Abstract
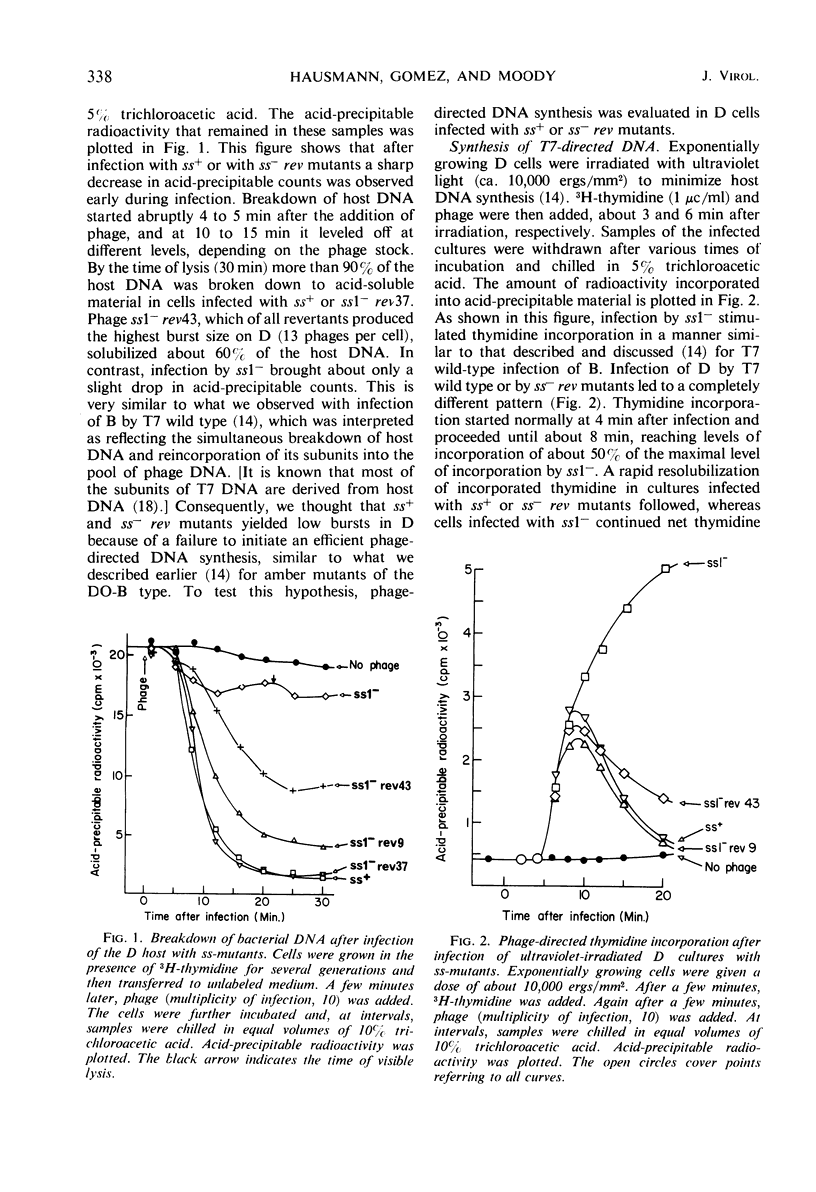
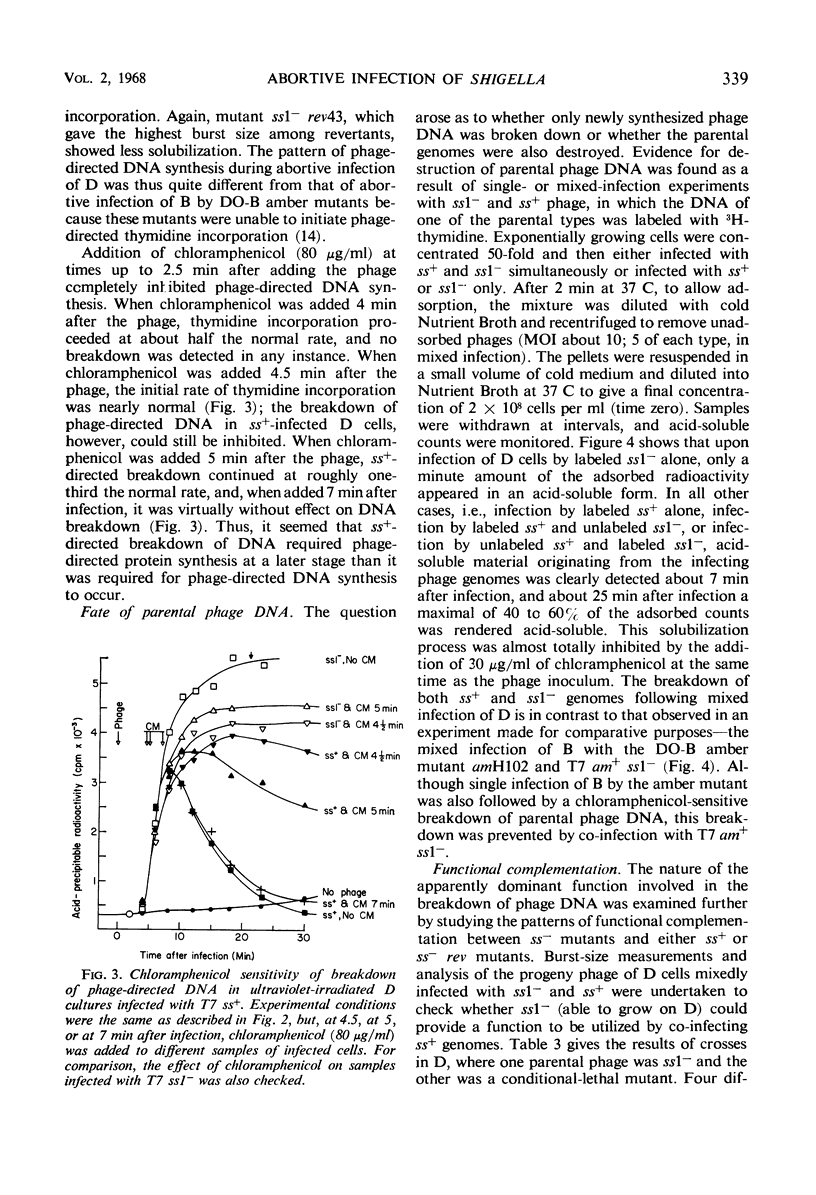
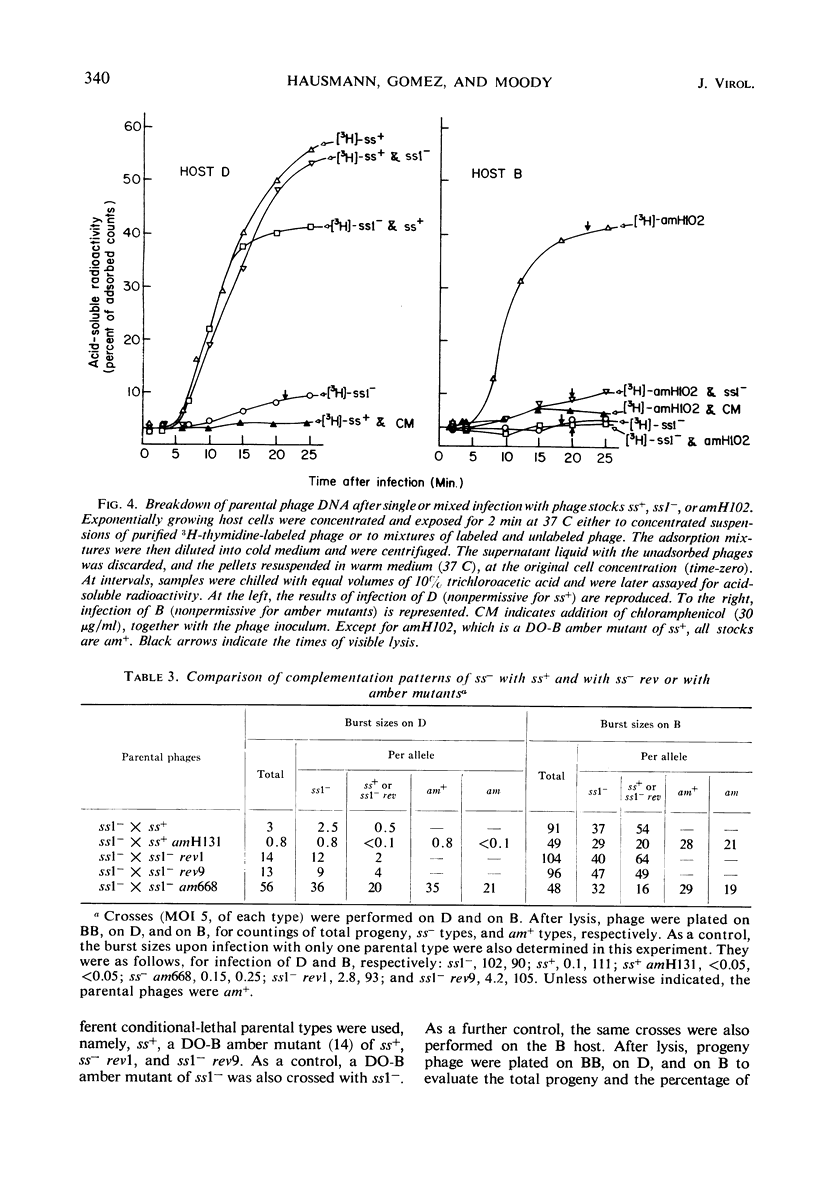
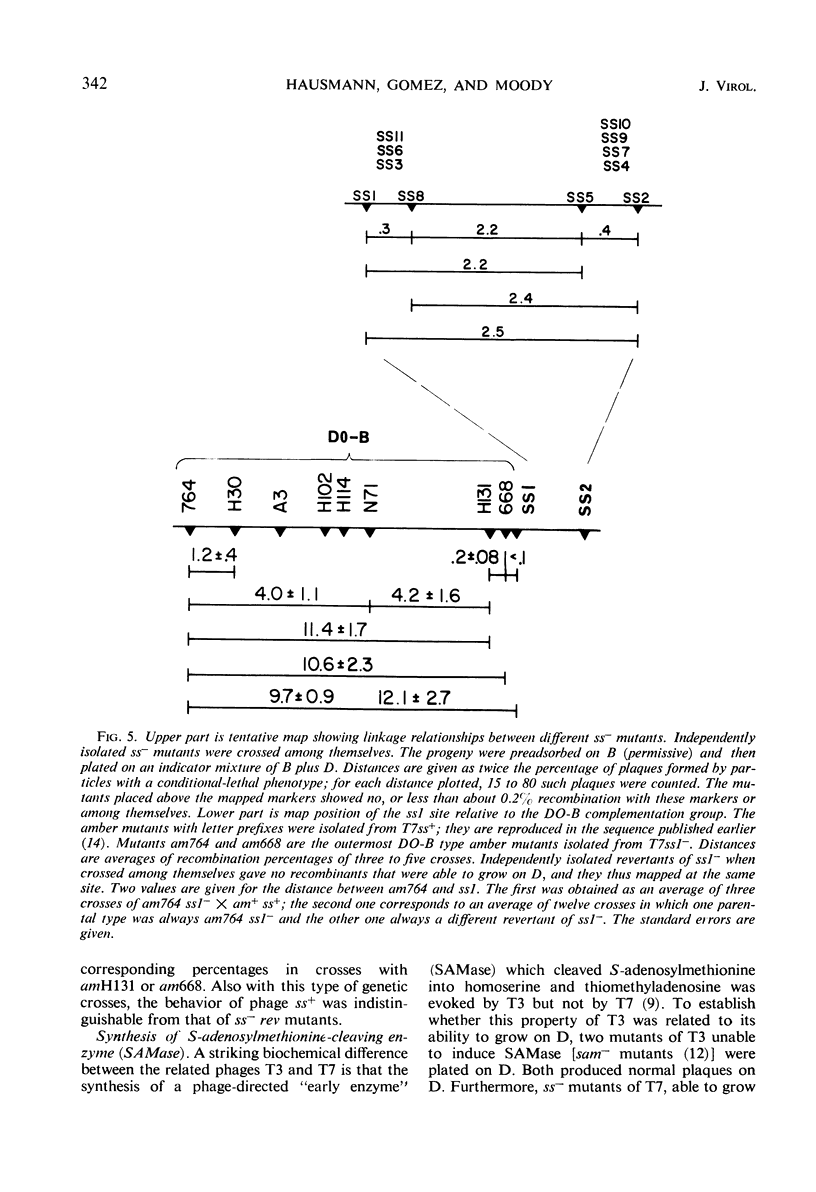
Phage T7 adsorbed to and lysed cells of Shigella sonnei D2 371-48, although the average burst size was only 0.1 phage per cell (abortive infection). No mechanism of host-controlled modification was involved. Upon infection, T7 rapidly degraded host deoxyribonucleic acid (DNA) to acid-soluble material. Phage-directed DNA synthesis was initiated normally, but after a few minutes the pool of phage DNA, including the parental DNA, was degraded. Addition of chloramphenicol, at the time of phage infection, prevented both the initiation of phage-directed DNA synthesis and the degradation of parental phage DNA. Addition of chloramphenicol 4.5 min after phage was added permitted the onset of phage-directed DNA synthesis but prevented breakdown of phage DNA. Mutants of T7 (ss− mutants) have been isolated which show normal growth in strain D2 371-48. Upon mixed infection of this strain with T7 wild type and an ss− mutant, infection was abortive; no complementation occurred. The DNA of the ss− mutants was degraded in mixed infection like that of the wild type. Revertant mutants which have lost their ability to grow on D2 371-48 were isolated from ss− mutants; they are, in essence, phenotypically like T7 wild type. Independently isolated revertants of ss− mutants did not produce ss− recombinants when they were crossed among themselves. When independently isolated ss− mutants were crossed with each other, wild-type recombinants were found; ss− mutants could then be mapped in a cluster compatible with the length of one cistron. We concluded that T7 codes for an active, chloramphenicol-sensitive function [ss+ function (for suicide in Shigella)] which leads to the breakdown of phage DNA in the Shigella host.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. Host specificity of DNA produced by Escherichia coli. II. Control over acceptance of DNA from infecting phage lambda. J Mol Biol. 1962 Jul;5:37–49. doi: 10.1016/s0022-2836(62)80059-x. [DOI] [PubMed] [Google Scholar]
- Eskridge R. W., Weinfeld H., Paigen K. Susceptibility of different coliphage genomes to host-controlled variation. J Bacteriol. 1967 Mar;93(3):835–844. doi: 10.1128/jb.93.3.835-844.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M., Hausmann R., Gold M., Hurwitz J. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. X. Bacteriophage T3-induced S-adenosylmethionine cleavage. J Biol Chem. 1966 May 10;241(9):1995–2006. [PubMed] [Google Scholar]
- HATTMAN S. THE FUNCTIONING OF T-EVEN PHAGES WITH UNGLUCOSYLATED DNA IN RESTRICTING ESCHERICHIA COLI HOST CELLS. Virology. 1964 Nov;24:333–348. doi: 10.1016/0042-6822(64)90171-0. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Gold M. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. IX. Deoxyribonucleic acid methylase in bacteriophage-infected Escherichia coli. J Biol Chem. 1966 May 10;241(9):1985–1994. [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R. Synthesis of an S-adenosylmethionine-cleaving enzyme in T3-infected Escherichia coli and its disturbance by co-infection with enzymatically incompetent bacteriophage. J Virol. 1967 Feb;1(1):57–63. doi: 10.1128/jvi.1.1.57-63.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kerszman G., Glover S. W., Aronovitch J. The restriction of bacteriophage lambda in Escherichia coli strain w. J Gen Virol. 1967 Jul;1(3):333–347. doi: 10.1099/0022-1317-1-3-333. [DOI] [PubMed] [Google Scholar]
- Klein A. Wirtskontrollierte Modifikation. Z Vererbungsl. 1965;96(4):324–345. [PubMed] [Google Scholar]
- LABAW L. W. The origin of phosphorus in Escherichia coli bacteriophages. J Bacteriol. 1951 Aug;62(2):169–173. doi: 10.1128/jb.62.2.169-173.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG S., MESELSON M. DEGRADATION OF NON-REPLICATING BACTERIOPHAGE DNA IN NON-ACCEPTING CELLS. J Mol Biol. 1964 May;8:623–628. doi: 10.1016/s0022-2836(64)80112-1. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. A nonhereditary, host-induced variation of bacterial viruses. J Bacteriol. 1952 Oct;64(4):557–569. doi: 10.1128/jb.64.4.557-569.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREISINGER G., FRANKLIN N. C. Mutation and recombination at the host range genetic region of phage T2. Cold Spring Harb Symp Quant Biol. 1956;21:103–111. doi: 10.1101/sqb.1956.021.01.009. [DOI] [PubMed] [Google Scholar]



