Gram-negative anaerobes of the Prevotella intermedia/nigrescens group are usually isolated from polymicrobial infections of periodontal origin, although recovery from healthy individuals is also possible [1, 2]. Only a limited number of isolates have been identified from extraoral infections, which causes difficulty in the assessment of the frequency of extraoral infections caused by P. intermedia and P. nigrescens. A previous study of 154 isolates of P. intermedia/P. nigrescens described a low frequency of extraoral infections recovered from sites of foot ulcer, blood stream infection, and lung aspirates [3]. Here we report a case of extraoral infection where P. nigrescens was identified at the strain level by 16S ribosomal RNA (rRNA) sequencing.
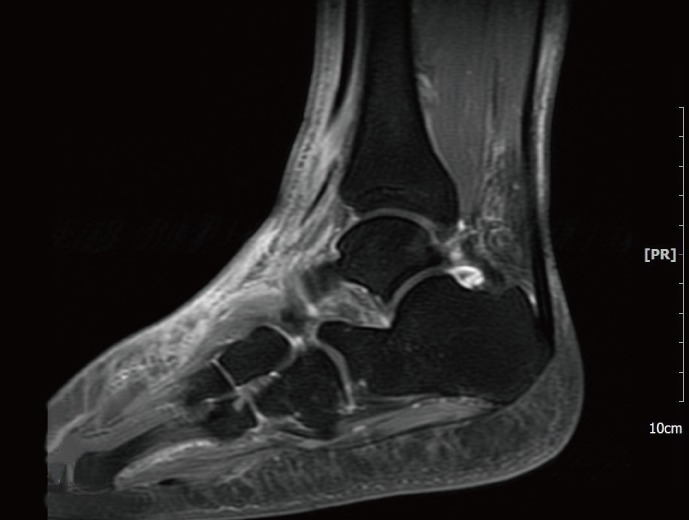
A 52-yr-old man with a history of diabetes and hypertension was referred to the department of orthopedics on December 18 complaining of cellulitis of the left ankle. The patient initially received acupuncture for his sprained ankle during a hiking trip in November 2012. As the symptoms did not improve, he visited a secondary hospital where he was diagnosed with cellulitis. Magnetic resonance imaging (MRI) taken on December 1 showed cellulitis with talus osteochondral lesions and a low probability of abscess or osteomyelitis (Fig. 1). In spite of a week of antibiotic treatment with cefazedone followed by another week with ceftazidime, the patient was referred to our hospital with symptoms of swelling, redness, and aggravated pain. Ultrasound sonography (US) performed on December 18 revealed an abscess on the left ankle, as suggested by fluid accumulation and diffuse inflammation of soft tissue. Complete blood counts were as follows: hemoglobin, 12.7 g/dL; white blood cells, 12.48×109/L; platelets, 514×109/L; and erythrocyte sedimentation rate (ESR), 75 mm/hr. The C-reactive protein (CRP) level was 4.68 mg/dL and the procalcitonin level was 0.068 µg/L (reference range: ~0.046 µg/L).
Fig. 1.
Magnetic Resonance Imaging: T1-weighted image showing cellulitis associated with talus osteochondral lesions.
Initial treatment with aminoglycoside was ineffective; the symptoms were aggravated and the CRP level rose to 7.46 mg/dL. US-guided aspiration and drainage were performed and the antibiotic regimen was changed to ampicillin/sulbactam, as the first set of culture results recovered very low levels of Streptococcus viridians. The symptoms of pain and swelling of the left ankle were persistent. MRI on January 8 revealed diffuse soft tissue edema, in addition to tenosynovitis with fluid collection and debris, which were signs of progressive infection. As the response to treatment was poor, surgical management was considered. On Jan 14, incision and drainage (I&D) with debridement of severely infected tissue was required, and a closed pus specimen was obtained during the procedure for follow-up culture.
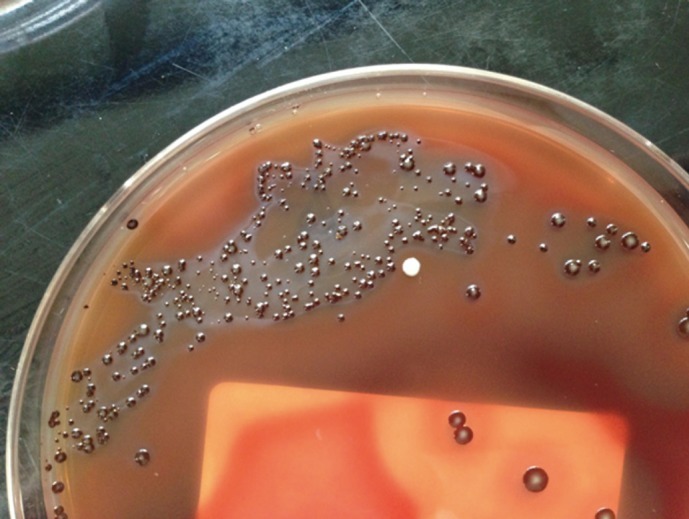
Black pigment-producing colonies with gram-negative rod-shaped anaerobes were recovered (Fig. 2), in which positive biochemical reactions were seen in α-galactosidase (αGAL), α-glucosidase (αGLU), mannose fermentation (MNF), and raffinose fermentation (RAF). The colonies were initially identified as Prevotella spp. using API rapid ID32A (API-bioMérieux UK Ltd., Basingstoke, UK). The reference biochemical profile used for comparison with the API rapid ID32A result is described in Table 1. The results of antimicrobial susceptibility testing are described in Table 2.
Fig. 2.
Recovery of black-pigmented anaerobe under anaerobic condition from blood agar plate. Black-pigmented Bacteroides including Prevotella nigrescens can be suspected.
Table 1.
Biochemical identification of Prevotella intermedia/nigrescens*
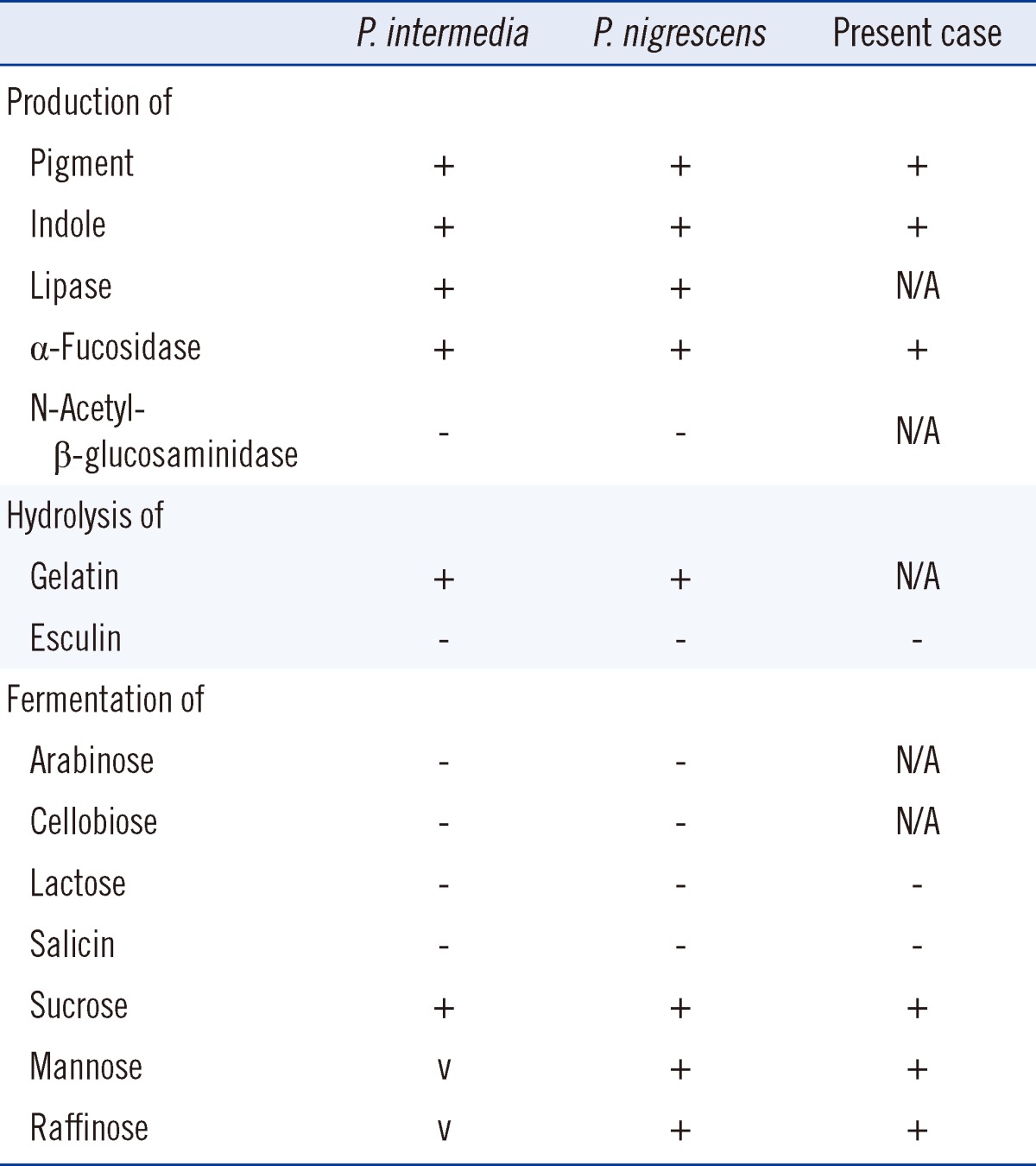
*Reference [12].
Abbreviations: P. intermedia, Prevotella intermedia; P. nigrescens, Prevotella nigrescens; v, variable; N/A, not available.
Table 2.
Antimicrobial susceptibility testing result
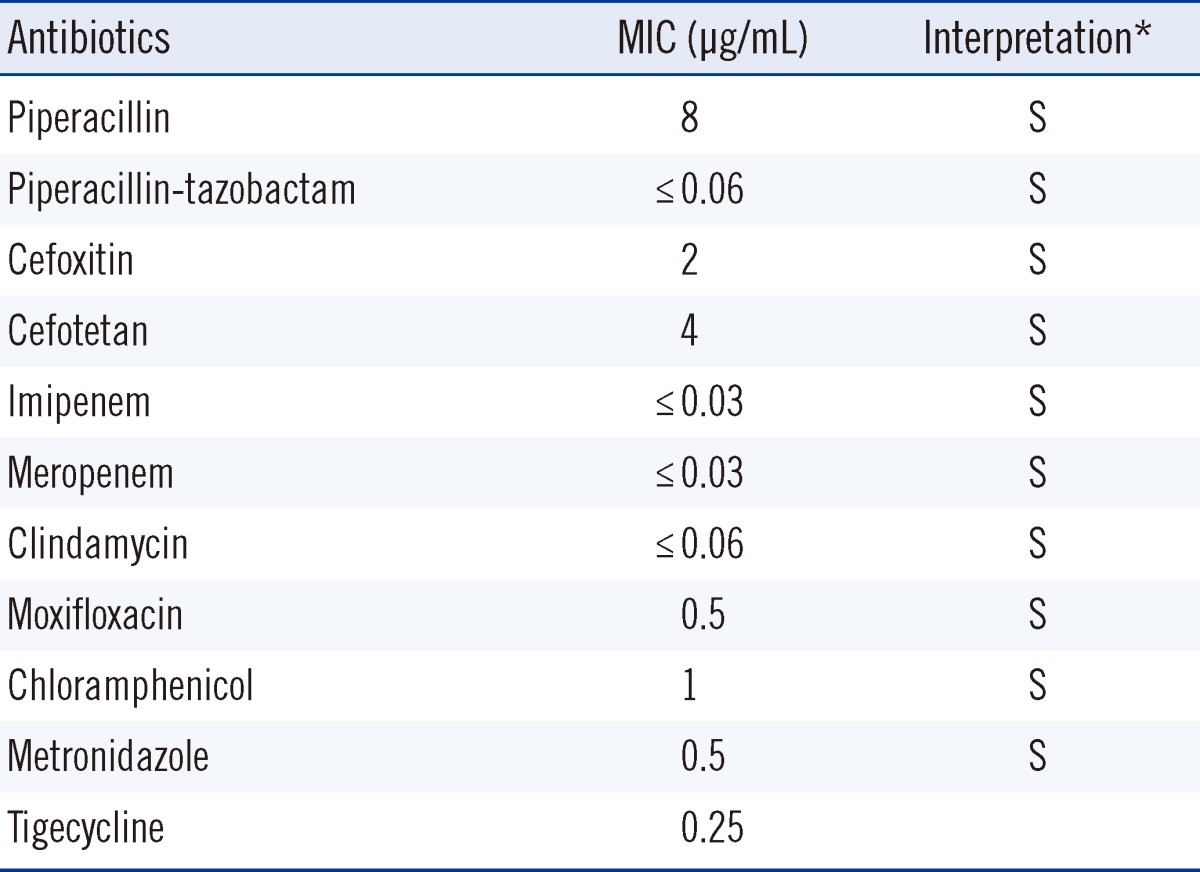
*Interpretations using breakpoints suggested by CLSI guidelines: antimicrobial susceptibility testing standards M02 and M07, 2012.
Abbreviations; MIC, minimal inhibitory concentration; S, susceptible.
The sequence obtained from the cultured isolates was a match to the P. nigrescens gene for 16S rRNA (Strain: JCM 6322, GenBank: AB547697.1). Capillary sequencing was performed by Macrogen Inc. (Seoul, Korea) using two primers (518F-forward: 5'-CCAGCAGCCGCGGTAATACG-3' and 800R-reverse 5'-TACCAGGGTATCTAATCC-3'). The sequencing service included quality control of template DNA, library preparation and filtering of low quality reads post-sequencing.
It was important to note the polymicrobial tendency of Prevotella infection, which in this case was present with S. viridans. Specifically, in all four results in which Prevotella was recovered, there was co-infection with S. viridians.
The antibiotic regimen was changed to tazobactam and piperacillin, as soft tissue inflammation was persistently observed on radiological evaluation with computed tomography (CT) and X-ray imaging. On February 6, a second I&D with curettage of the previously infected site was performed. The antibiotic regimen was reverted to ampicillin and sulbactam. Discharge gradually decreased and the patient was discharged on February 15.
Prevotella spp. is a clinically important pathogen, although it is rarely recovered from clinical specimens other than perioral locations [1, 2]. Although most previously reported extraoral infections involving the P. intermedia/nigrescens group are based on biochemical identification and morphological features of characteristic black pigment-producing gram-negative anaerobes, differentiation of P. intermedia and P. nigrescens should be based on molecular methods such as 16S rRNA sequencing [4, 5]. Importantly, the biochemical profiles of P. intermedia and P. nigrescens are almost identical, which complicates the differentiation between the two organisms (Table 1). Therefore, the API rapid ID32A system is unable to properly identify P. nigrescens, and indications of P. intermedia presence using this system should be further verified with additional tests [4, 6, 7]. As infections with Prevotella spp. are polymicrobial in the majority of cases (including the present case), careful identification of the causative organism(s) will be helpful in the treatment of infections [3].
In the present case, P. nigrescens was identified at the species level by 16S rRNA sequencing of an aspirated specimen and pus obtained by US-guided aspiration and two procedures of I&D, respectively. The clinical course of infection was incessant, which may be attributed to several factors. The first is the rather distal and deep location of infection site, which likely rendered conventional therapeutic doses of antibiotics ineffective. Secondly, the underlying hypertension and diabetes of this patient were adverse factors for treatment. Furthermore, patient compliance was also unfavorable, as he persistently ambulated and kept smoking throughout the admission period. Finally, polymicrobial infection, with a synergistic effect of P. nigrescens and S. viridans, may have been present. Prevotella spp. infections are often polymicrobial, which is frequently more pathogenic than infections involving a single organism [3, 8]. The precise cause of infection is difficult to identify, although both a history of acupuncture and aspiration are possible causes. Indeed, there is one case report of Prevotella spp. infection in which a history of acupuncture was also present, and early detection and aggressive drainage were important for treatment in that case [9].
Despite retrospective antimicrobial susceptibility testing, antimicrobial treatment using piperacillin and tazobactam seemed sufficient, as both agents were found to be effective, and had activity in this case with a low minimal inhibitory concentration. A high percentage of Prevotella spp. are known to produce β-lactamase [10], and therefore, antimicrobial susceptibility testing may be helpful in such cases, although it is not currently recommended as a routine procedure.
Although P. nigrescens is a phenotypically similar pathogen to P. intermedia with an indistinguishable biochemical profile, a greater likelihood of suppurative infection has been suggested [11]. Distinction by PCR-based methods such as 16S rRNA gene sequencing for detecting genotypic differences should be helpful in the management of such anaerobic infections.
Acknowledgements
We express our sincere gratitude to professor Kyungwon Lee, M.D., Ph.D. of Yonsei University for arrangements and conducting antimicrobial susceptibility testing.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Finegold SM, Strong CA, McTeague M, Marina M. The importance of black-pigmented gram-negative anaerobes in human infections. FEMS Immunol Med Microbiol. 1993;6:77–82. doi: 10.1111/j.1574-695X.1993.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 2.Jousimies-Somer H, Savolainen S, Mäkitie A, Ylikoski J. Bacteriologic findings in peritonsillar abscesses in young adults. Clin Infect Dis. 1993;16(Suppl 4):S292–S298. doi: 10.1093/clinids/16.supplement_4.s292. [DOI] [PubMed] [Google Scholar]
- 3.Mättö J, Asikainen S, Väisänen ML, Rautio M, Saarela M, Summanen P, et al. Role of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens in extraoral and some odontogenic infections. Clin Infect Dis. 1997;25(Suppl 2):S194–S198. doi: 10.1086/516205. [DOI] [PubMed] [Google Scholar]
- 4.Frandsen EV, Poulsen K, Kilian M. Confirmation of the species Prevotella intermedia and Prevotella nigrescens. Int J Syst Bacteriol. 1995;45:429–435. doi: 10.1099/00207713-45-3-429. [DOI] [PubMed] [Google Scholar]
- 5.Gharbia SE, Haapasalo M, Shah HN, Kotiranta A, Lounatmaa K, Pearce MA, et al. Characterization of Prevotella intermedia and Prevotella nigrescens isolates from periodontic and endodontic infections. J Periodontol. 1994;65:56–61. doi: 10.1902/jop.1994.65.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Mättö J, Saarela M, von Troil-Lindén B, Alaluusua S, Jousimies-Somer H, Asikainen S. Similarity of salivary and subgingival Prevotella intermedia and Prevotella nigrescens isolates by arbitrarily primed polymerase chain reaction. Oral Microbiol Immunol. 1996;11:395–401. doi: 10.1111/j.1399-302x.1996.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 7.Shah HN, Gharbia SE. Biochemical and chemical studies on strains designated Prevotella intermedia and proposal of a new pigmented species, Prevotella nigrescens sp. nov. Int J Syst Bacteriol. 1992;42:542–546. doi: 10.1099/00207713-42-4-542. [DOI] [PubMed] [Google Scholar]
- 8.Brook I. Microbiology of polymicrobial abscesses and implications for therapy. J Antimicrob Chemother. 2002;50:805–810. doi: 10.1093/jac/dkg009. [DOI] [PubMed] [Google Scholar]
- 9.Kim SS, Ha GI. Mediastinitis caused by Prevotella intermedia/nigrescens occurred after acupuncture: a case report. Korean J Thorac Cardiovasc Surg. 2000;33:440–444. [Google Scholar]
- 10.Lee Y, Park Y, Kim MS, Yong D, Jeong SH, Lee K, et al. Antimicrobial susceptibility patterns for recent clinical isolates of anaerobic bacteria in South Korea. Antimicrob Agents Chemother. 2010;54:3993–3997. doi: 10.1128/AAC.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stubbs S, Park SF, Bishop PA, Lewis MA. Direct detection of Prevotella intermedia and P. nigrescens in suppurative oral infection by amplification of 16S rRNA gene. J Med Microbiol. 1999;48:1017–1022. doi: 10.1099/00222615-48-11-1017. [DOI] [PubMed] [Google Scholar]
- 12.Könönen E, Wade WG, Citron DM. Versalovic J. Manual of clinical microbiology. 10th ed. Washington: ASM Press; 2011. Bacteroides, Porphyromonas, Prevotella, Fusobacterium, and other anaerobic gram-negative rods; pp. 858–880. [Google Scholar]