima H, Adra CN, Nakamura Y, Tamari M, Shirakawa T. Functional promoter polymorphism in the TBX21 gene associated with aspirin-induced asthma. Hum Genet. 2005;117:16–26. doi: 10.1007/s00439-005-1285-0. [DOI] [PubMed] [Google Scholar]
- 51.Kim SH, Bae JS, Holloway JW, Lee JT, Suh CH, Nahm DH, Park HS. A polymorphism of MS4A2 (- 109T > C) encoding the beta-chain of the high-affinity immunoglobulin E receptor (FcepsilonR1beta) is associated with a susceptibility to aspirin-intolerant asthma. Clin Exp Allergy. 2006;36:877–883. doi: 10.1111/j.1365-2222.2006.02443.x. [DOI] [PubMed] [Google Scholar]
- 52.Palikhe NS, Kim SH, Cho BY, Ye YM, Hur GY, Park HS. Association of three sets of high-affinity IgE receptor (FcepsilonR1) polymorphisms with aspirin-intolerant asthma. Respir Med. 2008;102:1132–1139. doi: 10.1016/j.rmed.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida S, Sakamoto H, Yamawaki Y, Shoji T, Akahori K, Onuma K, Nakagawa H, Hasegawa H, Amayasu H. Effect of acyclovir on bronchoconstriction and urinary leukotriene E4 excretion in aspirin-induced asthma. J Allergy Clin Immunol. 1998;102:909–914. doi: 10.1016/s0091-6749(98)70327-6. [DOI] [PubMed] [Google Scholar]
- 54.Palikhe NS, Kim SH, Kim JH, Losol P, Ye YM, Park HS. Role of toll-like receptor 3 variants in aspirin-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2011;3:123–127. doi: 10.4168/aair.2011.3.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Hitomi Y, Ebisawa M, Tomikawa M, Imai T, Komata T, Hirota T, Harada M, Sakashita M, Suzuki Y, Shimojo N, Kohno Y, Fujita K, Miyatake A, Doi S, Enomoto T, Taniguchi M, Higashi N, Nakamura Y, Tamari M. Associations of functional NLRP3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J Allergy Clin Immunol. 2009;124:779–785.e6. doi: 10.1016/j.jaci.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 57.Lee JY, Park SW, Chang HK, Kim HY, Rhim T, Lee JH, Jang AS, Koh ES, Park CS. A disintegrin and metalloproteinase 33 protein in patients with asthma: Relevance to airflow limitation. Am J Respir Crit Care Med. 2006;173:729–735. doi: 10.1164/rccm.200409-1175OC. [DOI] [PubMed] [Google Scholar]
- 58.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, Torrey D, Pandit S, McKenny J, Braunschweiger K, Walsh A, Liu Z, Hayward B, Folz C, Manning SP, Bawa A, Saracino L, Thackston M, Benchekroun Y, Capparell N, Wang M, Adair R, Feng Y, Dubois J, FitzGerald MG, Huang H, Gibson R, Allen KM, Pedan A, Danzig MR, Umland SP, Egan RW, Cuss FM, Rorke S, Clough JB, Holloway JW, Holgate ST, Keith TP. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Park HS, Park SW, Jang AS, Uh ST, Rhim T, Park CS, Hong SJ, Holgate ST, Holloway JW, Shin HD. ADAM33 polymorphism: association with bronchial hyper-responsiveness in Korean asthmatics. Clin Exp Allergy. 2004;34:860–865. doi: 10.1111/j.1365-2222.2004.01977.x. [DOI] [PubMed] [Google Scholar]
- 60.Sakagami T, Jinnai N, Nakajima T, Sekigawa T, Hasegawa T, Suzuki E, Inoue I, Gejyo F. ADAM33 polymorphisms are associated with aspirin-intolerant asthma in the Japanese population. J Hum Genet. 2007;52:66–72. doi: 10.1007/s10038-006-0081-6. [DOI] [PubMed] [Google Scholar]
- 61.Wen GY, Yang SY, Kaczmarski W, He XY, Pappas KS. Presence of hydroxysteroid dehydrogenase type 10 in amyloid plaques (APs) of Hsiao's APP-Sw transgenic mouse brains, but absence in APs of Alzheimer's disease brains. Brain Res. 2002;954:115–122. doi: 10.1016/s0006-8993(02)03354-1. [DOI] [PubMed] [Google Scholar]
- 62.Kim JY, Kim JH, Park TJ, Bae JS, Lee JS, Pasaje CF, Park BL, Cheong HS, Park JS, Park SW, Uh ST, Kim MK, Choi IS, Cho SH, Choi BW, Park CS, Shin HD. Positive association between aspirin-intolerant asthma and genetic polymorphisms of FSIP1: a case-case study. BMC Pulm Med. 2010;10:34. doi: 10.1186/1471-2466-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freishtat RJ, Benton AS, Watson AM, Wang Z, Rose MC, Hoffman EP. Delineation of a gene network underlying the pulmonary response to oxidative stress in asthma. J Investig Med. 2009;57:756–764. doi: 10.231/JIM.0b013e3181b91a83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fouladkou F, Lu C, Jiang C, Zhou L, She Y, Walls JR, Kawabe H, Brose N, Henkelman RM, Huang A, Bruneau BG, Rotin D. The ubiquitin ligase Nedd4-1 is required for heart development and is a suppressor of thrombospondin-1. J Biol Chem. 2010;285:6770–6780. doi: 10.1074/jbc.M109.082347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, Walters EH. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57:309–316. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leimeister C, Steidl C, Schumacher N, Erhard S, Gessler M. Developmental expression and biochemical characterization of Emu family members. Dev Biol. 2002;249:204–218. doi: 10.1006/dbio.2002.0764. [DOI] [PubMed] [Google Scholar]
- 67.Pasaje CF, Kim JH, Park BL, Cheong HS, Kim MK, Choi IS, Cho SH, Hong CS, Lee YW, Lee JY, Koh IS, Park TJ, Lee JS, Kim Y, Bae JS, Park CS, Shin HD. A possible association of EMID2 polymorphisms with aspirin hypersensitivity in asthma. Immunogenetics. 2011;63:13–21. doi: 10.1007/s00251-010-0490-8. [DOI] [PubMed] [Google Scholar]
- 68.Christiansen SC, Proud D, Cochrane CG. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J Clin Invest. 1987;79:188–197. doi: 10.1172/JCI112782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roisman GL, Danel CJ, Lacronique JG, Alhenc-Gelas F, Dusser DJ. Decreased expression of angiotensin-converting enzyme in the airway epithelium of asthmatic subjects is associated with eosinophil inflammation. J Allergy Clin Immunol. 1999;104:402–410. doi: 10.1016/s0091-6749(99)70385-4. [DOI] [PubMed] [Google Scholar]
- 70.Kim TH, Chang HS, Park SM, Nam BY, Park JS, Rhim T, Park HS, Kim MK, Choi IS, Cho SH, Chung IY, Park BL, Park CS, Shin HD. Association of angiotensin I-converting enzyme gene polymorphisms with aspirin intolerance in asthmatics. Clin Exp Allergy. 2008;38:1727–1737. doi: 10.1111/j.1365-2222.2008.03082.x. [DOI] [PubMed] [Google Scholar]
- 71.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 72.Fozard JR, Ellis KM, Villela Dantas MF, Tigani B, Mazzoni L. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. Eur J Pharmacol. 2002;438:183–188. doi: 10.1016/s0014-2999(02)01305-5. [DOI] [PubMed] [Google Scholar]
- 73.Kim SH, Kim YK, Park HW, Kim SH, Kim SH, Ye YM, Min KU, Park HS. Adenosine deaminase and adenosine receptor polymorphisms in aspirin-intolerant asthma. Respir Med. 2009;103:356–363. doi: 10.1016/j.rmed.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S, Nagata K. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang JL, Gao PS, Mathias RA, Yao TC, Chen LC, Kuo ML, Hsu SC, Plunkett B, Togias A, Barnes KC, Stellato C, Beaty TH, Huang SK. Sequence variants of the gene encoding chemoattractant receptor expressed on Th2 cells (CRTH2) are associated with asthma and differentially influence mRNA stability. Hum Mol Genet. 2004;13:2691–2697. doi: 10.1093/hmg/ddh279. [DOI] [PubMed] [Google Scholar]
- 76.Palikhe NS, Kim SH, Cho BY, Ye YM, Choi GS, Park HS. Genetic variability in CRTH2 polymorphism increases eotaxin-2 levels in patients with aspirin exacerbated respiratory disease. Allergy. 2010;65:338–346. doi: 10.1111/j.1398-9995.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 77.Ueki S, Matsuwaki Y, Kayaba H, Oyamada H, Kanda A, Usami A, Saito N, Chihara J. Peroxisome proliferator-activated receptor gamma regulates eosinophil functions: a new therapeutic target for allergic airway inflammation. Int Arch Allergy Immunol. 2004;134(Suppl 1):30–36. doi: 10.1159/000077790. [DOI] [PubMed] [Google Scholar]
- 78.Min JW, Jang AS, Park SM, Lee SH, Lee JH, Park SW, Park CS. Comparison of plasma eotaxin family level in aspirin-induced and aspirin-tolerant asthma patients. Chest. 2005;128:3127–3132. doi: 10.1378/chest.128.5.3127. [DOI] [PubMed] [Google Scholar]
- 79.Palmer CN, Doney AS, Ismail T, Lee SP, Murrie I, Macgregor DF, Mukhopadhyay S. PPARG locus haplotype variation and exacerbations in asthma. Clin Pharmacol Ther. 2007;81:713–718. doi: 10.1038/sj.clpt.6100119. [DOI] [PubMed] [Google Scholar]
- 80.Oh SH, Park SM, Park JS, Jang AS, Lee YM, Uh ST, Kim YH, Choi IS, Kim MK, Park BL, Shin HD, Park CS. Association analysis of peroxisome proliferator-activated receptors gamma gene polymorphisms with asprin hypersensitivity in asthmatics. Allergy Asthma Immunol Res. 2009;1:30–35. doi: 10.4168/aair.2009.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shah AS, Farmen SL, Moninger TO, Businga TR, Andrews MP, Bugge K, Searby CC, Nishimura D, Brogden KA, Kline JN, Sheffield VC, Welsh MJ. Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proc Natl Acad Sci U S A. 2008;105:3380–3385. doi: 10.1073/pnas.0712327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sivaprasad U, Gibson AM, Wang N, Khurana Hershey GK. Expression of cilia structural genes is downregulated in asthma and single nucleotide polymorphisms in these genes correlate with asthma. J Allergy Clin Immunol. 2009;123:S81. [Google Scholar]
- 83.Kim JH, Cha JY, Cheong HS, Park JS, Jang AS, Uh ST, Kim MK, Choi IS, Cho SH, Park BL, Bae JS, Park CS, Shin HD. KIF3A, a cilia structural gene on chromosome 5q31, and its polymorphisms show an association with aspirin hypersensitivity in asthma. J Clin Immunol. 2011;31:112–121. doi: 10.1007/s10875-010-9462-x. [DOI] [PubMed] [Google Scholar]
- 84.Picado C, Bioque G, Roca-Ferrer J, Pujols L, Mullol J, Benitez P, Bulbena O. Nuclear factor-kappaB activity is down-regulated in nasal polyps from aspirin-sensitive asthmatics. Allergy. 2003;58:122–126. doi: 10.1034/j.1398-9995.2003.23792.x. [DOI] [PubMed] [Google Scholar]
- 85.Liu M, Wang L, Holm BA, Enhorning G. Dysfunction of guinea-pig pulmonary surfactant and type II pneumocytes after repetitive challenge with aerosolized ovalbumin. Clin Exp Allergy. 1997;27:802–807. doi: 10.1046/j.1365-2222.1997.420885.x. [DOI] [PubMed] [Google Scholar]
- 86.Rückert P, Bates SR, Fisher AB. Role of clathrin- and actin-dependent endocytotic pathways in lung phospholipid uptake. Am J Physiol Lung Cell Mol Physiol. 2003;284:L981–L989. doi: 10.1152/ajplung.00392.2002. [DOI] [PubMed] [Google Scholar]
- 87.Natsume W, Tanabe K, Kon S, Yoshida N, Watanabe T, Torii T, Satake M. SMAP2, a novel ARF GTPase-activating protein, interacts with clathrin and clathrin assembly protein and functions on the AP-1-positive early endosome/trans-Golgi network. Mol Biol Cell. 2006;17:2592–2603. doi: 10.1091/mbc.E05-10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JY, Kim JH, Park BL, Cheong HS, Park JS, Jang AS, Uh ST, Choi JS, Kim YH, Kim MK, Choi IS, Cho SH, Choi BW, Park CS, Shin HD. Putative association of SMAPIL polymorphisms with risk of aspirin intolerance in asthmatics. J Asthma. 2010;47:959–965. doi: 10.1080/02770903.2010.514637. [DOI] [PubMed] [Google Scholar]
- 89.Rasola A, Galietta LJ, Barone V, Romeo G, Bagnasco S. Molecular cloning and functional characterization of a GABA/betaine transporter from human kidney. FEBS Lett. 1995;373:229–233. doi: 10.1016/0014-5793(95)01052-g. [DOI] [PubMed] [Google Scholar]
- 90.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O'Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 91.Golovko AI, Sofronov GA, Kliuntina TV. The effect of salicylates on the toxicity of GABAlytics for mice. Biull Eksp Biol Med. 1995;119:619–620. [PubMed] [Google Scholar]
- 92.Pasaje CF, Kim JH, Park BL, Cheong HS, Chun JY, Park TJ, Lee JS, Kim Y, Bae JS, Park JS, Yoon SH, Uh ST, Choi JS, Kim YH, Kim MK, Choi IS, Cho SH, Choi BW, Park CS, Shin HD. Association of SLC6A12 variants with aspirin-intolerant asthma in a Korean population. Ann Hum Genet. 2010;74:326–334. doi: 10.1111/j.1469-1809.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 93.Kimura H, Takeda M, Narikawa S, Enomoto A, Ichida K, Endou H. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J Pharmacol Exp Ther. 2002;301:293–298. doi: 10.1124/jpet.301.1.293. [DOI] [PubMed] [Google Scholar]
- 94.Gosens R, Zaagsma J, Grootte Bromhaar M, Nelemans A, Meurs H. Acetylcholine: a novel regulator of airway smooth muscle remodelling? Eur J Pharmacol. 2004;500:193–201. doi: 10.1016/j.ejphar.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 95.Kang HJ, Song IS, Shin HJ, Kim WY, Lee CH, Shim JC, Zhou HH, Lee SS, Shin JG. Identification and functional characterization of genetic variants of human organic cation transporters in a Korean population. Drug Metab Dispos. 2007;35:667–675. doi: 10.1124/dmd.106.013581. [DOI] [PubMed] [Google Scholar]
- 96.Park TJ, Kim JH, Bae JS, Park BL, Cheong HS, Chun JY, Lee JS, Kim JY, Pasaje CF, Cho SH, Uh ST, Kim MK, Choi IS, Koh IS, Park CS, Shin HD. Possible association of SLC22A2 polymorphisms with aspirin-intolerant asthma. Int Arch Allergy Immunol. 2011;155:395–402. doi: 10.1159/000321267. [DOI] [PubMed] [Google Scholar]
- 97.Brueggemann LI, Mani BK, Mackie AR, Cribbs LL, Byron KL. Novel actions of nonsteroidal anti-inflammatory drugs on vascular ion channels: accounting for cardiovascular side effects and identifying new therapeutic applications. Mol Cell Pharmacol. 2010;2:15–19. [PMC free article] [PubMed] [Google Scholar]
- 98.Wang IM, Stepaniants S, Boie Y, Mortimer JR, Kennedy B, Elliott M, Hayashi S, Loy L, Coulter S, Cervino S, Harris J, Thornton M, Raubertas R, Roberts C, Hogg JC, Crackower M, O'Neill G, Paré PD. Gene expression profiling in patients with chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med. 2008;177:402–411. doi: 10.1164/rccm.200703-390OC. [DOI] [PubMed] [Google Scholar]
- 99.Lee JS, Kim JH, Bae JS, Kim JY, Park TJ, Pasaje CF, Park BL, Cheong HS, Uh ST, Park JS, Jang AS, Kim MK, Choi IS, Park CS, Shin HD. Association of CACNG6 polymorphisms with aspirin-intolerance asthmatics in a Korean population. BMC Med Genet. 2010;11:138. doi: 10.1186/1471-2350-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, Heinzmann A, Simma B, Frischer T, Willis-Owen SA, Wong KC, Illig T, Vogelberg C, Weiland SK, von Mutius E, Abecasis GR, Farrall M, Gut IG, Lathrop GM, Cookson WO. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 101.Himes BE, Hunninghake GM, Baurley JW, Rafaels NM, Sleiman P, Strachan DP, Wilk JB, Willis-Owen SA, Klanderman B, Lasky-Su J, Lazarus R, Murphy AJ, Soto-Quiros ME, Avila L, Beaty T, Mathias RA, Ruczinski I, Barnes KC, Celedón JC, Cookson WO, Gauderman WJ, Gilliland FD, Hakonarson H, Lange C, Moffatt MF, O'Connor GT, Raby BA, Silverman EK, Weiss ST. Genome-wide association analysis identifies PDE4D as an asthma-susceptibility gene. Am J Hum Genet. 2009;84:581–593. doi: 10.1016/j.ajhg.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jönsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 103.Kim JH, Park BL, Cheong HS, Bae JS, Park JS, Jang AS, Uh ST, Choi JS, Kim YH, Kim MK, Choi IS, Cho SH, Choi BW, Park CS, Shin HD. Genome-wide and follow-up studies identify CEP68 gene variants associated with risk of aspirin-intolerant asthma. PLoS One. 2010;5:e13818. doi: 10.1371/journal.pone.0013818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360:1696–1698. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]
- 105.Kraft P, Hunter DJ. Genetic risk prediction--are we there yet? N Engl J Med. 2009;360:1701–1703. doi: 10.1056/NEJMp0810107. [DOI] [PubMed] [Google Scholar]
- 106.Holloway JW, Barton SJ, Holgate ST, Rose-Zerilli MJ, Sayers I. The role of LTA4H and ALOX5AP polymorphism in asthma and allergy susceptibility. Allergy. 2008;63:1046–1053. doi: 10.1111/j.1398-9995.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 107.Higuchi T, Seki N, Kamizono S, Yamada A, Kimura A, Kato H, Itoh K. Polymorphism of the 5'-flanking region of the human tumor necrosis factor (TNF)-alpha gene in Japanese. Tissue Antigens. 1998;51:605–612. doi: 10.1111/j.1399-0039.1998.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 108.Shin HD, Park BL, Kim LH, Jung JH, Wang HJ, Kim YJ, Park HS, Hong SJ, Choi BW, Kim DJ, Park CS. Association of tumor necrosis factor polymorphisms with asthma and serum total IgE. Hum Mol Genet. 2004;13:397–403. doi: 10.1093/hmg/ddh036. [DOI] [PubMed] [Google Scholar]
- 109.Hong SJ, Kim HB, Kang MJ, Lee SY, Kim JH, Kim BS, Jang SO, Shin HD, Park CS. TNF-alpha (-308 G/A) and CD14 (-159T/C) polymorphisms in the bronchial responsiveness of Korean children with asthma. J Allergy Clin Immunol. 2007;119:398–404. doi: 10.1016/j.jaci.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 110.Kim SH, Ye YM, Lee SK, Choi JH, Holloway JW, Park CS, Park HS. Association of TNF-alpha genetic polymorphism with HLA DPB1*0301. Clin Exp Allergy. 2006;36:1247–1253. doi: 10.1111/j.1365-2222.2006.02567.x. [DOI] [PubMed] [Google Scholar]
- 111.Kim SH, Park HS, Holloway JW, Shin HD, Park CS. Association between a TGFbeta1 promoter polymorphism and rhinosinusitis in aspirin-intolerant asthmatic patients. Respir Med. 2007;101:490–495. doi: 10.1016/j.rmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 113.Hobbs K, Negri J, Klinnert M, Rosenwasser LJ, Borish L. Interleukin-10 and transforming growth factor-beta promoter polymorphisms in allergies and asthma. Am J Respir Crit Care Med. 1998;158:1958–1962. doi: 10.1164/ajrccm.158.6.9804011. [DOI] [PubMed] [Google Scholar]
- 114.Chatterjee R, Batra J, Kumar A, Mabalirajan U, Nahid S, Niphadkar PV, Ghosh B. Interleukin-10 promoter polymorphisms and atopic asthma in North Indians. Clin Exp Allergy. 2005;35:914–919. doi: 10.1111/j.1365-2222.2005.02273.x. [DOI] [PubMed] [Google Scholar]
- 115.Lim S, Crawley E, Woo P, Barnes PJ. Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet. 1998;352:113. doi: 10.1016/S0140-6736(98)85018-6. [DOI] [PubMed] [Google Scholar]
- 116.Kim SH, Yang EM, Lee HN, Cho BY, Ye YM, Park HS. Combined effect of IL-10 and TGF-beta1 promoter polymorphisms as a risk factor for aspirin-intolerant asthma and rhinosinusitis. Allergy. 2009;64:1221–1225. doi: 10.1111/j.1398-9995.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- 117.Szczeklik A. The cyclooxygenase theory of aspirin-induced asthma. Eur Respir J. 1990;3:588–593. [PubMed] [Google Scholar]
- 118.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 119.Park JS, Jang AS, Park SW, Lee YM, Uh ST, Kim YH, Cha JY, Park SM, Park CS. Protection of leukotriene receptor antagonist against aspirin-induced bronchospasm in asthmatics. Allergy Asthma Immunol Res. 2010;2:48–54. doi: 10.4168/aair.2010.2.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi JH, Lee KW, Oh HB, Lee KJ, Suh YJ, Park CS, Park HS. HLA association in aspirin-intolerant asthma: DPB1*0301 as a strong marker in a Korean population. J Allergy Clin Immunol. 2004;113:562–564. doi: 10.1016/j.jaci.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 121.Kim SH, Ye YM, Hur GY, Lee SK, Sampson AP, Lee HY, Park HS. CysLTR1 promoter polymorphism and requirement for leukotriene receptor antagonist in aspirin-intolerant asthma patients. Pharmacogenomics. 2007;8:1143–1150. doi: 10.2217/14622416.8.9.1143. [DOI] [PubMed] [Google Scholar]
- 122.Pasaje CF, Kim JH, Park BL, Cheong HS, Park TJ, Lee JS, Kim Y, Bae JS, Kim JM, Park JS, Park CS, Shin HD. Association of the variants in AGT gene with modified drug response in Korean aspirin-intolerant asthma patients. Pulm Pharmacol Ther. 2011;24:595–601. doi: 10.1016/j.pupt.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 123.Thomsen SF, van der, Kyvik KO, Skytthe A, Backer V. Estimates of asthma heritability in a large twin sample. Clin Exp Allergy. 2010;40:1054–1061. doi: 10.1111/j.1365-2222.2010.03525.x. [DOI] [PubMed] [Google Scholar]
- 124.Miller RL. Prenatal maternal diet affects asthma risk in offspring. J Clin Invest. 2008;118:3265–3268. doi: 10.1172/JCI37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheong HS, Park SM, Kim MO, Park JS, Lee JY, Byun JY, Park BL, Shin HD, Park CS. Genome-wide methylation profile of nasal polyps: relation to aspirin hypersensitivity in asthmatics. Allergy. 2011;66:637–644. doi: 10.1111/j.1398-9995.2010.02514.x. [DOI] [PubMed] [Google Scholar]
- 126.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11:415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 127.Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, He W, Chen YJ, Makhijani V, Roth GT, Gomes X, Tartaro K, Niazi F, Turcotte CL, Irzyk GP, Lupski JR, Chinault C, Song XZ, Liu Y, Yuan Y, Nazareth L, Qin X, Muzny DM, Margulies M, Weinstock GM, Gibbs RA, Rothberg JM. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- 128.Wellcome Trust Case Control Consortium. Craddock N, Hurles ME, Cardin N, Pearson RD, Plagnol V, Robson S, Vukcevic D, Barnes C, Conrad DF, Giannoulatou E, Holmes C, Marchini JL, Stirrups K, Tobin MD, Wain LV, Yau C, Aerts J, Ahmad T, Andrews TD, Arbury H, Attwood A, Auton A, Ball SG, Balmforth AJ, Barrett JC, Barroso I, Barton A, Bennett AJ, Bhaskar S, Blaszczyk K, Bowes J, Brand OJ, Braund PS, Bredin F, Breen G, Brown MJ, Bruce IN, Bull J, Burren OS, Burton J, Byrnes J, Caesar S, Clee CM, Coffey AJ, Connell JM, Cooper JD, Dominiczak AF, Downes K, Drummond HE, Dudakia D, Dunham A, Ebbs B, Eccles D, Edkins S, Edwards C, Elliot A, Emery P, Evans DM, Evans G, Eyre S, Farmer A, Ferrier IN, Feuk L, Fitzgerald T, Flynn E, Forbes A, Forty L, Franklyn JA, Freathy RM, Gibbs P, Gilbert P, Gokumen O, Gordon-Smith K, Gray E, Green E, Groves CJ, Grozeva D, Gwilliam R, Hall A, Hammond N, Hardy M, Harrison P, Hassanali N, Hebaishi H, Hines S, Hinks A, Hitman GA, Hocking L, Howard E, Howard P, Howson JM, Hughes D, Hunt S, Isaacs JD, Jain M, Jewell DP, Johnson T, Jolley JD, Jones IR, Jones LA, Kirov G, Langford CF, Lango-Allen H, Lathrop GM, Lee J, Lee KL, Lees C, Lewis K, Lindgren CM, Maisuria-Armer M, Maller J, Mansfield J, Martin P, Massey DC, McArdle WL, McGuffin P, McLay KE, Mentzer A, Mimmack ML, Morgan AE, Morris AP, Mowat C, Myers S, Newman W, Nimmo ER, O'Donovan MC, Onipinla A, Onyiah I, Ovington NR, Owen MJ, Palin K, Parnell K, Pernet D, Perry JR, Phillips A, Pinto D, Prescott NJ, Prokopenko I, Quail MA, Rafelt S, Rayner NW, Redon R, Reid DM, Renwick, Ring SM, Robertson N, Russell E, St Clair D, Sambrook JG, Sanderson JD, Schuilenburg H, Scott CE, Scott R, Seal S, Shaw-Hawkins S, Shields BM, Simmonds MJ, Smyth DJ, Somaskantharajah E, Spanova K, Steer S, Stephens J, Stevens HE, Stone MA, Su Z, Symmons DP, Thompson JR, Thomson W, Travers ME, Turnbull C, Valsesia A, Walker M, Walker NM, Wallace C, Warren-Perry M, Watkins NA, Webster J, Weedon MN, Wilson AG, Woodburn M, Wordsworth BP, Young AH, Zeggini E, Carter NP, Frayling TM, Lee C, McVean G, Munroe PB, Palotie A, Sawcer SJ, Scherer SW, Strachan DP, Tyler-Smith C, Brown MA, Burton PR, Caulfield MJ, Compston A, Farrall M, Gough SC, Hall AS, Hattersley AT, Hill AV, Mathew CG, Pembrey M, Satsangi J, Stratton MR, Worthington J, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand W, Parkes M, Rahman N, Todd JA, Samani NJ, Donnelly P. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature. 2010;464:713–720. doi: 10.1038/nature08979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Toft M, Ross OA. Copy number variation in Parkinson's disease. Genome Med. 2010;2:62. doi: 10.1186/gm183. [DOI] [PMC free article] [PubMed] [Google Scholar]