Abstract
Leprechaunism is a rare autosomal recessive disease that is characterized by severe insulin resistance. This disease is caused by a defective insulin receptor and features abnormal glucose metabolism and retarded intrauterine and postnatal growth. However, there are few reports on the long-term course of leprechaunism. We reported the long-term clinical course and rh-IGF-1 treatment in a patient with leprechaunism. During follow-up her diabetes gradually deteriorated despite of treatment of rh-IGF-1. Furthermore, she developed endometrioid adenocarcinoma at the age of 24 yr. The development of endometrial disease must be carefully followed up in this disease.
Keywords: Leprechaunism, insulin receptor, rh-IGF-1, endometrioid adenocarcinoma
Introduction
Leprechaunism is a rare autosomal recessive disease that is characterized by severe insulin resistance (1,2,3). This disease is caused by a defective insulin receptor and features abnormal glucose metabolism and retarded intrauterine and postnatal growth (1,2,3,4).
Insulin-like growth factor I (IGF-I), a 70-amino acid polypeptide with extensive, structural homology to insulin (48%), exerts its biological effects by binding to IGF-1 receptor (IGF-1R), IR, or IGF-1R/IR hybrid receptor on the surface of target cells (3, 5, 6). It is well known that the insulin and IGF-1 receptors are structurally related and share common post-receptor signaling pathways (5, 6). Therefore, exogenous administration of recombinant human IGF-1 (rh-IGF-1) has been used in patients with leprechaunism (3, 7,8,9,10,11). However, there are few reports on the long-term effects of rh-IGF-1 and clinical course of leprechaunism. We previously reported the effect of rh-IGF-1 and clinical course in a patient up to 7 yr of age (9). Furthermore, we and others reported retinal neovascularization during treatment with rh-IGF-1 when this patient was 12 yr old (12, 13). We have continued to follow this patient. To our knowledge, the patient is the longest follow-up case so far.
Case Report
The patient is a Japanese female who is now 24 yr old. Her clinical course until 12 yr old was previously described (9, 12, 13). In brief, she was born after 39 weeks of gestation as a small for age with a birth weight and length of 1,552 g and 44 cm, respectively. She had typical multiple phenotypic symptoms of leprechaunism and showed very high immunoreactive insulin levels. Based on these findings, she was diagnosed as having leprechaunism. She was treated with rh-IGF-1 beginning at 6 mo of age. Molecular analysis of her insulin receptor gene revealed a missense mutation (P87L) in the paternal allele and a 1.3-kb deletion between exons 4 and 6 in the maternal allele (14).
Clinical course of the patient
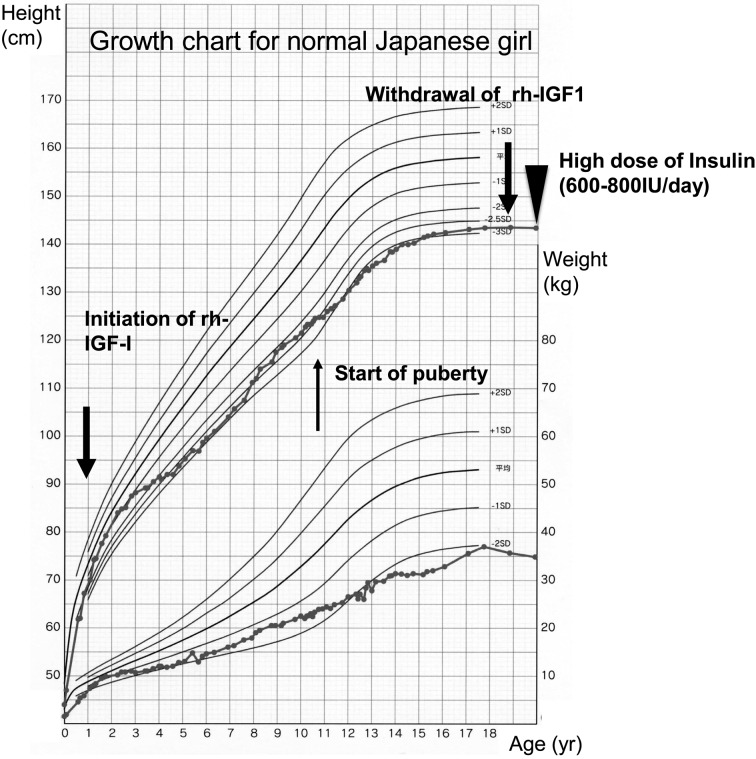
Thereafter, she was treated with rh-IGF-1, and the dosage was gradually increased to 24 mg/d at 11 yr of age to reduce her blood glucose levels. The rh-IGF-1 was given by a subcutaneous bolus injection before each meal and continuous subcutaneous infusion. However, it became difficult to control her diabetes despite the high dose of rh-IGF-1. Her fasting blood glucose levels were 100–200 mg/dl, and her postprandial blood glucose levels were 200–300 mg/dl. Her HbA1c (JDS) levels increased to 10–11%. She was prescribed metformin, voglibose and pioglitazone; however, these medications were ineffective. At the age of 12 yr, she showed neovascular glaucoma and retinal neovascularization in both eyes. Despite treatment with various medications and cryoretinopexy, she lost her vision as reported previously (12, 13). Thereafter, her diabetes further deteriorated, and her fasting blood glucose levels were 200–250 mg/dl and postprandial blood glucose levels were 250–350 mg/dl. Her HbA1C levels increased to 12–13% (JDS). During this period, her fasting insulin levels remained high (800–1,000 IU/ml). Her growth hormone and IGFBP3 levels remained undetectable, suggesting GH resistance as reported previously (9). At the age of 11 yr, her breast development started, and her pubertal stage gradually developed. At the age of 15 yr, she reached her adult height (143 cm, –2.7 SD for normal Japanese girl) (Fig. 1). Finally, at the age of 18 yr, she developed diabetic ketoacidosis because of gastroenteritis. At this time, her blood glucose was 835 mg/dl, blood vein pH was 6.895, HCO3- was 3.1 mEq/L and urinary ketone body test was markedly positive. Saline infusion and continuous intravenous regular insulin (100–200 IU/h) were started. Thereafter her condition improved; however, despite a high dose of insulin infusion, the control of her fasting and postprandial blood glucose levels was difficult. After this episode, rh-IGF-1 treatment was withdrawn, and high-dose insulin was started (120 IU of insulin aspart and 160 IU of insulin glargine simultaneously before each meal). Despite the massive dose of insulin, her fasting and postprandial blood glucose levels were 200–300 mg/dl and 300–350 mg/dl, respectively. First morning urine ketone body tests were sometimes positive. At the age of 22 yr, she developed diabetic ketoacidosis again because of exacerbation of mastitis. She is now treated high-dose insulin (total daily dose of 800IU), but the control of her diabetes remains difficult.
Fig. 1.
Growth chart of the patient.
Diseases of the breast and uterus
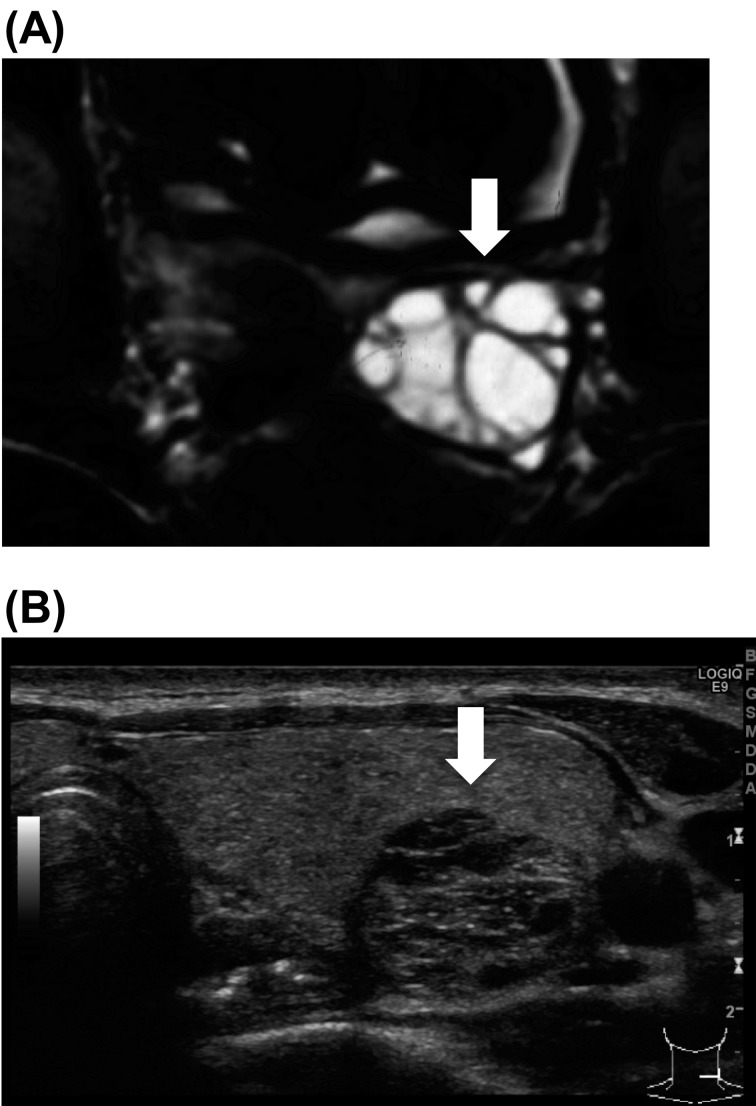
At the age of 3 yr, she underwent tonsillectomy as described previously(9). Regular radiological examination of each organ showed enlargement of the spleen, kidney, ovary, thyroid gland, submandibular, parotid, and lacrimal glands since 9 yr old. Her ovary became polycystic beginning at 9 yr of age (Fig. 2A). At the age of 14 yr, ultrasonography of the thyroid gland identified several nodules. The size of a nodule in the left lobe was 19 × 16 × 12 mm, and it was diagnosed as adenomatous goiter (Fig. 2B). Beginning at 16 yr of age, she frequently complained of painful distention of the bilateral mammary glands, and purulent effusion form the mammary glands was frequently observed. Ultrasonography of the mammary glands demonstrated dilation of lactiferous ducts and several small low echogenic masses. These findings indicated mastitis and fibroadenoma. After the withdrawal of rh-IGF-1, the size of enlarged organs did not change.
Fig. 2.
(A) MRI findings of the ovary. She developed a polycystic ovary. (B) Ultrasonography of the thyroid gland identified several nodules. The size of the nodule in the left lobe was 19 × 16 × 12 mm, and it was diagnosed as adenomatous goiter.
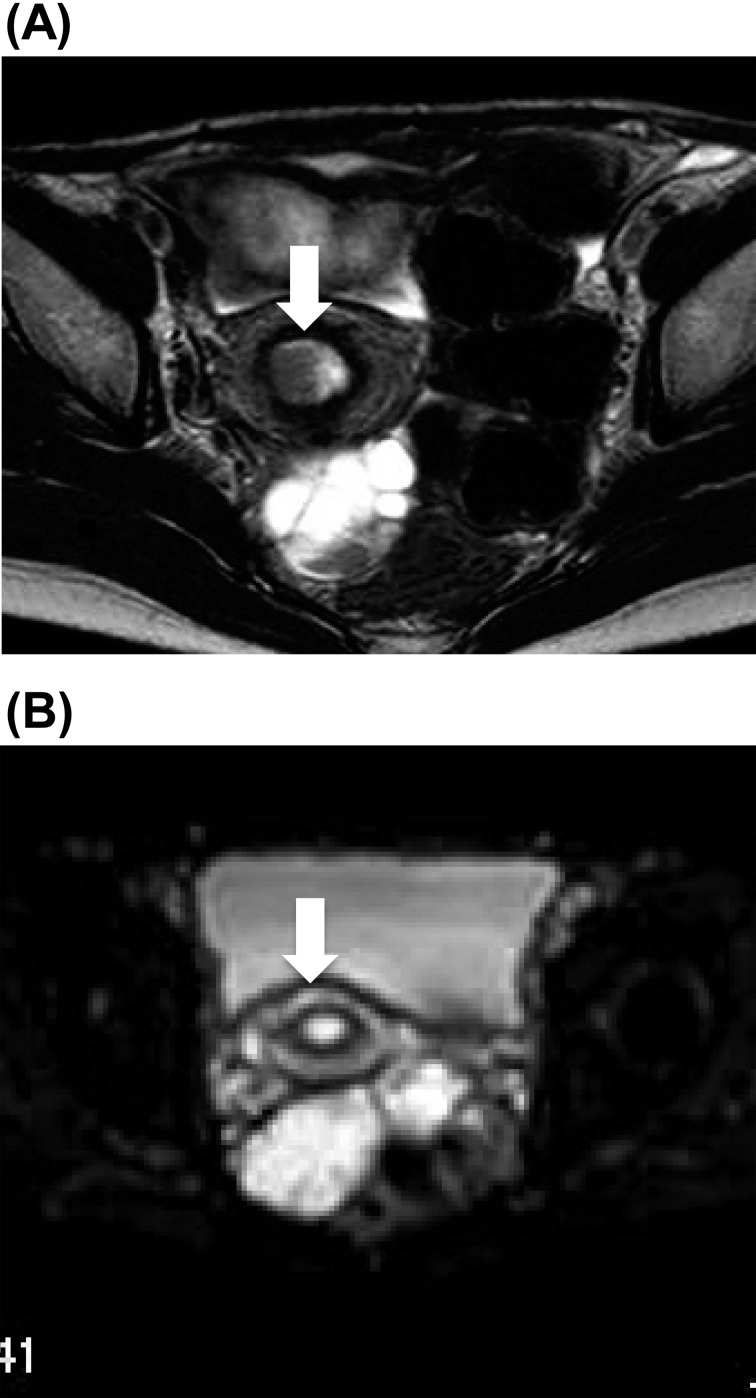
In addition, she had an irregular menstrual cycle due to polycystic ovaries, and so she was monitored regularly in our gynecologic outpatient department. At the age of 24 yr, magnetic resonance image (MRI) demonstrated a thickened endometrium with a low-intensity nodule of 14 × 12 × 18 mm in size in a T2-weighted image (Fig. 3). The nodule, which showed a high intensity in diffusion-weighted image, strongly suggested uterine endometrial neoplasm. Intrauterine endometrial curettage was performed, and the histological diagnosis was grade 1 endometrioid adenocarcinoma. Therefore, she underwent ovariohysterectomy, and the surgical staging was FIGO IA. Stage IA involves the endometrium and/or less than one-half myometrial invasion. Because her breast diseases improved after operation presumably due to a lack of estrogen, estrogen replacement therapy was not applied. She was treated with eldecalcitol© to prevent osteoporosis due to gonadectomy.
Fig. 3.
MRI of the uterus (A) A T2-weighted image showed a low-density nodule of 14 × 12 × 18 mm in size. (B) The nodule showed a high-intensity signal in a diffusion weighted image.
Discussion
In this report, we described the long-term clinical course and rh-IGF-1 treatment in a patient with leprechaunism. To our knowledge, she is the longest surviving patient with severe insulin receptor mutation.
Initially, rh-IGF-1 therapy was effective; however, rh-IGF-1 became gradually ineffective. Disruption of the insulin signaling pathway is thought to be a mechanism leading to the development of insulin resistance; however, this mechanism is complex because of the number of independent factors in the pathway (15). For example, persistent hyperglycemia may cause oxidative stress and inhibit insulin signaling through IR, resulting in insulin resistance (16). In addition, continued exposure to insulin leads to a downregulation of receptor numbers (17). Furthermore, propagation of the signal from the insulin receptor to downstream effectors is negatively regulated by hyperinsulinism (18). In this patient, glucose transport is a cascade of events starting from the interaction of rh-IGF-1 with its own receptor and ending with intracellular glucose metabolism using the identical post-receptor signaling pathways as insulin. Similar to the mechanism of the development of insulin resistance mentioned above, hyperglycemia and high IGF-1 levels may be responsible for a deterioration of rh-IGF-1 action.
In her clinical course, she developed mastitis and finally endometrial cancer. The IGF-1 system has been implicated in several different malignancies (19, 20). In the uterus, cyclic changes in IGF-1 expression and signaling play a pivotal role in regulating the transition of the premenopausal endometrium through proliferative, secretory, and menstrual cycles (19). Although the relation between blood level of IGF-1 and incidence of endometrial cancer is controversial in epidemiological studies (21, 22), in vitro studies indicated that autocrine stimulation of IGF-1 enhanced estrogen-induced proliferation of endometrial carcinoma (23, 24). Since the present case had very high levels of insulin after birth, continuous signaling through IGF-1R by excessive insulin may be related to the development of endometrial cancer.
Regarding her mammary glands, the patient suffered from repeated mastitis and fibroadenoma. Hyperplasia of the mammary gland has been reported in leprechaunism (1). It is also known that the IGF-1 and IGF-1R system is important for mammary gland development (25). Taken together, it is plausible that persistent high levels of insulin may act through IGF-1R in the mammary gland, and this may have been the reason for the breast disease in the present case.
To our knowledge, the long-term effect of administration of high-dose rh-IGF-1 for the treatment for leprechaunism has not been reported so far. Whereas the development of her complications in the breast and uterus may have been affected by this medication in addition to hyperinsulinism, there is no direct evidence for this speculation.
In conclusion, we reported the long-term course of a patient with leprechaunism who had been treated with rh-IGF-1 and had persisted hyperinsulinism. The development of breast and endometrial diseases must be carefully followed up in this disease.
References
- 1.Donohue WL, Uchida I. Leprechaunism: a euphuism for a rare familial disorder. J Pediatr 1954;45:505–19 10.1016/S0022-3476(54)80113-2 [DOI] [PubMed] [Google Scholar]
- 2.Kosztolányi G. Leprechaunism/Donohue syndrome/insulin receptor gene mutations: a syndrome delineation story from clinicopathological description to molecular understanding. Eur J Pediatr 1997;156:253–5 10.1007/s004310050594 [DOI] [PubMed] [Google Scholar]
- 3.Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, et al. Clinical course of genetic diseases of the insulin receptor (type A and Rabson -Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 2004;83:209–22 10.1097/01.md.0000133625.73570.54 [DOI] [PubMed] [Google Scholar]
- 4.Fujieda K. Leprechaunism (Donohue syndrome). Nihon Rinsho 2006;28:94–9 (in Japanese). [PubMed] [Google Scholar]
- 5.Di Cola G, Cool MH, Accili D. Hypoglycemic effect of insulin-like growth factor-1 in mice lacking insulin receptors. J Clin Invest 1997;99:2538–44 10.1172/JCI119438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology 2011;152:2546–51 10.1210/en.2011-0231 [DOI] [PubMed] [Google Scholar]
- 7.Schoenle EJ, Zenobi PD, Torresani T, Werder EA, Zachmann M, Froesch ER. Recombinant human insulin like growth factor I (rhIGF-I) reduces hyperglycaemia in patients with extreme insulin resistance. Diabetologia 1991;34:675–9 10.1007/BF00400998 [DOI] [PubMed] [Google Scholar]
- 8.Kuzuya H, Matsuura N, Sakamoto M, Makino H, Sakamoto Y, Kadowaki T, et al. Trial of insulin-like growth factor 1 therapy for patients with extreme insulin resistance syndromes. Diabetes 1993;42:696–705 10.2337/diabetes.42.5.696 [DOI] [PubMed] [Google Scholar]
- 9.Nakae J, Kato M, Murashita M, Shinohara N, Tajima T, Fujieda K. Long-term effect of recombinant human insulin-like growth factor I on metabolic and growth control in a patient with leprechaunism. J Clin Endocrinol Metab 1998;83:542–9 10.1210/jc.83.2.542 [DOI] [PubMed] [Google Scholar]
- 10.McDonald A, Williams R, Regan F, Semple R, Dunger D. IGF-1 treatment of insulin resistance. Eur J Endocrinol 2007;157:S51–6 10.1530/EJE-07-0271 [DOI] [PubMed] [Google Scholar]
- 11.Kawashima Y, Nishimura R, Utsunomiya A, Kagawa R, Funata H, Fujimoto M, et al. Leprechaunism (Donohue syndrome): a case bearing novel compound heterozygous mutations in the insulin receptor gene. Endocr J 2013;60:107–12 10.1507/endocrj.EJ12-0289 [DOI] [PubMed] [Google Scholar]
- 12.Okuhara K, Tsubaki J, Satoh K, Murashita M, Tajima T. Long-Term effects of rh IGF-I treatment in a patient with Leprechaunism. The 2nd International Congress of the GRS and IGF Society, Boston, Abstract P-18, 2004
- 13.Kitamei H, Yokoi M, Kase M, Ohno S. Retinal neovascularization during treatment with IGF-1 for insulin resistance syndrome. Graefes Arch Clin Exp Ophthalmol 2005;243:715–7 10.1007/s00417-004-1093-6 [DOI] [PubMed] [Google Scholar]
- 14.Nakae J, Morioka H, Ohtsuka E, Fujieda K. Replacements of leucine 87 in human insulin receptor alter affinity for insulin. J Biol Chem 1995;270:22017–22 10.1074/jbc.270.37.22017 [DOI] [PubMed] [Google Scholar]
- 15.Schofield CJ, Sutherland C. Disordered insulin secretion in the development of insulin resistance and Type 2 diabetes. Diabet Med 2012;29:972–9 10.1111/j.1464-5491.2012.03655.x [DOI] [PubMed] [Google Scholar]
- 16.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 2011;18:139–43 10.1097/MED.0b013e3283444b09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha MK, Taylor LG, Pories WJ, Flickinger EG, Meelheim D, Atkinson S, et al. Long-term effect of insulin on glucose transport and insulin binding in cultured adipocytes from normal and obese humans with and without non-insulin-dependent diabetes. J Clin Invest 1987;80:1073–81 10.1172/JCI113163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusari J, Kenner KA, Suh KI, Hill DE, Henry RR. Skeletal muscle protein tyrosine phosphatase activity and tyrosine phosphatase 1B protein content are associated with insulin action and resistance. J Clin Invest 1994;93:1156–62 10.1172/JCI117068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene 2012;31:2703–14 10.1038/onc.2011.447 [DOI] [PubMed] [Google Scholar]
- 20.Malaguarnera R, Morcavallo A, Belfiore A. The insulin and IGF-I pathway in endocrine glands carcinogenesis. J Oncol 2012;2012:635614. 10.1155/2012/635614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petridou E, Koukoulomatis P, Alexe DM, Voulgaris Z, Spanos E, Trichopoulos D. Endometrial cancer and the IGF system: a case-control study in Greece. Oncology 2003;64:341–5 10.1159/000070291 [DOI] [PubMed] [Google Scholar]
- 22.Weiderpass E, Brismar K, Bellocco R, Vainio H, Kaaks R. Serum levels of insulin-like growth factor-I, IGF-binding protein 1 and 3, and insulin and endometrial cancer risk. Br J Cancer 2003;89:1697–704 10.1038/sj.bjc.6601312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strissel PL, Ellmann S, Loprich E, Thiel F, Fasching PA, Stiegler E, et al. Early aberrant insulin-like growth factor signaling in the progression to endometrial carcinoma is augmented by tamoxifen. Int J Cancer 2008;123:2871–9 10.1002/ijc.23900 [DOI] [PubMed] [Google Scholar]
- 24.Kashima H, Shiozawa T, Miyamoto T, Suzuki A, Uchikawa J, Kurai M, et al. Autocrine stimulation of IGF1 in estrogen-induced growth of endometrial carcinoma cells: involvement of the mitogen-activated protein kinase pathway followed by up-regulation of cyclin D1 and cyclin E. Endocr Relat Cancer 2009;16:113–22 10.1677/ERC-08-0117 [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Shushanov S, LeRoith D, Wood TL. Decreased IGF type 1 receptor signaling in mammary epithelium during pregnancy leads to reduced proliferation, alveolar differentiation, and expression of insulin receptor substrate (IRS)-1 and IRS-2. Endocrinology 2011;152:3233–45 10.1210/en.2010-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]