Hereditary elliptocytosis (HE) is a group of disorders characterized by abnormal erythrocyte shapes that occur mainly in individuals of African and Mediterranean ancestry. In Korea, HE is the cause of 1.4% (6/431) cases of hereditary hemolytic anemia (HHA) [1]. HE causes mechanical weakness or fragility of the erythrocytic membrane skeleton because of defects in spectrin or protein 4.1. The erythrocyte spectrin is a scaffold protein composed of 2 subunits, α- and β-spectrin, maintains the cellular shape, regulates the lateral mobility of integral membrane proteins, and provides structural support for the lipid bilayer [2, 3]. The mutations responsible for HE are located in several genes encoding membrane proteins, including protein 4.1, α-spectrin, β-spectrin, band 3, and glycophorin C. Mutations in α-spectrin are the most common, occurring in 65% of the HE cases, followed by mutations in β-spectrin (30%) and protein 4.1 (5%) [4].
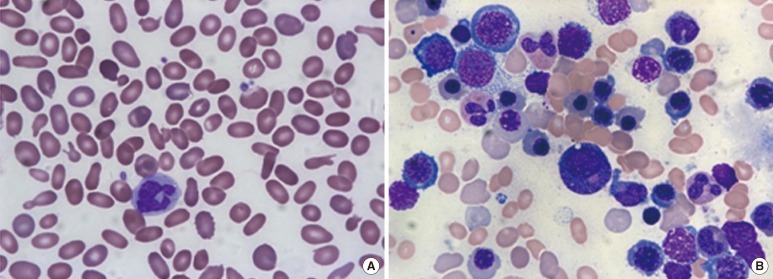
We studied a Korean family presenting with HE. To our knowledge, this is the first report to describe a family of Korean descent diagnosed with HE by molecular analysis. A 28-yr-old woman visited our hospital and was found to have anemia during a routine health screening. She did not complain of symptoms related to anemia. Abdominal ultrasonography revealed splenomegaly (craniocaudal length, 13.8 cm). Marked anisopoikilocytosis, including elliptocytes, schistocytes, and teardrop cells, were detected on a peripheral blood smear (Fig. 1A). A complete blood count revealed pancytopenia (white blood cell count, 3.88×109/L; hemoglobin level, 7.6 g/dL; and platelet count, 95×109/L). Red cell indices revealed macrocytic normochromic anemia (red blood cell count, 2.20×1012/L; reticulocyte count, 0.968×1012/L; hematocrit value, 24%; mean corpuscular volume, 109.1 fL; and mean corpuscular hemoglobin concentration, 31.7%). We ruled out iron deficiency or megaloblastic anemia based on the following laboratory findings: serum iron level, 96 µg/dL (reference range: 50-150 µg/dL); ferritin level, 104 ng/mL (10-120 ng/mL); total iron-binding capacity, 204 µg/dL (250-450 µg/dL); vitamin B12 concentration, >1,500 pg/mL (180-914 pg/mL); and folate concentration, 7.47 ng/mL (3.1-19.9 ng/mL). The serum concentrations of total bilirubin (1.02 mg/dL), haptoglobin (<30 mg/dL), and lactate dehydrogenase (556 U/L) confirmed the presence of a hemolytic process. Bone marrow studies showed hypercellularity and erythroid hyperplasia with G:E ratio of 0.67:1 (Fig. 1B), and cytogenetic studies revealed a normal karyotype (46,XX[20]).
Fig. 1.
(A) Marked anisopoikilocytosis, including elliptocytosis, schistocytes, and teardrop cells, on a peripheral blood smear. (B) Bone marrow aspirates showing hypercellularity and erythroid hyperplasia (Wright-Giemsa Stain, ×1,000).
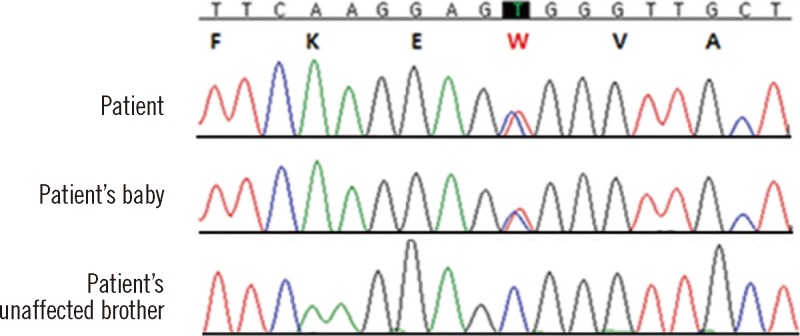
The patient's mother was also diagnosed with anemia and elliptocytosis based on peripheral blood films. Therefore, we suspected familial transmission of elliptocytosis. At the time of this study, the patient was pregnant, and after delivery, the peripheral blood smears from the newborn were analyzed, which revealed elliptocytosis. Autosomal dominant inheritance was suspected, and spectrin-gene-mutation analysis of the patient and her family (affected mother, her baby, and unaffected brother) was performed. Genomic DNA was prepared using the QIAamp DNA Mini Kit (Qiagen, Hamburg, Germany). All coding exons and flanking intronic sequences of the spectrin, alpha, erythrocytic 1 gene (SPTA1) and spectrin, beta, erythrocytic gene (SPTB) (reference cDNA sequence, NM_003126.2 and NM_000347.5) were amplified and sequenced using primers as previously described [5, 6]. In particular, the region encoding exon 2 of SPTA1 was amplified by PCR using the following forward and reverse primers: 5'-GGTCCAACATGAGTAAACACCTTGACA-3' and 5'-TCTCACCTCTCCAACTTCATAAGGGA-3', respectively. Direct sequencing of the SPTA1 gene revealed a heterozygous missense mutation in exon 2, resulting in a C to T substitution at nucleotide position 121 and an amino acid change of arginine to tryptophan (c.121C>T; p.Arg41Trp) at amino acid residue 41 in the alpha l domain in both the patient and her baby (Fig. 2). This missense mutation induces spectrin Tunis, which reduces the binding affinity for the spectrin tetramer assembly. Spectrin Tunis was designated as αI/78 because of its location in the first alpha domain of SPTA1 [7]. In addition, we analyzed the sequences of SLC4A1 (solute carrier family 4, anion exchanger, member 1) and EPB41 (erythrocyte membrane protein band 4.1) but found no mutations in these genes.
Fig. 2.
DNA sequence analysis of the SPTA1 gene. The patient and her baby carried a heterozygous missense mutation in exon 2 (c.121C>T; p.Arg41Trp) in the alpha 1 domain. Protein changes are indicated by red lettering.
HE is a group of disorders characterized by the presence of elliptical erythrocytes on a peripheral blood smear. Disorders in which elliptocytosis may be prominent also include iron deficiency, leukemia, megaloblastic anemia, myelofibrosis, myelophthisic anemia, myelodysplastic syndromes, polycythemia, pyruvate kinase deficiency, and sickle cell disease [8]. Examination of family history is the most reliable method for differentiating HE from other disorders, in which elliptocytosis may be prominent [9]. Biochemical and mechanical methods can be used to determine defects in erythrocyte membrane proteins, such as spectrin or protein 4.1. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) is used in qualitative and quantitative detection of alterations in membrane proteins. In addition, assessment of thermal sensitivity, in vitro studies of spectrin self-association, and trypsin peptide mapping of spectrin can assist in the diagnosis of HE [8].
Currently, many genetic studies have been designed to detect various genetic mutations in HE [9]. All spectrin mutations associated with HE are located in or near self-association sites between the α- or β-chains. As a result, the corresponding subset of HE is derived from an impaired self-association process, which is a critical process for red cell deformability. Erythroid spectrin α- and β-chains are encoded by the SPTA1 gene on 1q22-23 and the SPTB gene on 14q23-24.2, respectively. Many SPTA1 gene mutations are single-nucleotide substitutions, whereas others are intronic mutations that cause errors in gene splicing [10-14]. SPTA1-related HE is characterized by marked clinical, biochemical, and genetic heterogeneity. Clinically, the presentation varies from a nearly complete absence of symptoms to transfusion-dependent hemolytic anemia. The location of the change within the spectrin α-chain and the homozygous or compound heterozygous status affect clinical manifestations. Remarkably, both mildly and severely affected patients are found in almost all affected kindred [15].
In a review of Korean literature on HHA, we found 15 case reports on HE, including our case [1, 16]. Red-cell-membrane defects were the most common (88.6%) in HHA. Hereditary spherocytosis accounted for 87.2% of red-cell-membrane defects, whereas HE accounted for 1.4% of the defects [1]. Lee et al. [16] detected protein 4.1 deficiency in 6 Korean patients with HE by using SDS-PAGE analysis.
In this study, we identified the first SPTA1 gene mutation in a Korean family diagnosed with HE. The heterozygous c.121C>T mutation induces an amino acid change p.Arg41Trp in the α1 domain of the α-spectrin protein. Morlé et al. [7] first described this mutation and called this disease spectrin Tunis, which causes asymptomatic HE (OMIM 130600) in patients with a heterozygous mutation. A variant was also found in a white North African man and his mother. The αI/78 variant (rs121918640) exhibits reduced binding affinity, generates an abnormal spectrin protein with an αI 78-kDa fragment, and results in a mutation, which partially destroys the ability of the dimer to form a tetramer [7]. Most spectrin mutations are private, and it is interesting that spectrin Tunis was detected in a Korean family. However, some mutations in SPTA1 are encountered more frequently than originally thought, such as the case of a mutation affecting codon α28, which contains a CpG hot spot [17]. The CpG dinucleotide has also been implicated in a number of band 3 gene hot spots, most of which encode arginine (CGN, N indicating any nucleotide). The mutation resulting in spectrin Tunis occurs in the CpG dinucleotide at codon 41, which also encodes arginine (CGG).
The patient who transmits the production-defective spectrin allele is clinically normal with unremarkable erythrocyte morphology because α-spectrin is normally synthesized in a 2- to 3-fold excess, and the output from a single normal α-spectrin allele is sufficient to maintain membrane integrity [18, 19]. Because this α-spectrin variant is related to asymptomatic HE in a heterozygous state, the outcomes may include mild elliptocytosis in the proband and her baby. To our knowledge, this is the first report on HE confirmed by mutation analysis in a Korean family. More data need to be collected from HE patients to understand the genetic distributions and genotype-phenotype correlations.
Acknowledegments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120175).
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Cho HS, Hah JO, Kang IJ, Kang HJ, Kwak JY, Koo HH, et al. Hereditary hemolytic anemia in Korea: a retrospective study from 1997 to 2006. Korean J Hematol. 2007;42:197–205. [Google Scholar]
- 2.Lux SE, Palek J. Disorders of the red cell membrane. In: Handin RI, Lux SE, Stossel TP, editors. Blood principles and practice of hematology. Philadelphia: Lippincott; 1995. p. 1701. [Google Scholar]
- 3.Becker PS, Lux SE. Disorders of the red cell membrane skeleton: hereditary spherocytosis and hereditary elliptocytosis. In: Scriver CS, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 7th ed. New York: McGraw-Hill; 1995. p. 529. [Google Scholar]
- 4.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141:367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 5.Costa DB, Lozovatsky L, Gallagher PG, Forget BG. A novel splicing mutation of the alpha-spectrin gene in the original hereditary pyropoikilocytosis kindred. Blood. 2005;106:4367–4369. doi: 10.1182/blood-2005-05-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhermy D, Galand C, Bournier O, Cynober T, Méchinaud F, Tchemia G, et al. Hereditary spherocytosis with spectrin deficiency related to null mutations of the beta-spectrin gene. Blood Cells Mol Dis. 1998;24:251–261. doi: 10.1006/bcmd.1998.0190. [DOI] [PubMed] [Google Scholar]
- 7.Morlé L, Morlé F, Roux AF, Godet J, Forget BG, Denoroy L, et al. Spectrin Tunis (Sp alpha I/78), an elliptocytogenic variant, is due to the CGG----TGG codon change (Arg----Trp) at position 35 of the alpha I domain. Blood. 1989;74:828–832. [PubMed] [Google Scholar]
- 8.Gallagher PG. Hereditary elliptocytosis: spectrin and protein 4.1R. Semin Hematol. 2004;41:142–164. doi: 10.1053/j.seminhematol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007;21:1–20. doi: 10.1016/j.blre.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Maillet P, Alloisio N, Morlé L, Delaunay J. Spectrin mutations in hereditary elliptocytosis and hereditary spherocytosis. Hum Mutat. 1996;8:97–107. doi: 10.1002/(SICI)1098-1004(1996)8:2<97::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Alloisio N, Wilmotte R, Maréchal J, Texier P, Denoroy L, Féo C, et al. A splice site mutation of alpha-spectrin gene causing skipping of exon 18 in hereditary elliptocytosis. Blood. 1993;81:2791–2798. [PubMed] [Google Scholar]
- 12.Baklouti F, Maréchal J, Wilmotte R, Alloisio N, Morlé L, Ducluzeau MT, et al. Elliptocytogenic alpha I/36 spectrin Sfax lacks nine amino acids in helix 3 of repeat 4. Evidence for the activation of a cryptic 5'-splice site in exon 8 of spectrin alpha-gene. Blood. 1992;79:2464–2470. [PubMed] [Google Scholar]
- 13.Fournier CM, Nicolas G, Gallagher PG, Dhermy D, Grandchamp B, Lecomte MC. Spectrin St Claude, a splicing mutation of the human alpha-spectrin gene associated with severe poikilocytic anemia. Blood. 1997;89:4584–4590. [PubMed] [Google Scholar]
- 14.Costa DB, Lozovatsky L, Gallagher PG, Forget BG. A novel splicing mutation of the alpha-spectrin gene in the original hereditary pyropoikilocytosis kindred. Blood. 2005;106:4367–4369. doi: 10.1182/blood-2005-05-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iolascon A, King MJ, Robertson S, Avvisati RA, Vitiello F, Asci R, et al. A genomic deletion causes truncation of α-spectrin and ellipto-poikilocytosis. Blood Cells Mol Dis. 2011;46:195–200. doi: 10.1016/j.bcmd.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Lee YK, Cho HI, Park SS, Ra ER, Chang YH, Huh MN, et al. SDS-PAGE analysis of red cell membrane proteins in hereditary hemolytic anemia. Korean J Hematol. 1999;34:559–567. [Google Scholar]
- 17.Coetzer TL, Sahr K, Prchal J, Blacklock H, Peterson L, Koler R, et al. Four different mutations in codon 28 of alpha spectrin are associated with structurally and functionally abnormal spectrin alpha I/74 in hereditary elliptocytosis. J Clin Invest. 1991;88:743–749. doi: 10.1172/JCI115371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowles WJ, Morrow JS, Speicher DW, Zarkowsky HS, Mohandas N, Mentzer WC, et al. Molecular and functional changes in spectrin from patients with hereditary pyropoikilocytosis. J Clin Invest. 1983;71:1867–1877. doi: 10.1172/JCI110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanspal M, Palek J. Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J Cell Biol. 1987;105:1417–1424. doi: 10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]