Abstract
Objectives
The study tested the hypothesis that augmentation of the left ventricular (LV) wall thickness with direct intramyocardial injections of alginate hydrogel implants (AHI) reduces LV cavity size, restores LV shape, and improves LV function in dogs with heart failure (HF).
Background
Progressive LV dysfunction, enlargement, and chamber sphericity are features of HF associated with increased mortality and morbidity.
Methods
Studies were performed in 14 dogs with HF produced by intracoronary microembolizations (LV ejection fraction [EF] <30%). Dogs were randomized to AHI treatment (n = 8) or to sham-operated control (n = 6). During an open-chest procedure, dogs received either intramyocardial injections of 0.25 to 0.35 ml of alginate hydrogel (Algisyl-LVR, LoneStar Heart, Inc., Laguna Hills, California) or saline. Seven injections were made ∼1.0 to 1.5 cm apart (total volume 1.8 to 2.1 ml) along the circumference of the LV free wall halfway between the apex and base starting from the anteroseptal groove and ending at the posteroseptal groove. Hemodynamic and ventriculographic measurements were made before treatment (PRE) and repeated post-surgery for up to 17 weeks (POST).
Results
Compared to control, AHI significantly reduced LV end-diastolic and end-systolic volumes and improved LV sphericity. AHI treatment significantly increased EF (26 ± 0.4% at PRE to 31 ± 0.4% at POST; p < 0.05) compared to the decreased EF seen in control dogs (27 ± 0.3% at PRE to 24 ± 1.3% at POST; p < 0.05). AHI treatment was well tolerated and was not associated with increased LV diastolic stiffness.
Conclusions
In HF dogs, circumferential augmentation of LV wall thickness with AHI improves LV structure and function. The results support continued development of AHI for the treatment of patients with advanced HF.
Keywords: animal models, congestive heart failure, functional mitral regurgitation, left ventricular function, pressure-volume relationship
Despite considerable progress over the past 3 decades in the development and availability of drugs and devices for treating heart failure (HF) (1), the incidence of HF continues to increase and has, by all counts, reached epidemic proportions. During the course of evolving HF, the left ventricle (LV) progressively enlarges and its shape transforms from elliptical to one that more closely approximates a sphere (2). These structural changes promote worsening HF symptoms and predict poor long-term outcome (3). An important contributor to progressive LV systolic dysfunction in HF and supported by Laplace's law, is elevation of LV wall stress that results from progressive LV enlargement and wall thinning. Wall stress or tension is a major determinant of myocardial oxygen consumption (MVO2). Structural LV changes that develop during disease progression can, therefore, lead to increased wall stress and consequently increased MVO2 with increased energy demands; a state that the failing heart can ill afford.
Several studies have used biopolymer materials alone or in combination with various cell types for myocardial repair. Biopolymer scaffolds can act to alter the material properties of the injured myocardial wall (4) and provide a platform for cellular repopulation of the injured myocardium (5). Passive polymer materials injected into the infarcted myocardial wall of the failing LV have been suggested to reduce the elevated levels of myofiber stress during the cardiac cycle (6–9). The present study tested the hypothesis that augmentation of thickness of the LV free wall with direct intramyocardial injections of alginate hydrogel implant (AHI) in dogs with advanced HF reduces LV chamber size and restores chamber geometry, consequently reducing LV wall stress and improving cardiac function.
Methods
The study was approved by Henry Ford Health System Institutional Animal Care and Use Committee. The canine model of chronic HF used in this study was previously described in detail (10). Briefly, studies were performed in 15 dogs with HF produced by intracoronary microembolizations (LV ejection fraction [EF] <30%). Dogs were randomized to AHI treatment (n = 9) or to sham-operated control (n = 6). During an open-chest procedure, dogs received either intramyocardial injections of 0.25 to 0.35 ml of alginate hydrogen (Algisyl-LVR, LoneStar Heart Inc., Laguna Hills, California) or saline. Seven injections were made ∼1.0 to 1.5 cm apart (total volume 1.8 to 2.1 ml) along the circumference of the LV free wall halfway between the apex and base starting from the anteroseptal groove and ending at the posteroseptal groove (Fig. 1). Hemodynamic, ventriculographic, echocardiographic, and Doppler measurements including LV pressure-volume loops as well as ambulatory 24-h Holter monitoring studies were made at baseline, before treatment (PRE) and repeated for up to 17 weeks thereafter (POST). Histomorphometric measurements were performed on LV tissue obtained at the end of the study. For more details on the methods in this study, please see the Online Appendix.
Figure 1. Diagram of a Cross-Sectional View of the Heart at Midventricular Level Showing AHI Location.
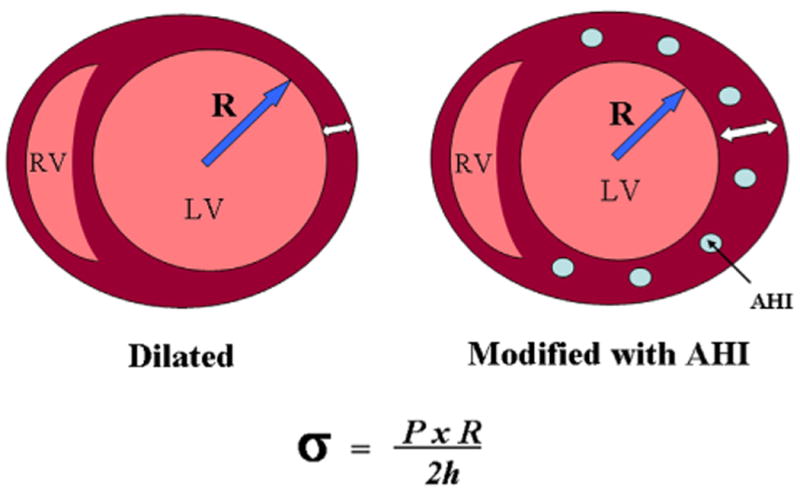
Left: untreated. Right: after treatment. Bottom: calculation of wall stress from Laplace law. σ = wall stress, AHI = alginate hydrogel implants; h = wall thickness; LV = left ventricle; P = intracavitary pressure, R = cavity radius; RV = right ventricle.
Statistical analysis
Within group comparisons were made using repeated measures analysis of variance with alpha set at 0.05. If significance was attained, pair-wise comparisons were made using the Student-Newman-Keuls test between post-treatment measurements and pre-treatment measurements with p < 0.05 considered significant. To assess treatment effects, the change (Δ) in each measure from PRE to POST (17 weeks) was calculated and comparisons were made using a t statistic for 2 means with significance set at p < 0.05. A t statistic for 2 means with significance set at p < 0.05 was also used to compare the absolute measures at week 17 between the 2 study groups.
Histomorphometric and electrocardiographic (ECG) Holter monitoring results were examined using ANOVA with alpha set at 0.05 and pairwise comparisons performed using the Student-Newman-Keuls test. All data are reported as the mean ± SEM.
Results
Needle penetration during intra-myocardial delivery of AHI or saline was associated with ventricular arrhythmias including couplets, triplets and rarely non-sustained ventricular tachycardia. Fourteen of 15 dogs entered into the study completed the 17-week follow-up period. One dog randomized to AHI died intraoperatively from ventricular fibrillation. Arrhythmias subsided in all dogs within 10 to 15 min without use of anti-arrhythmic drugs. None of the dogs developed signs or symptoms of cardiac decompensation, none experience sudden death, and none received cardioactive medication during follow-up. There were no significant differences in any of the baseline and pre-treatment measures between study groups by analysis of variance (Table 1).
Table 1. Ventriculographic, Echocardiographic, and Doppler Measures in Control Dogs and Dogs Treated With AHI Obtained at Baseline, PRE, and at 2, 6, 12, and 17 Weeks POST.
| Sham-Operated Controls | Baseline (n = 6) | PRE (n = 6) | 2 Weeks POST (n = 6) | 6 Weeks POST (n = 6) | 12 Weeks POST (n = 6) | 17 Weeks POST (n = 6) |
|---|---|---|---|---|---|---|
| LV EDV, ml | 53 ± 1.3 | 66 ± 2.0 | 66 ± 1.8 | 67 ± 1.8 | 68 ± 1.7* | 69 ± 1.7* |
| LV ESV, ml | 26 ± 0.8 | 48 ± 1.6 | 48 ± 1.4 | 49 ± 1.3 | 51 ± 1.5* | 53 ± 1.8* |
| LV EF, % | 52 ± 0.8 | 27 ± 0.3 | 27 ± 0.3 | 26 ± 0.4 | 25 ± 0.9 | 24 ± 1.3* |
| SV, ml | 27 ± 0.8 | 18 ± 0.5 | 18 ± 0.5 | 17 ± 0.6 | 17 ± 0.7 | 16 ± 0.8* |
| LV ESSI | 1.88 ± 0.05 | 1.58 ± 0.03 | 1.58 ± 0.03 | 1.58 ± 0.03 | 1.58 ± 0.03 | 1.55 ± 0.04 |
| LV EDP, mm Hg | 9 ± 0.4 | 14 ± 0.7 | 11 ± 0.7* | 12 ± 0.6* | 12 ± 1.0* | 13 ± 0.9* |
| SV/EDP, ml/mm Hg | 2.88 ± 0.09 | 1.35 ±0.1 | 1.62 ± 0.11 | 1.48 ± 0.08 | 1.42 ± 0.16 | 1.33 ± 0.12 |
| DT, ms | 132 ± 6 | 102 ± 5 | 101 ± 4 | 98 ± 2 | 92 ± 2 | 93 ± 3 |
| EDWS, g/cm2 | 30.2 ± 1.9 | 53.6 ± 3.5 | 47.4 ± 2.9 | 48.1 ± 2.1 | 54.3 ± 4.6 | 57.0 ± 4.4 |
| MR, % | 0 ± 0.0 | 10 ± 1.9 | 10 ± 2.1 | 11 ± 2.3 | 13 ± 2.0* | 13 ± 1.9* |
| Slope of ESPVR, mm Hg/ml | – | 1.96 ± 0.20 | – | – | – | 1.66 ± 0.12 |
| LV AW EDWT, cm | – | 0.85 ± 0.02 | – | – | – | 0.83 ± 0.02 |
| LV PW EDWT, cm | – | 0.98 ± 0.02 | – | – | – | 0.92 ± 0.04 |
| AHI-Treated Dogs | (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) |
|---|---|---|---|---|---|---|
| LV EDV, ml | 53 ± 1.3 | 63 ± 1.0 | 63 ± 1.0 | 62 ± 0.9 | 62 ± 0.9 | 62 ± 1.1*† |
| LV ESV, ml | 25 ± 0.6 | 47 ± 0.8 | 44 ± 0.7* | 43 ± 0.9* | 44 ± 0.9* | 43 ± 0.8*† |
| LV EF, % | 52 ± 0.9 | 26 ± 0.4 | 30 ± 0.8* | 31 ± 0.6* | 31 ± 0.5* | 31 ± 0.4*† |
| SV, ml | 28 ± 1 | 17 ± 0.4 | 19 ± 0.6* | 19 ± 0.3* | 19 ± 0.3* | 19 ± 0.3*† |
| LV ESSI | 1.76 ± 0.03 | 1.53 ± 0.03 | 1.65 ± 0.05* | 1.58 ± 0.05 | 1.63 ± 0.05* | 1.64 ± 0.05* |
| LV EDP, mm Hg | 10 ± 0.4 | 15 ± 0.9 | 13 ± 1.1 | 13 ± 1.0 | 13 ± 1.1 | 11 ± 0.5 |
| SV/EDP, ml/mm Hg | 2.98 ± 0.19 | 1.1 ± 0.06 | 1.51 ± 0.12* | 1.54 ± 0.1* | 1.51 ± 0.11* | 1.87 ± 0.07*† |
| DT, ms | 132 ± 6 | 83 ± 6 | 103 ± 5* | 103 ± 6* | 103 ± 6* | 110 ± 5*† |
| EDWS, g/cm2 | 32.1 ± 1.9 | 64.5 ± 7.8 | 49.4 ± 5.5* | 48.4 ± 5.2* | 51.1 ± 5.9* | 41.4 ± 3.6*† |
| MR, % | 0.0 | 11 ± 2.0 | 7 ± 1.3* | 7 ± 1.3* | 7 ± 1.4* | 7 ± 1.3*† |
| Slope of ESPVR, mm Hg/ml | – | 1.28 ± 0.17 | – | – | – | 1.86 ± 0.21* |
| LV AW EDWT, cm | – | 0.89 ± 0.05 | – | – | – | 1.03 ± 0.05*† |
| LV PW EDWT, cm | – | 0.94 ± 0.04 | – | – | – | 1.09 ± 0.04*† |
Values are mean ± SEM.
p < 0.05 PRE versus POST.
p<0.05 for comparisons between control dogs at week 17 and AHI-treated dogs at week 17.
Measures across time without any *or †showed a nonsignificant p value in the repeated measures analysis of variance.
AHI = alginate hydrogel implant; AW EDWT = anterior wall end-diastolicwall thickness; DT = early mitral inflow deceleration time; EDP = end-diastolic pressure; EDV = end-diastolic volume; EDWS = left ventricular end-diastolic circumferential wall stress; EF = ejection fraction; ESPVR = end-systolic pressure-volume relation; ESSI = end-systolic sphericity index; ESV = end-systolic volume; LV = left ventricular; MR = severity of mitral valve functional regurgitation; POST = post-treatment; PRE = pre-treatment; PW EDWT = posterior wall end-diastolic wall thickness; SV = stroke volume.
Changes in LV wall thickness, size, and shape
Compared to controls, AHI-treated dogs showed a significant increase of both end-systolic and end-diastolic wall thickness of both the anterior and posterior LV walls (Table 1). Control dogs showed a trend across time for increased LV end-diastolic (EDV) and end-systolic (ESV) volumes and a trend for decreased stroke volume (SV) while AHI-treated dogs showed a trend for decreased volumes and a trend for increased SV (Table 1, Fig. 2). Treatment with AHI significantly increased end-systolic sphericity index, thus partially restoring LV ellipsoidal shape (Table 1).
Figure 2. Line Graphs Illustrating Changes in Dogs.
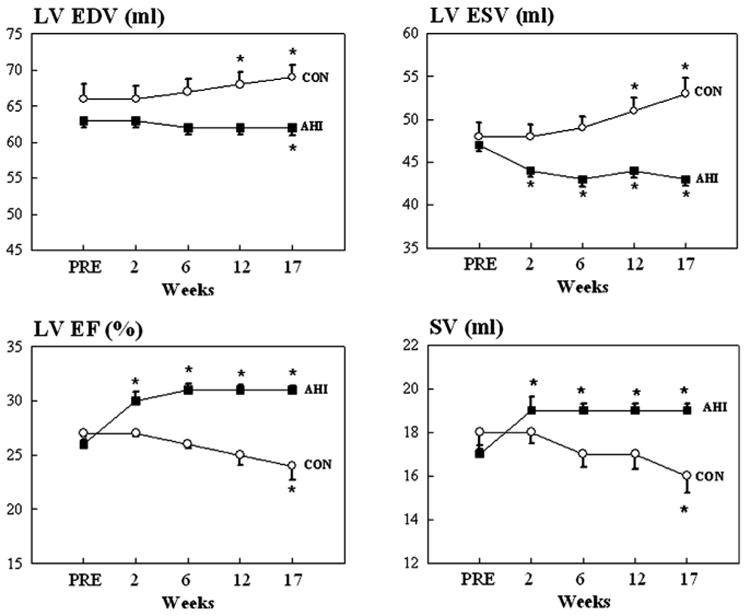
Changes in left ventricular (LV) end-diastolic volume (EDV) (top left), end-systolic volume (ESV) (top right), ejection fraction (EF) (bottom left), and stroke volume (SV) (bottom right) in control dogs (CON) and dogs treated with alginate hydrogel implants (AHI). Values are as mean ± SEM. PRE = before delivery of AHI. *p < 0.05 versus PRE; p values are based on sequential testing with Student-Newman-Keuls test.
Changes in LV systolic and diastolic function
Left ventricular EF decreased in control dogs but increased significantly in AHI-treated dogs (Fig. 2) and was accompanied by a significant reduction of functional mitral regurgitation (MR) (Table 1). The slope of the LV end-systolic pressure-volume relationship, a measure of load-independent contractility, decreased modestly but not significantly in controls but increased in AHI-treated dogs (Table 1). The ratio SV/end-diastolic pressure (EDP) was unchanged in controls but increased significantly in AHI-treated dogs (Table 1, Fig. 3). AHI therapy also produced significant improvements in measures of LV diastolic function. Deceleration time (DT) of mitral inflow velocity increased while end-diastolic circumferential wall stress (EDWS) decreased significantly at pre-treatment compared to post-treatment. LV end-diastolic pressure (EDP), a measure of preload, did not change significantly in controls but decreased in AHI-treated dogs (Table 1).
Figure 3. Bar Graphs Depicting the Treatment Effect Change.
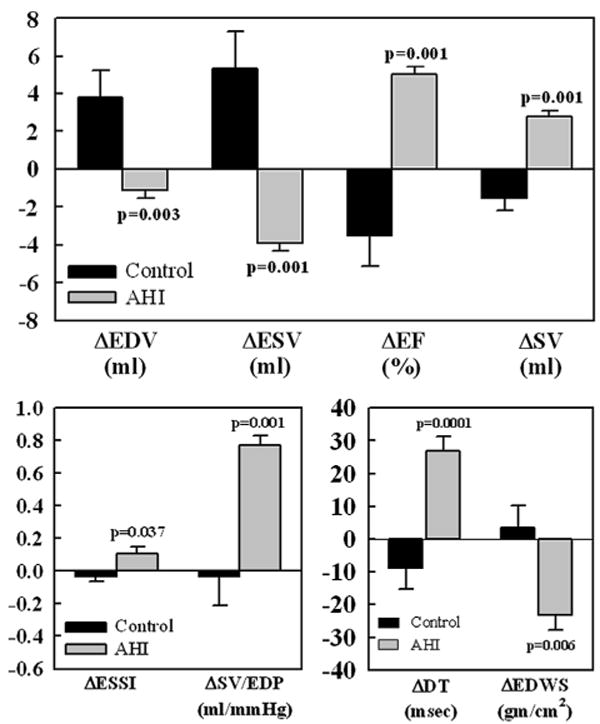
Change (Δ) between control dogs (black bars) and AHI-treated dogs (gray bars). The Δs represent the difference between pre-treatment and 17 weeks among the 2 treatment groups. Top: LV EDV, LV ESV, LV EF, and SV. Bottom left: LV end-systolic sphericity index (ESSI) and ratio of SV to LV end-diastolic pressure (SV/EDP). Bottom right: deceleration time of early mitral inflow velocity (DT) and LV end-diastolic circumferential wall stress (EDWS). Data are shown as mean ± SEM. P = Probability value between control and AHI. p Values are based on a t statistic for 2 means. Abbreviations as in Figure 2.
Comparisons of treatment effect
Compared with controls, AHI therapy significantly increased LV wall thickness and decreased EDV, ESV, EDP, and EDWS, and significantly increased EF, end-systolic sphericity index, SV/ EDP ratio and DT (Fig. 3). The slope of the end-systolic pressure-volume relationship increased in AHI-treated dogs compared to controls (p = 0.001) and the severity of functional MR decreased (p = 0.001). Compared to controls, AHI treatment resulted in increased LV anterior and posterior wall at sites of AHI implants (p = 0.001). Comparisons between measures obtained in control dogs and dogs treated with AHI at week 17 also showed significant differences. At week 17, EDV, ESV, EDWS, and MR were significantly lower in dogs treated with AHI whereas EF, SV/EDP, DT, and LV wall thickness at site of AHI delivery were significantly higher (Table 1). Compared to controls, AHI-treated dogs, showed a leftward and downward shift of the pressure-volume relationship consistent with LV reverse remodeling.
Ambulatory 24-h Holters showed no significant differences between groups with respect to minimum, average, and maximum heart rate and frequency and severity of ventricular arrhythmias (Online Table 1). In AHI-treated dogs, trichrome stained LV transmural sections obtained at post-treatment showed that pockets of AHI material were still within the LV free wall and were encapsulated by a thin layer of connective tissue (Fig. 4). There was no evidence of ongoing inflammation based on absence of mononuclear cells. There was no difference between groups in volume fraction of replacement fibrosis between groups. Volume fraction of interstitial fibrosis, cardiomyocyte cross-sectional area (MCSA), and oxygen diffusion distance decreased significantly in AHI-treated dogs compared to controls and capillary density increased (Online Table 2). Detailed results on pressure-volume relationships (Online Fig. 1), arrhythmias, and histomorphometry are provided in the Online Appendix.
Figure 4. Trichrome-Stained Transmural Section From the Left Ventricular Free Wall in a Control Dog and a Dog Treated With Alginate Hydrogel Implant.
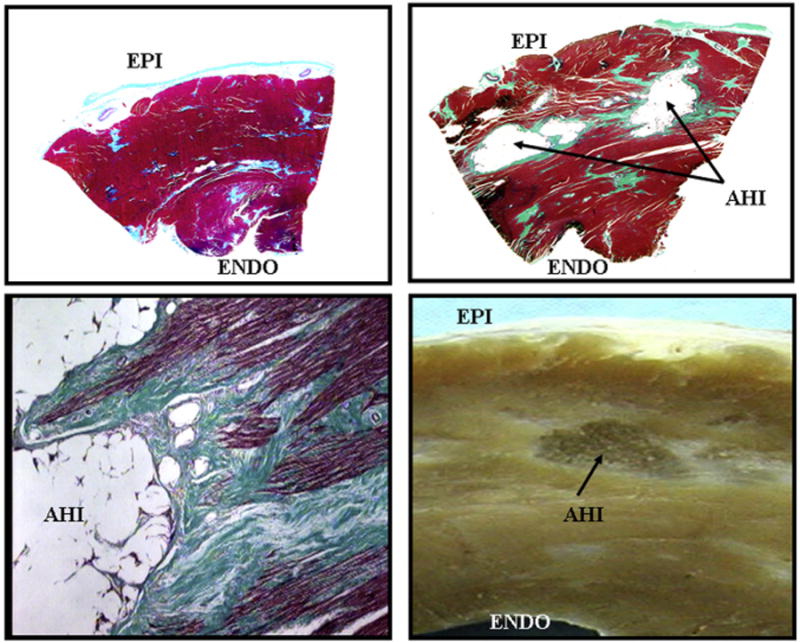
Trichrome-stained transmural section from left ventricular free wall in a control dog (upper left) and a dog treated with alginate hydrogel implant (AHI) (upper right) at end of study. AHI are encapsulated in thin layer of connective tissue (light green). Bottom left: high magnification trichrome-stained myocardial section showing the border between myocardium and AHI. Bottom right: photograph of gross LV free wall formalin-fixed section showing an AHI within the wall. EPI = epicardial surface; ENDO = endocardial surface; other abbreviations as in Figure 2.
Discussion
In dogs with advanced HF, augmentation of LV wall thickness at midventricular level with intramyocardial AHI significantly improves LV systolic and diastolic function. The magnitude of improvement was similar to that seen following long-term therapy with beta-blockers in this animal model (11). Treatment with AHI significantly increased SV/EDP suggesting a preload independent improvement of LV performance. Therapy with AHI also reduced LV size, partially restored physiologic LV shape, and had a favorable impact at the cellular level consistent with reverse LV remodeling. The results also indicate that the benefits of AHI therapy are elicited early after AHI delivery (2 weeks) and persist during long-term follow-up (17 weeks).
Several studies have used alginates or other biopolymers to alter LV wall geometry in animal models of myocardial infarction (4–6,9,12–14). Leor and colleagues injected alginate into the culprit coronary artery following myocardial infarction in pigs and showed that the resulting alginate scaffold increased scar thickness and prevented LV dilation (13). Mukherjee et al. (14) made direct injections of fibrin-alginate composite into the infarcted area in pigs and showed attenuation of post-infarction LV wall thinning and infarct expansion. Others also used direct myocardial injection of calcium cross-linked alginate solution into recent and old infarcts in rats and showed prevention of adverse cardiac remodeling and dysfunction (12). The approach used in the present study differs in that the injections were made without distinction to viable or scarred myocardium thus setting the stage for a therapy that can be applied to HF regardless of etiology.
Heart failure is invariably associated with elevation of LV wall stress. Because wall stress and tension are determinants of MVO2, an increase of wall stress increases energy demands on the failing myocardium, a condition the failing heart can ill afford. Any reduction of wall stress through reduction of LV size and restoration of LV shape can improve LV function. Reducing wall stress may not be the only mechanism that underlies the benefits of AHI therapy. Augmentation of the LV wall thickness with a subsequent reduction of LV size can also lead to reduced radius of curvature along the LV endocardial surface and hence to a shorter end-diastolic length of contractile elements. Because the myocardium is essentially incompressible, for the same intrinsic contractile shortening, a greater radial deformation is likely to occur leading to greater encroachment of the wall on the LV cavity (much smaller ESV). These pure geometrical alterations can also explain, albeit in part, the observed early increase of LV EF with AHI therapy.
Therapy with AHI also reduced MCSA, a measure of cardiomyocyte hypertrophy. Progressive LV enlargement and subsequent increase of mechanical stretch can stimulate cardiomyocyte hypertrophy through up-regulation of stretch response proteins (15). The resulting maladaptive hypertrophy is invariably associated with abnormal sarcoplasmic reticulum calcium cycling and shifts in myosin isoforms (16,17). Thus, in the long-term reducing LV size and preventing excessive myocardial stretch may down-regulate stretch response proteins and block an important signaling pathway for HF progression. Structural maladaptations of the cardiac interstitium also occur in HF and can adversely influence global LV function. A maladaptation of the failing heart is the accumulation of collagen in the interstitium termed “reactive interstitial fibrosis” that can lead to decreased capillary density and increased oxygen diffusion distance (18). These structural/cellular changes can lead to increased LV stiffness and can promote hypoxia of the collagen-encircled cardiomyocytes (18,19). In the present study, the effects of AHI therapy included a significant reduction of interstitial fibrosis and oxygen diffusion distance and a significant increase of capillary density. The present study also showed that AHI therapy is associated with reduced severity of functional MR. The presence of functional MR is attributable to several factors that include LV dilation and sphericity and dilation of the mitral valve annulus (20). In this study, AHI therapy reduced LV size and partially reversed maladaptive LV chamber sphericity. The passive mechanical alteration of LV geometry employed in the present study differs from previous approaches. Studies have shown that progressive LV dilation in HF can be prevented or attenuated by wrapping synthetic materials around the cardiac ventricles to elicit containment as was the case with the Myosplint (Myocor Inc., Maple Grove, Minnesota), the Acorn CorCap (Acorn Cardiovascular, Inc., St. Paul, Minnesota), and the Paracor HeartNET (Paracor Medical, Inc., Sunnyvale, California), all of which had a favorable impact on LV size and LV shape (21–24). However, in all of these applications, the benefits often manifested months after implantation in contrast to the early benefit seen with AHI therapy.
Several observations made in the study support a favorable safety profile for AHI therapy. The improvement in indexes of LV diastolic function and those seen from pressure-volume loops provide support that AHI therapy does not negatively impact LV relaxation or filling. Results of ambulatory electrocardiographic Holter monitoring also suggest that AHI is not pro-arrhythmic in the long-term. This is in contrast to myoblast therapy, for example, that produces areas of heterogeneous myocardial conduction potentially leading to areas of slow conduction and ventricular arrhythmias (25). Nevertheless, a limitation of the study is absence of programmed electrical stimulation studies that may have been useful in uncovering potential changes in substrate for lethal ventricular arrhythmias.
Alginates are known to degrade and be resorbed (12). In this study, the AHI implants were present even after 3 months of implantation. Studies conducted in normal dogs by the manufacturer with intramyocardial injections of Algisyl-LVR as part of regulatory safety and toxicity studies, showed persistence of the AHI implant for up to 2 years (Frank Ahmann and Sam Helgerson, personal communication, 2010) with histologic appearance similar to that seen at 3 months post implantation. In the present study, histologic sections examined at 3 months post-implantation showed no evidence of ongoing inflammation and/or increased macrophage activity in the vicinity of the AHI implants. The lack of AHI degradation may be due to encapsulation of the AHI in a thin layer of fibrotic tissue consistently observed in myocardial tissue samples from treated hearts. The long-term improvement in LV function, seen in the present study, likely reflects the persistence of AHI in the LV myocardium.
Observation made in this pre-clinical study and others led to a first-in-man study. Patients received therapy with AHI with either concomitant coronary artery bypass or valve surgery (26) and were followed for 3 months. Results showed a reduction in LV volumes and an increase in EF that was accompanied by improvements in clinical status and quality-of-life indicators with no apparent serious adverse events (26). The encouraging first-in-man results, while not confirmatory of efficacy, led to the initiation of a Phase-II clinical trial, the AUGMENT-HF (A Randomized, Controlled Study to Evaluate Algisyl-LVR as a Method of Left Ventricular Augmentation for Heart Failure) study (NCT01311791), a randomized, controlled study to evaluate Algisyl-LVR in patients with advanced symptomatic HF.
Conclusions
Augmentation of LV wall thickness at midventricular level with intramyocardial AHI delivery improves LV function in dogs with advanced HF. This strategically targeted AHI delivery elicits early beneficial effects that persist in the long term. The study results corroborate and extend previous observations of the use of biopolymers to favorably alter geometry of the failing LV. The results favor continued development of this therapeutic approach for the treatment of patients with chronic HF.
Supplementary Material
Acknowledgments
The authors acknowledge the assistance of Robert Peterson, PhD, Norman Tarazona, DVM, and Andrew Hinson, BS.
The study was supported, in part, by research grants from LoneStar Heart, Inc. and National Heart, Lung, and Blood Institute PO1 HL074237-09. Dr. Sabbah has received research grants from and is a consultant to LoneStar Heart, Inc. At the time of research Dr. Helgerson was an employee of Lonestar Heart, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- AHI
alginate hydrogel implant
- DT
deceleration time
- EDP
end-diastolic pressure
- EDV
end-diastolic volume
- EDWS
end-diastolic circumferential wall stress
- EF
ejection fraction
- ESV
end-systolic volume
- HF
heart failure
- LV
left ventricle/ ventricular
- MR
mitral regurgitation
- MVO2
myocardial oxygen consumption
- SV
stroke volume
Footnotes
Appendix: For an expanded Methods and Results sections as well as supplemental tables and a figure, please see the online version of this article.
References
- 1.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102:IV14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 2.Sabbah HN, Kono T, Stein PD, Mancini GB, Goldstein S. Left ventricular shape changes during the course of evolving heart failure. Am J Physiol. 1992;263:H266–70. doi: 10.1152/ajpheart.1992.263.1.H266. [DOI] [PubMed] [Google Scholar]
- 3.Douglas PS, Morrow R, Ioli A, Reichek N. Left ventricular shape, afterload and survival in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1989;13:311–5. doi: 10.1016/0735-1097(89)90504-4. [DOI] [PubMed] [Google Scholar]
- 4.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 5.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–13. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 7.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 8.Wenk JF, Wall ST, Peterson RC, et al. A method for automatically optimizing medical devices for treating heart failure: designing polymeric injection patterns. J Biomech Eng. 2009;131:121011. doi: 10.1115/1.4000165. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Christman KL, Chin E, Sievers RE, Saeed M, Lee RJ. Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2009;137:180–7. doi: 10.1016/j.jtcvs.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Sabbah HN, Stein PD, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–84. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 11.Sabbah HN, Shimoyama H, Kono T, et al. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation. 1994;89:2852–9. doi: 10.1161/01.cir.89.6.2852. [DOI] [PubMed] [Google Scholar]
- 12.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 13.Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee R, Zavadzkas JA, Saunders SM, et al. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg. 2008;86:1268–76. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–92. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain gene expression in human heart failure. J Clin Invest. 1997;100:2362–70. doi: 10.1172/JCI119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadoshima J, Qiu Z, Morgan JP, Izumo S. Angiotensin II and other hypertrophic stimuli mediated by G protein-coupled receptors activate tyrosine kinase, mitogen-activated protein kinase, and 90-kD S6 kinase in cardiac myocytes. The critical role of Ca(2+)-dependent signaling. Circ Res. 1995;76:1–15. doi: 10.1161/01.res.76.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem. 1995;147:29–34. doi: 10.1007/BF00944780. [DOI] [PubMed] [Google Scholar]
- 19.Sabbah HN, Sharov VG, Goldstein S. Cell death, tissue hypoxia and the progression of heart failure. Heart Fail Rev. 2000;5:131–8. doi: 10.1023/A:1009880720032. [DOI] [PubMed] [Google Scholar]
- 20.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–8. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 21.Sabbah HN. The cardiac support device and the myosplint: treating heart failure by targeting left ventricular size and shape. Ann Thorac Surg. 2003;75:S13–9. doi: 10.1016/s0003-4975(03)00463-6. [DOI] [PubMed] [Google Scholar]
- 22.Sabbah HN, Sharov VG, Gupta RC, et al. Reversal of chronic molecular and cellular abnormalities due to heart failure by passive mechanical ventricular containment. Circ Res. 2003;93:1095–101. doi: 10.1161/01.RES.0000101932.70443.FE. [DOI] [PubMed] [Google Scholar]
- 23.Klodell CT, Jr, Aranda JM, Jr, McGiffin DC, et al. Worldwide surgical experience with the Paracor HeartNet cardiac restraint device. J Thorac Cardiovasc Surg. 2008;135:188–95. doi: 10.1016/j.jtcvs.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Mann DL, Acker MA, Jessup M, Sabbah HN, Starling RC, Kubo SH. Clinical evaluation of the CorCap Cardiac Support Device in patients with dilated cardiomyopathy. Ann Thorac Surg. 2007;84:1226–35. doi: 10.1016/j.athoracsur.2007.03.095. [DOI] [PubMed] [Google Scholar]
- 25.Hagege AA, Marolleau JP, Vilquin JT, et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114:I108–13. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 26.Lee RJ, Hinson A, Helgerson S, Bauerschmitt R, Sabbah HN. Polymer-based restoration of left ventricular mechanics. Cell Transpl. 2013;22:529–33. doi: 10.3727/096368911X637461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


