Abstract
Purpose
Allergen immunotherapy (AIT) has been used as a curative and specific treatment of allergic diseases. However, no data on the prescription patterns of AIT in Korea is available. Therefore, we surveyed the prescription patterns of AIT by allergy specialists in Korea.
Methods
We emailed a questionnaire on AIT prescription patterns to the 690 members of the Korean Academy of Asthma, Allergy and Clinical Immunology with clinical practice experience. All returned answers were evaluated.
Results
The response rate was 21.0%. Only 69.0% of the respondents performed AIT in practice. Hindrance factors for performing AIT in the practice included a lack of facilities (21%), lack of practical experience during their subspecialty or postgraduate educational training programs (15.8%), no need for AIT because of sufficient pharmacotherapy (14.5%), insufficient economic profits (14.5%), and risks for adverse reactions (13.2%). Ninety-two allergy specialists (82%) performed AIT subcutaneously subcutaneous immunotherapy (SCIT) and 20 allergy specialists (18%) performed it sublingually sublingual immunotherapy (SLIT). Only 8 specialists performed both SCIT and SLIT. The allergens used for SCIT were house dust mites (98.9%), pollens (72.8%), and animal dander (23.9%). SLIT was prescribed only for house dust mites. Twenty-eight physicians (30.4%) observed anaphylactic reactions during SCIT. Eight physicians (40.0%) who prescribed SLIT observed adverse reactions, including local reactions, but none of them observed anaphylactic reactions.
Conclusions
In this survey, 69.0% of the respondents performed AIT in clinical practice. SCIT prescription is more popular than SLIT. The Lack of facilities and clinical education is a critical barrier to performing AIT. Therefore, proper clinical education of AIT is necessary for Korean allergists.
Keywords: Allergen immunotherapy, allergic rhinitis, asthma, survey
INTRODUCTION
Allergen immunotherapy (AIT) with allergens has been used as the only specific treatment of allergic diseases, such as allergic mild asthma, allergic rhinitis, and bee venom anaphylaxis.1,2 There have been several reports of the positive effects of AIT in atopic dermatitis, suggesting that AIT may also have a therapeutic value in the treatment of atopic dermatitis.3,4 AIT may change the natural course of the disease and has several disease-modifying effects. Randomized clinical trials have shown that AIT can decrease symptom-medication scores by 30%-40%.5,6 In addition, the effects of AIT can be maintained long after the discontinuation of AIT, particularly when it is performed for 3 years or longer.7,8 Furthermore, AIT can prevent the development of allergic asthma in allergic rhinitis patients9-11 and new sensitization in children with house dust mite mono-sensitized asthma.12,13
In Korea, prescription of AIT is not popular compared to that in Western countries, and data on the current prescription pattern of AIT in Korea are not available. Therefore, we conducted a survey of the prescription pattern of Korean allergy specialists and analyzed underlying causes of the low AIT prescription rate in Korea.
MATERIALS AND METHODS
We emailed a questionnaire on AIT prescription patterns to the 690 members of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI) with clinical practice experience. We obtained responses to the questionnaire by August 2009. The questionnaire was again circulated to the same Academy members at 1-month intervals in order to increase the response rate. The questionnaire consisted of 24 questions regarding practice patterns. The questionnaire included demographics, insight into AIT for the treatment of allergic disease, practice patterns of AIT, and adverse reactions during AIT. The detailed questionnaire is shown in Figure. All returned answers were evaluated and analyzed using descriptive statistics.
Figure.
Questionnaire regarding allergen immunotherapy (AIT) sent to the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI) members.
SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy.
RESULTS
Characteristics of the respondents
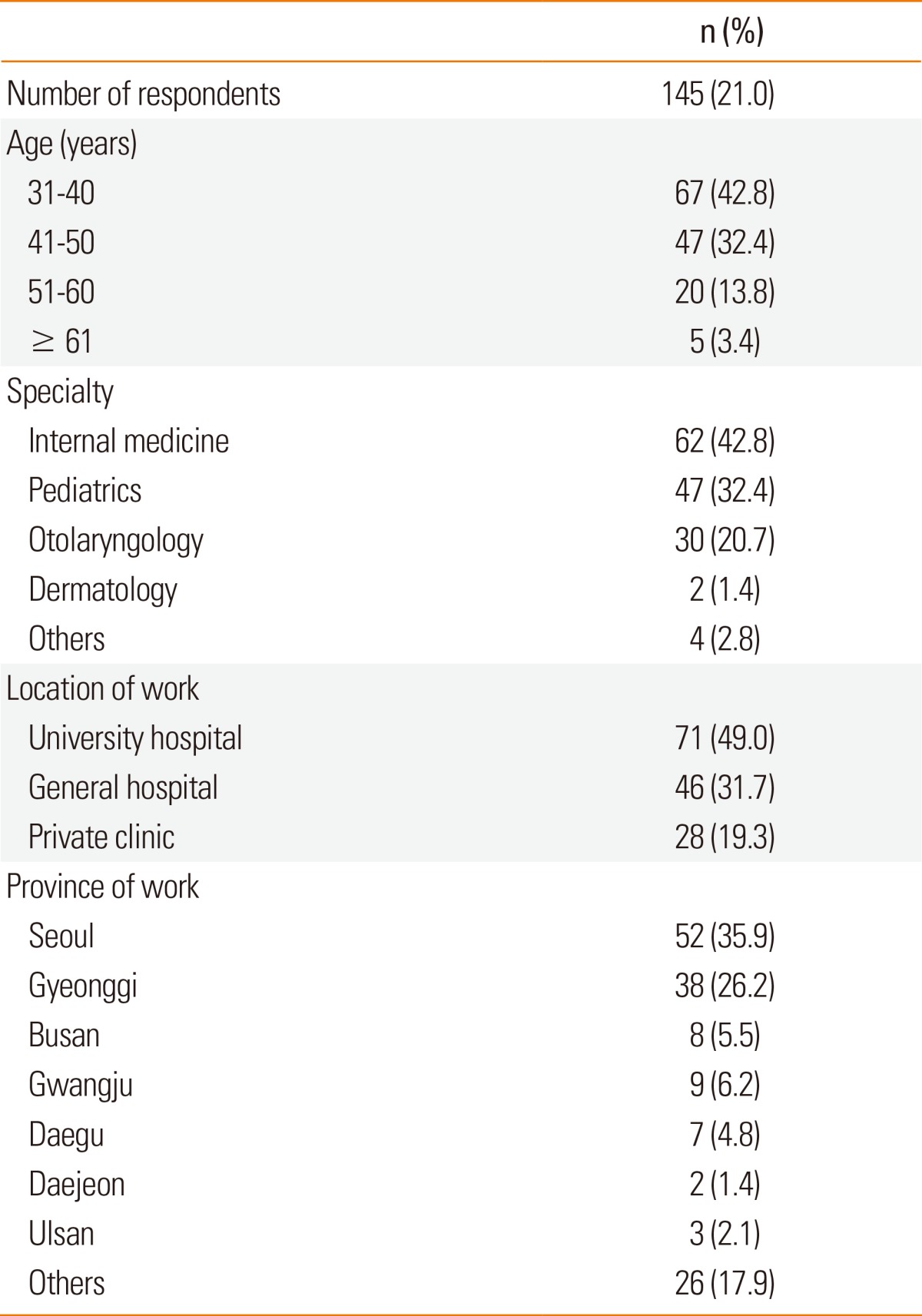
Table 1 shows the characteristics of the respondents. We obtained responses from 145 (21.0%) of the 690 members members. Of these who respondents, 67 (42.8%) were in their 30s, 47 (32.4%) were in their 40s, and 20 (13.8%) were in their 50s. Five respondents (3.4%) were at the age of over 60 years. The majority (75.2%) of the respondents were in their 30s and 40s. Of these respondents, 62 (42.8%) were physicians, 47 (32.4%) were pediatricians, 30 (20.7%) were otolaryngologists, and 2 (1.4%) were dermatologists. Approximately half of the respondents (49.0%) worked at university hospitals, 46 (31.7%) worked at general hospitals, and the remaining (19.3%) worked at private clinics. The institutes of more than half (62.1%) of the respondents were located in Seoul and its metropolitan area.
Table 1.
Characteristics of the survey respondents
Insights into AIT
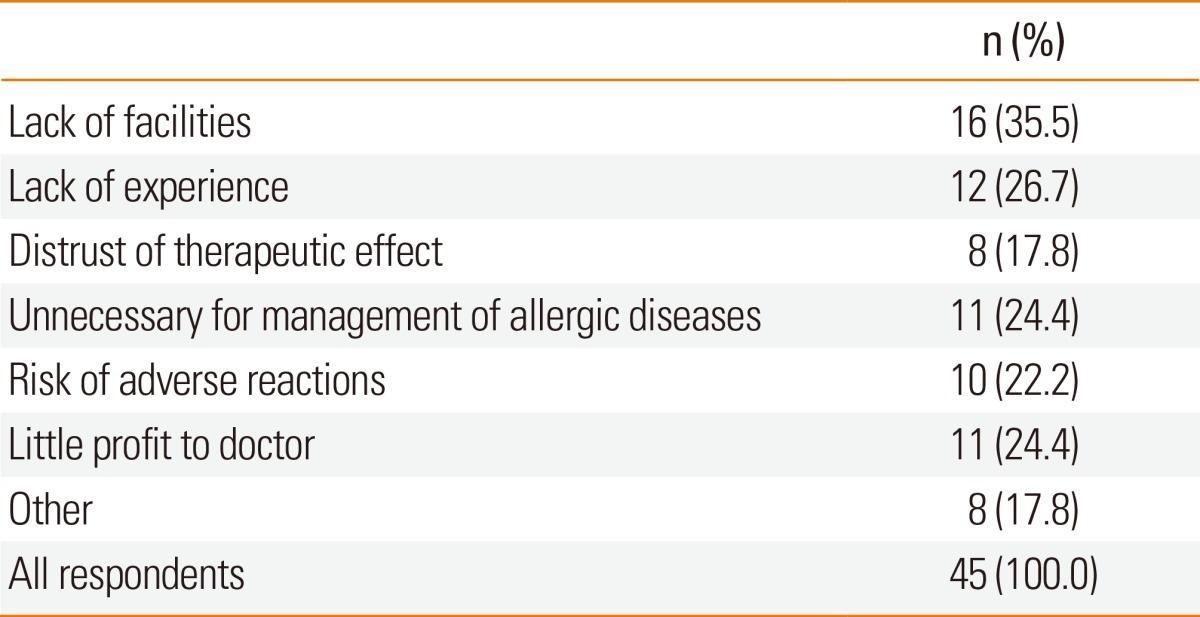
Most respondents agreed on the necessity of AIT to treat allergic diseases (143/145, 98.6%). However, only 100 (69.0%) were currently using AIT in their practice. Although the remaining 45 (31.0%) were not yet using AIT, 30 (20.7%) respondents desired to use it in the future practice. Table 2 shows the obstacles to starting AIT in their own practice based on responses from the members without AIT experience. The most frequent reason was lack of facilities, including lack of trained health professionals (35.5%), and lack of practical AIT experience during the training course of their subspecialty (26.7%). Some respondents answered that they did not trust therapeutic effects of AIT (17.8%); they thought AIT was unnecessary because pharmacotherapy alone was sufficient for the management of allergic diseases (24.4%). These respondents also reported that AIT did not provide enough economic profits (24.4%) considering the risks of serious adverse events (22.2%).
Table 2.
Reasons for not performing allergen immunotherapy
Numbers are not mutually exclusive.
AIT in practice
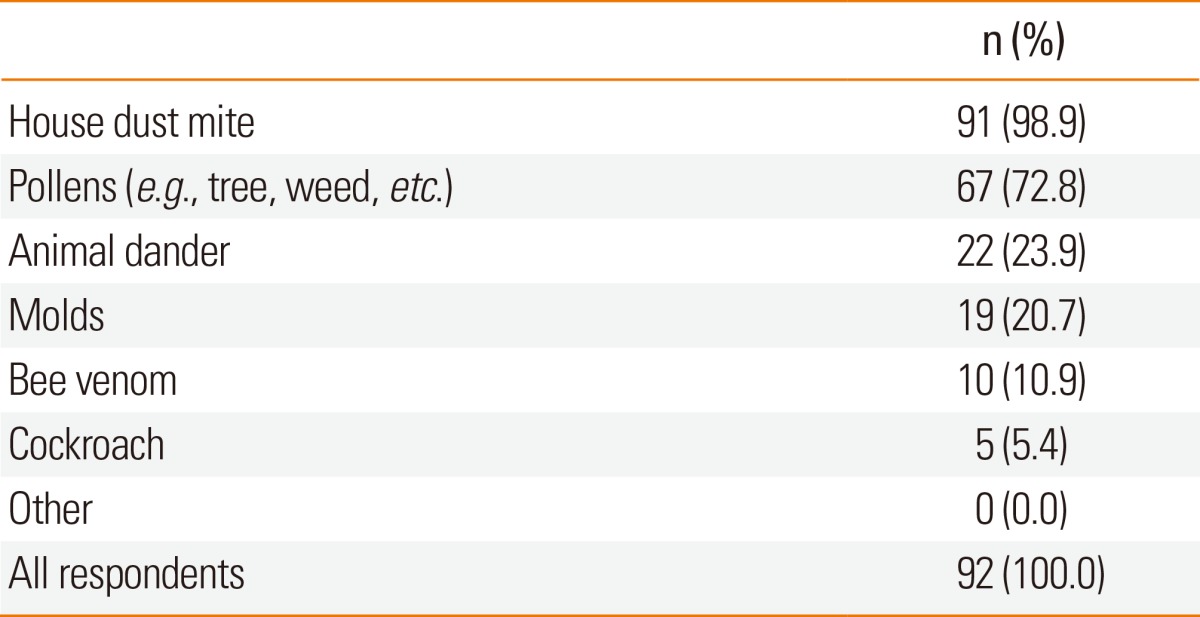
Table 3 shows target diseases for which the respondents used AIT. Allergic rhinoconjunctivitis (92.0%), allergic asthma (76.0%), atopic dermatitis (20.0%), and bee sting anaphylaxis (10.0%) were ranked as the major indications. We asked the methods used to detect causative allergens and found that skin prick tests and serologic tests, including ImmunoCAP, RAST, and MAST, were all popular for the diagnosis of causative agents (94.0% vs. 100.0%). Approximately 94.0% of the respondents used both skin prick tests and serologic tests for the identification of culprit allergens. 92 of all allergy specialists, (82.0%) performed subcutaneous immunotherapy (SCIT) and 20 (18.0%) performed sublingual immunotherapy (SLIT). Only 8 allergy specialists performed both SCIT and SLIT. The respondents who performed SCIT were asked as to which allergens they used for SCIT. House dust mite (98.9%) was the most common allergen for SCIT, followed by pollen allergens (72.8%), animal dander (23.9%), and fungal species (20.7%) (Table 4). In addition, 13.2% of the respondents did not prescribe poly allergens for SCIT, whereas the remaining respondents prescribed combined up to poly allergens containing 5 different allergen extracts. Only 10.9% of the respondents mixed and prepared the allergen extract from bulk extracts at their clinics. The remaining respondents used a tailor-made extract set provided by pharmaceutical companies. For the build-up phase, 72.3% of the respondents used a conventional schedule of increasing doses of allergen immunotherapy extract administered 1-2 times per week, whereas 16.0% used a rush protocol and 11.8% used a cluster schedule protocol. Only house dust mites were prescribed for SLIT in Korea.
Table 3.
Diseases indicated for allergen immunotherapy

Numbers are not mutually exclusive.
Table 4.
Culprit allergens for subcutaneous immunotherapy
Numbers are not mutually exclusive.
Adverse reactions during AIT
Twenty-eight (30.4%) of the 92 SCIT respondents reported anaphylaxis during the SCIT. Of the 20 SLIT respondents, none reported anaphylaxis, but 8 (40.0%) reported adverse local reactions, including oral swelling and itching sensations of the throat or tongue.
DISCUSSION
Numerous similar surveys of AIT prescription patterns have been conducted in Mexico,14 Italy,15 and the United States.16,17 In addition, a worldwide survey of AIT practice patterns which focused on AIT doses and durations has been published recently.18 In this study, 69.0% of the allergy specialists in Korea used AIT. This figure may be higher than that of all Korean allergy practitioners. Most respondents to our survey were physicians, pediatricians, and otolaryngologists. They were generally young (between ages of 30 and 40) and worked at general hospitals located in Seoul and Gyeonggi-do Province, the metropolitan area of Korea. Young allergists are willing to accept recent information and to update their medical skills. People living in Seoul and its vicinity show higher prevalences of atopic diseases, including allergic rhinitis, asthma, and atopic dermatitis. They had higher socioeconomic statuses and were more interested in AIT than people living in other regions in Korea. Most respondents practiced at university hospitals and only a few worked at private clinics. This reflects the composition of KAAACI members. Most Korean allergy specialists are working at university hospitals, which is quite different from Western allergy specialists.
Regarding the need for AIT, most Korean allergists (98.6%) agreed to its role in the treatment of allergic diseases. However, some were reluctant to recognize this role because 17.8% of the respondents did not believe the efficacy of AIT in managing allergic diseases. One-third of the respondents did not prescribe AIT in clinical practice. Lack of facilities, including lack of trained health professionals, and lack of instruction on how to perform AIT through training or postgraduate programs were the most common reasons for not prescribing it.
Diseases indicated for SCIT include allergic rhinoconjunctivitis (92.0%), asthma (76.0%), atopic dermatitis (20.0%), and bee sting anaphylaxis (10.0%) in this study. It suggests that allergic asthma and rhinoconjunctivitis are the main diseases treated with AIT in Korea. The role of AIT for the management of allergic rhinoconjunctivitis and bee sting anaphylaxis is apparent,19,20 but it is controversial for asthma management.20-22 AIT is prescribed more frequently for allergic asthma in Korea than in other countries. This may be because the majority of KAAACI members are physicians trained in the Department of Internal Medicine and they more focus on the treatment of allergic asthma than on allergic rhinoconjunctivitis. Another reason is that Korean allergy specialists are in favor of AIT for the management of atopic dermatitis. Recently, favorable data from randomized and nonrandomized clinical trials of AIT in atopic dermatitis have been reported.4,23-25
Both skin prick and serologic tests are popularly used to determine offending allergens. Skin tests are performed for screening in most allergy clinics (94%) and serologic tests, including ImmunoCAP, RAST, and MAST, are used to confirm the presence of specific IgE.
House dust mites are the most common culprit allergen, followed by pollen allergens, animal dander, and fungal species, which are consistent with the prevalences of offending allergens in Korea.26 Korean allergists are prudent in the identification of offending allergens. They use skin prick tests for screening and confirm the presence of specific IgE by serologic tests. In Korea, poly allergen immunotherapy is more popular than mono-allergen immunotherapy. Only 13.2% of the respondents used a single allergen extract, such as house dust mite alone, whereas most respondents prescribed mixed allergen extracts in multiple-sensitized patients. The cross-reactivity of allergens, dose optimization of each constituent, and enzymatic degradation of allergens must be considered when allergen extracts are mixed.27 KAAACI recommended a mixture of up to 5 different allergens.28 For the immunotherapy schedule, only 25% of the respondents performed rush or cluster AIT in the build-up phase. AIT was administered by physicians (37%) or nurses (63%).
Only 13.8% of the respondents prescribed SLIT in clinical practice; most were otolaryngologists. Because SLIT was introduced in Korea 2 years prior to our study, a relatively low prescription rate was expected compared to SCIT. More clinical experiences are needed for SLIT to be prescribed widely by physicians and pediatricians.
The frequency of adverse systemic reactions during SCIT has been reported to be 2.1%-2.9% of all patients.29 In this survey, ~30% of the respondents observed severe systemic reactions after SCIT. SLIT is known to be safer than SCIT because few cases of severe systemic reactions have been reported during SLIT.30 In this study, no respondents observed severe systemic reactions to SLIT, but 40% had local adverse reactions, including oral swelling and itching.
In conclusion, 69.0% of the respondents performed AIT in clinical practice. The prescription of SCIT is more popular than SLIT; however, SLIT is increasingly prescribed, particularly among otorhinolaryngologists. Lack of facilities, including trained health professionals, and lack of clinical experience and instruction on AIT are the critical barriers to the prescription of AIT by Korean allergy specialists. Therefore, proper clinical instruction that introduces and updates information on AIT is necessary to encourage AIT in Korea.
ACKNOWLEDGMENTS
This work was supported by a grant from the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Creticos PS, Adkinson NF, Jr, Kagey-Sobotka A, Proud D, Meier HL, Naclerio RM, Lichtenstein LM, Norman PS. Nasal challenge with ragweed pollen in hay fever patients. Effect of immunotherapy. J Clin Invest. 1985;76:2247–2253. doi: 10.1172/JCI112233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 3.Bussmann C, Böckenhoff A, Henke H, Werfel T, Novak N. Does allergen-specific immunotherapy represent a therapeutic option for patients with atopic dermatitis? J Allergy Clin Immunol. 2006;118:1292–1298. doi: 10.1016/j.jaci.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 4.Cadario G, Galluccio AG, Pezza M, Appino A, Milani M, Pecora S, Mastrandrea F. Sublingual immunotherapy efficacy in patients with atopic dermatitis and house dust mites sensitivity: a prospective pilot study. Curr Med Res Opin. 2007;23:2503–2506. doi: 10.1185/030079907X226096. [DOI] [PubMed] [Google Scholar]
- 5.Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2003;33:1076–1082. doi: 10.1046/j.1365-2222.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 6.Blumberga G, Groes L, Haugaard L, Dahl R. Steroid-sparing effect of subcutaneous SQ-standardised specific immunotherapy in moderate and severe house dust mite allergic asthmatics. Allergy. 2006;61:843–848. doi: 10.1111/j.1398-9995.2006.01088.x. [DOI] [PubMed] [Google Scholar]
- 7.Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Høst A, Koivikko A, Koller D, Norberg LA, Urbanek R, Valovirta E, Wahn U, Möller C PAT Investigator Group. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–859. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 8.Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, Wurtzen PA, Andersen JS, Tholstrup B, Riis B, Dahl R. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–725.e5. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, Koivikko A, Norberg LA, Valovirta E, Wahn U, Möller C The PAT investigator group. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 10.Dinakar C, Portnoy JM. Allergen immunotherapy in the prevention of asthma. Curr Opin Allergy Clin Immunol. 2004;4:131–136. doi: 10.1097/00130832-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, Burastero SE, Calori G, Benetti L, Bonazza P, Puccinelli P, Parmiani S, Bernardini R, Vierucci A. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851–857. doi: 10.1016/j.jaci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Inal A, Altintas DU, Yilmaz M, Karakoc GB, Kendirli SG, Sertdemir Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J Investig Allergol Clin Immunol. 2007;17:85–91. [PubMed] [Google Scholar]
- 13.Reha CM, Ebru A. Specific immunotherapy is effective in the prevention of new sensitivities. Allergol Immunopathol (Madr) 2007;35:44–51. doi: 10.1157/13101337. [DOI] [PubMed] [Google Scholar]
- 14.Larenas Linnemann D, Guidos Fogelbach GA, Arias Cruz A. Practice patterns in Mexican allergologists about specific immunotherapy with allergens. Rev Alerg Mex. 2008;55:53–61. [PubMed] [Google Scholar]
- 15.Lombardi C, Senna G, Passalacqua G. Specific immunotherapy among Italian specialists. Allergy. 2006;61:898–899. doi: 10.1111/j.1398-9995.2006.01085.x. [DOI] [PubMed] [Google Scholar]
- 16.Tucker MH, Tankersley MS ACAAI Immunotherapy and Diagnostics Committee. Perception and practice of sublingual immunotherapy among practicing allergists. Ann Allergy Asthma Immunol. 2008;101:419–425. doi: 10.1016/S1081-1206(10)60320-1. [DOI] [PubMed] [Google Scholar]
- 17.Coifman RE, Cox LS Immunotherapy and Allergy Diagnostics Committee of the AAAAI. 2006 American Academy of Allergy, Asthma & Immunology member immunotherapy practice patterns and concerns. J Allergy Clin Immunol. 2007;119:1012–1013. doi: 10.1016/j.jaci.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Larenas-Linnemann DE, Gupta P, Mithani S, Ponda P. Survey on immunotherapy practice patterns: dose, dose adjustments, and duration. Ann Allergy Asthma Immunol. 2012;108:373–378.e3. doi: 10.1016/j.anai.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Bousquet J, Lockey R, Malling HJ, Alvarez-Cuesta E, Canonica GW, Chapman MD, Creticos PJ, Dayer JM, Durham SR, Demoly P, Goldstein RJ, Ishikawa T, Ito K, Kraft D, Lambert PH, Løwenstein H, Müller U, Norman PS, Reisman RE, Valenta R, Valovirta E, Yssel H World Health Organization; American Academy of Allergy, Asthma and Immunology. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Ann Allergy Asthma Immunol. 1998;81:401–405. doi: 10.1016/s1081-1206(10)63136-5. [DOI] [PubMed] [Google Scholar]
- 20.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 21.National Asthma Education; Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 22.British Thoracic Society Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Thorax. 2008;63(Suppl 4):iv1–iv121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 23.Nahm DH, Lee ES, Park HJ, Kim HA, Choi GS, Jeon SY. Treatment of atopic dermatitis with a combination of allergen-specific immunotherapy and a histamine-immunoglobulin complex. Int Arch Allergy Immunol. 2008;146:235–240. doi: 10.1159/000115892. [DOI] [PubMed] [Google Scholar]
- 24.Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, Ruzicka T, Brehler R, Wolf H, Schnitker J, Kapp A. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006;61:202–205. doi: 10.1111/j.1398-9995.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 25.Bussmann C, Maintz L, Hart J, Allam JP, Vrtala S, Chen KW, Bieber T, Thomas WR, Valenta R, Zuberbier T, Sager A, Novak N. Clinical improvement and immunological changes in atopic dermatitis patients undergoing subcutaneous immunotherapy with a house dust mite allergoid: a pilot study. Clin Exp Allergy. 2007;37:1277–1285. doi: 10.1111/j.1365-2222.2007.02783.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim TB, Kim KM, Kim SH, Kang HR, Chang YS, Kim CW, Bahn JW, Kim YK, Kang HT, Cho SH, Park HS, Lee JM, Choi IS, Min KU, Hong CS, Kim NS, Kim YY. Sensitization rates for inhalant allergens in Korea; a multi-center study. J Asthma Allergy Clin Immunol. 2003;23:483–493. [Google Scholar]
- 27.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–S85. doi: 10.1016/j.jaci.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 28.National guideline for the management of asthma. J Asthma Allergy Clin Immunol. 1998;18:345–390. [Google Scholar]
- 29.Nelson HS. Allergen immunotherapy: where is it now? J Allergy Clin Immunol. 2007;119:769–779. doi: 10.1016/j.jaci.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Cox L. Sublingual immunotherapy in pediatric allergic rhinitis and asthma: efficacy, safety, and practical considerations. Curr Allergy Asthma Rep. 2007;7:410–420. doi: 10.1007/s11882-007-0063-6. [DOI] [PubMed] [Google Scholar]



