Mean platelet volume (MPV) reflects the size of platelets and is accepted as a marker of platelet function. Platelets secrete and express a large number of substances that are crucial mediators of coagulation, inflammation, thrombosis, and atherosclerosis [1].
Hip fractures in the elderly are associated with impaired mobility and increased morbidity and mortality. Chronic disease has one of the most potentially harmful impacts on bone health in the elderly. The detrimental factors involved in chronic diseases are often multifactorial, including poor nutrition, diminished physical activity, inflammatory cytokine exposure, and/or medications [2, 3]. These factors lead to reduced bone mineralization and increased osteoporosis, which is marked by decreased density of the bone infrastructure, leading to a decrease in overall bone strength. In this study, we investigated whether fractures in the femur neck and intertrochanteric region are related to MPV.
Patients above the age of 65 who were treated for femur neck or intertrochanteric region fractures between 2006 and 2012 were screened in this study. Exclusion criteria were as follows: a fracture due to a traffic accident or other major trauma and femur fractures in other regions. A total of 102 patients were included in the femur neck group, and 95 patients were included in the intertrochanteric group.
Age- and gender-matched control subjects (98 patients) without fractures were also enrolled. The study was performed according to the guidelines of the Helsinki Declaration and was approved by our local ethics committee. Hematologic tests were performed using CELL-DYN Ruby analyzer (Abbott Laboratories, Abbott Park, IL, USA). All biochemical tests were performed using Abbott Laboratories Architect C16000 analyzer (Abbott Laboratories).
The data were analyzed using SPSS 13.1 for Windows (Chicago, IL, USA). Differences between the 2 groups were analyzed using an independent sample t-test. The differences were considered significant at P<0.05.
The results for age, gender, and chronic diseases between the controls and patients are shown in Table 1. The mean MPV values for the femur neck and intertrochanteric region groups were significantly higher than those for the control group (Table 2). The mean Hb levels in the femur neck and intertrochanteric region groups were lower than that in the control group (11.0±1.5 g/dL, P<0.001; 11.7±1.7 g/dL, P=0.007; 12.4±1.4 g/dL, respectively).
Table 1.
The main characteristics and chronic disease in all groups
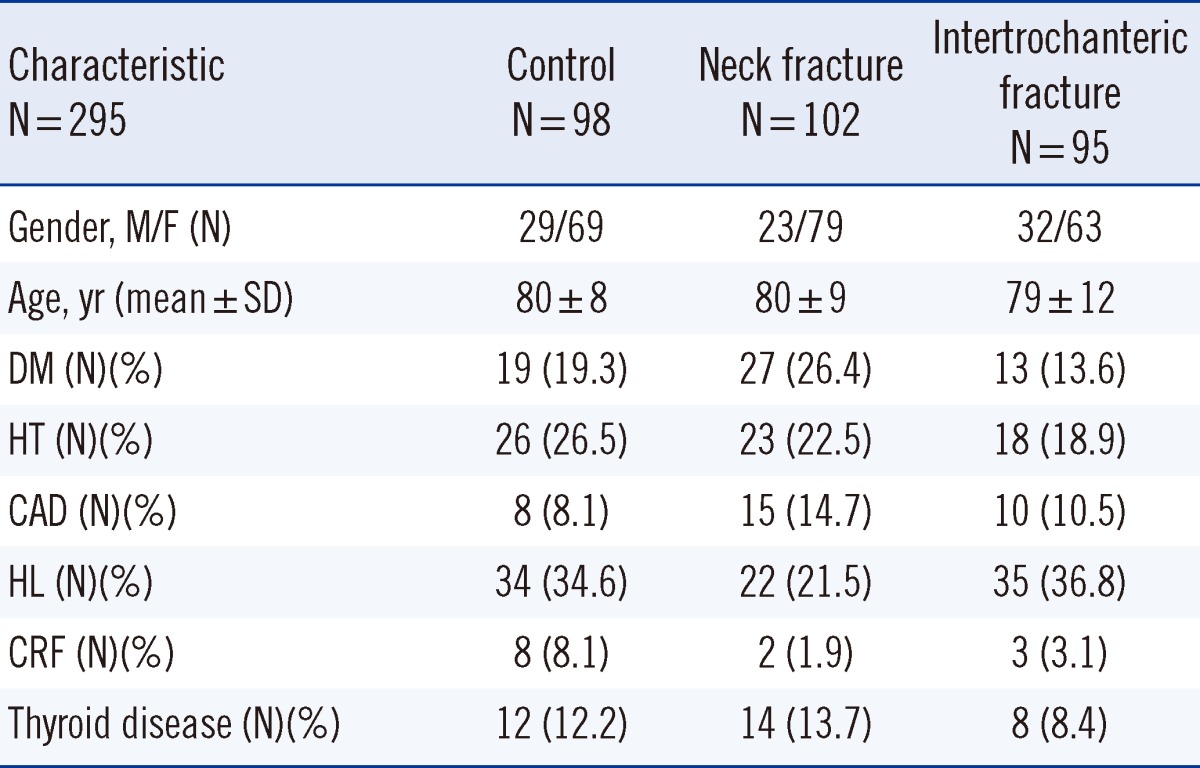
Abbreviations: M, male; F, female; DM, diabetes mellitus; HT, hypertension; CAD, coronary artery disease; HL, hyperlipidemia; CRF, chronic renal failure.
Table 2.
Laboratory parameters for the 3 groups
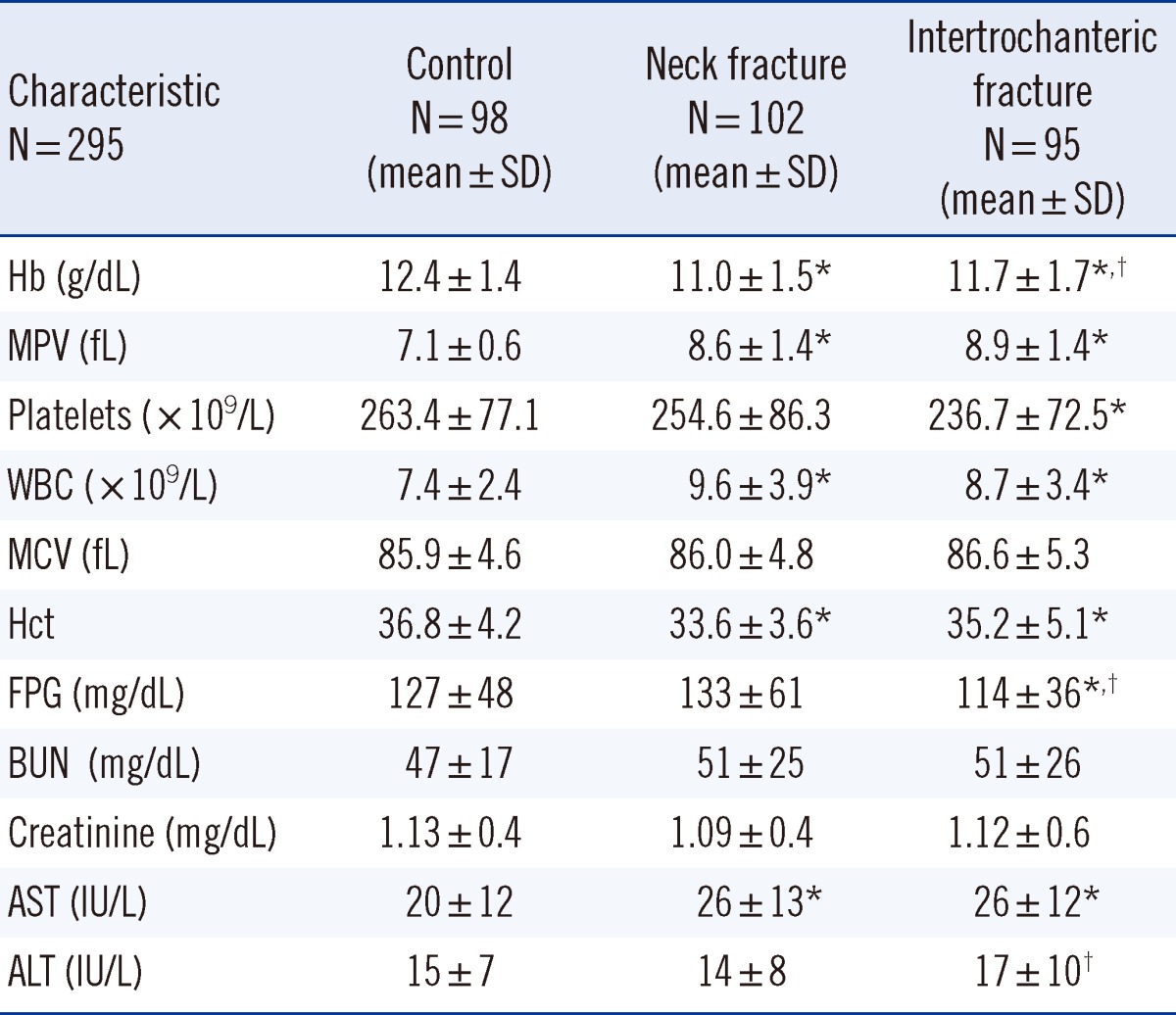
*P<0.05 vs. control group; †P<0.05 vs. neck fracture group.
Abbreviations: MPV, mean platelet volume; WBC, white blood cells; MCV, mean corpuscular volume; FPG, fasting plasma glucose; BUN, blood urea nitrogen.
The femur neck, intertrochanteric, and control groups were further divided into subgroups depending on the presence of chronic diseases such as diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, chronic renal failure, and thyroid disease. In the chronic disease group, the mean MPV values for the femur neck and intertrochanteric region groups were significantly higher than that for the control group (8.5±1.3 fL, P<0.001; 8.8±1.5 fL, P<0.001; and 7.1±0.6 fL, respectively). In the group without chronic diseases, the MPV values in the femur neck and intertrochanteric region groups were significantly higher than that in the control group (8.7±1.6 fL, P<0.001; 8.8±1.5 fL, P<0.001; and 7.1±0.6 fL, respectively). The MPV values for women (8.7±1.5 fL and 9.0±1.2 fL) in the femur neck and intertrochanteric groups were higher than those for men (8.4±1.3 fL and 8.5±1.5 fL); however, these results were not significantly different (Table 3).
Table 3.
Mean platelet volume (MPV) levels of neck, intertrochanteric and control groups subdivided according to gender and the presence of chronic disease
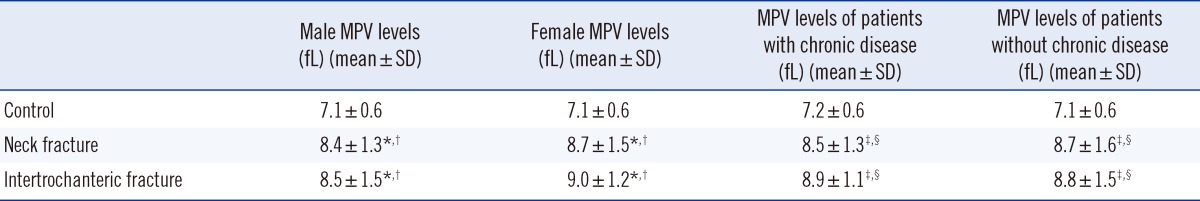
*P<0.001 vs. control male group; †P<0.001 vs. control female group; ‡P<0.001 vs. control without chronic disease group; §P<0.001 vs. control chronic disease group.
MPV was negatively correlated with occurrence of fracture (r2=0.273, P<0.001), Hb level (r2=0.038, P=0.001), and platelet count (r2=0.078, P<0.001). There was a positive correlation between AST level (r=0.171, P=0.003), ALT level (r=0.130, P<0.026), and MPV. There was a negative correlation between white blood cells (r2=0.058, P<0.001) and the occurrence of fractures and a positive correlation between Hb levels (r=0.290, P<0.001) and chronic renal disease (r=0.129, P=0.027). There was a negative correlation between Hb levels (r2=0.070, P<0.001) and age. By linear regression analysis, MPV, age, Hb level, and creatinine level were significant independent predictors of fractures (for MPV, odds ratio [OR]=-0.194, confidence interval [CI],=-0.235 to -0.153, P=0.001; for age, OR=0.009, CI=0.003 to 0.015, P=0.002; and for Hb level, OR=0.073, CI=0.034 to 0.111, P=0.001; for creatinine, OR=0.238, CI=0.083 to 0.393, P=0.003).
In the present study, a high MPV was found in patients with the femur neck fracture and as well as those with intertrochanteric fracture as compared to the control group. In addition, the Hb levels were lower in the intertrochanteric fracture group as well as the femur neck fracture group. In the control group, the MPV level in subjects without chronic disease was 7.1 fL, whereas in the chronic disease group, it was 7.2 fL. In the fracture group, the MPV was higher in those without chronic disease than in those with chronic disease. The mean MPV in the fracture group was higher than that in the control group. These findings suggest that high MPV is related to femur fractures.
The MPV values of women were higher than those of men in both fracture groups. A previous study reported a relationship between osteoporosis and MPV in postmenopausal women [4]. Since the MPVs of women were higher than those of men, fractures in women can be assumed to be associated with osteoporosis.
Larger platelets are more active than smaller ones and produce more pro-thrombotic factors. Additionally, endothelial dysfunction is related to platelet activation, and thrombotic factors released from platelets are related to many diseases. The increased MPV in patients with femur fractures suggests that the increase in the proportion of large platelets in these patients may lead to microvascular thrombosis in the arteries and veins of the femur neck and intertrochanteric region. As a result, deterioration of the bone nutrient content leads to fractures spontaneously or even with a small impact. Endothelial cell damage could be followed by abnormal blood coagulation and thrombus formation with resulting degeneration distal to the site of vascular occlusion.
Aging and chronic diseases increase the release of proinflammatory cytokines [5, 6]. Tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 are the major triggers for osteoclast activation, which may enhance bone loss during inflammation and lead to the development of osteopenia and osteoporosis, both of which increase the risk of hip fractures.
Li et al. [4] found that patients with osteoporosis had increased MPVs, leading to osteoporosis in postmenopausal women. They also noted that the increase in MPV was related to an increase in proinflammatory cytokine levels. TNF-α and IL-6 are the cytokines that cause osteoporosis, and they are linked with enhanced oxidative stress, which contributes to platelet activation and stimulates megakaryopoiesis [7].
In our study, the presence of fractures in patients without chronic disease suggests that the other mechanisms mentioned are effective such as osteoporosis, immobility, poor nutrition, poor calcium intake, diathesis, etc. Majority of the patients in our study had anemia, as iron and vitamin B12 deficiencies, and chronic disease anemia are quite common in elderly patients. In patients with anemia, a low Hb level is related to increased IL-6 levels [8]. IL-6, which leads to bone resorption, is also known to increase MPVs.
MPV is affected by medications, such as rosuvastatin, aspirin, clopidogrel, and metformin [9-11]. Most chronic disease patients in our study were under these medications. Some diabetes patients were taking pioglitazone, which is known to contribute to osteoporosis and bone fracture. In contrast, aspirin is known to protect against osteoporosis, and most of the chronic disease patients were using aspirin [12]. Thus, our results may have been affected by these drugs. However, most of the chronic disease patients in the control group were also using these drugs, and hence the relationship between MPV and femur factors persisted.
In conclusion, we demonstrated that a high MPV was associated with fractures, even in old age. A high MPV may be related to osteoporosis and an impaired blood supply; consequently, the risk of fracture may be increased. Since our case-control study involved a small study sample, additional large prospective studies are needed to confirm these results.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Cure MC, Cure E, Kirbas A, Cicek AC, Yuce S. The effects of Gilbert's syndrome on the mean platelet volume and other hematological parameters. Blood Coagul Fibrinolysis. 2013;24:484–488. doi: 10.1097/MBC.0b013e32835e4230. [DOI] [PubMed] [Google Scholar]
- 2.Westenbrink BD, Voors AA, de Boer RA, Schuringa JJ, Klinkenberg T, van der Harst P, et al. Bone marrow dysfunction in chronic heart failure patients. Eur J Heart Fail. 2010;12:676–684. doi: 10.1093/eurjhf/hfq061. [DOI] [PubMed] [Google Scholar]
- 3.Shiraki M, Kuroda T, Shiraki Y, Aoki C, Sasaki K, Tanaka S. Effects of bone mineral density of the lumbar spine and prevalent vertebral fractures on the risk of immobility. Osteoporos Int. 2010;21:1545–1551. doi: 10.1007/s00198-009-1121-9. [DOI] [PubMed] [Google Scholar]
- 4.Li XS, Zhang JR, Meng SY, Li Y, Wang RT. Mean platelet volume is negatively associated with bone mineral density in postmenopausal women. J Bone Miner Metab. 2012;30:660–665. doi: 10.1007/s00774-012-0362-4. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 6.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 7.Broudy VC, Lin NL, Fox N, Taga T, Saito M, Kaushansky K. Thrombopoietin stimulates colony-forming unit-megakaryocyte proliferation and megakaryocyte maturation independently of cytokines that signal through the gp130 receptor subunit. Blood. 1996;88:2026–2032. [PubMed] [Google Scholar]
- 8.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 9.De Luca G, Secco GG, Iorio S, Verdoia M, Bellomo G, Marino P. Short-term effects of aspirin and clopidogrel on mean platelet volume among patients with acute coronary syndromes. A single-center prospective study. Blood Coagul Fibrinolysis. 2012;23:756–759. doi: 10.1097/MBC.0b013e328358e941. [DOI] [PubMed] [Google Scholar]
- 10.Coban E, Afacan B. The effect of rosuvastatin treatment on the mean platelet volume in patients with uncontrolled primary dyslipidemia with hypolipidemic diet treatment. Platelets. 2008;19:111–114. doi: 10.1080/09537100701230444. [DOI] [PubMed] [Google Scholar]
- 11.Dolasık I, Sener SY, Celebı K, Aydın ZM, Korkmaz U, Canturk Z. The effect of metformin on mean platelet volume in diabetic patients. Platelets. 2013;24:118–121. doi: 10.3109/09537104.2012.674165. [DOI] [PubMed] [Google Scholar]
- 12.Shi S, Yamaza T, Akiyama K. Is aspirin treatment an appropriate intervention to osteoporosis? Fut Rheumatol. 2008;3:499–502. doi: 10.2217/17460816.3.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]


