Abstract
β-amyloid (Aβ) plaque accumulation is a hallmark of Alzheimer's disease (AD). It is believed to start many years prior to symptoms and is reflected by reduced cerebrospinal fluid (CSF) levels of the peptide Aβ1–42 (Aβ42). Here we tested the hypothesis that baseline levels of CSF proteins involved in microglia activity, synaptic function and Aβ metabolism predict the development of Aβ plaques, assessed by longitudinal CSF Aβ42 decrease in cognitively healthy people. Forty-six healthy people with three to four serial CSF samples were included (mean follow-up 3 years, range 2–4 years). There was an overall reduction in Aβ42 from a mean concentration of 211–195 pg ml−1 after 4 years. Linear mixed-effects models using longitudinal Aβ42 as the response variable, and baseline proteins as explanatory variables (n=69 proteins potentially relevant for Aβ metabolism, microglia or synaptic/neuronal function), identified 10 proteins with significant effects on longitudinal Aβ42. The most significant proteins were angiotensin-converting enzyme (ACE, P=0.009), Chromogranin A (CgA, P=0.009) and Axl receptor tyrosine kinase (AXL, P=0.009). Receiver-operating characteristic analysis identified 11 proteins with significant effects on longitudinal Aβ42 (largely overlapping with the proteins identified by linear mixed-effects models). Several proteins (including ACE, CgA and AXL) were associated with Aβ42 reduction only in subjects with normal baseline Aβ42, and not in subjects with reduced baseline Aβ42. We conclude that baseline CSF proteins related to Aβ metabolism, microglia activity or synapses predict longitudinal Aβ42 reduction in cognitively healthy elders. The finding that some proteins only predict Aβ42 reduction in subjects with normal baseline Aβ42 suggest that they predict future development of the brain Aβ pathology at the earliest stages of AD, prior to widespread development of Aβ plaques.
Keywords: Alzheimer's disease, beta-amyloid, biomarker, cerebrospinal fluid, longitudinal, microglia
Introduction
β-amyloid (Aβ) plaques is a hallmark of Alzheimer's disease (AD) and are reflected by reduced levels of cerebrospinal fluid (CSF) Aβ42.1, 2, 3 Reduced CSF Aβ42 has high diagnostic accuracy for AD as determined by autopsy4 and agrees well with the results of positron emission tomography (PET) using Aβ tracers.5, 6, 7 Although somewhat controversial, many believe that biomarkers reflecting the accumulation of brain Aβ in plaques are the earliest detectable biomarker signs of AD in elders, reaching pathological levels several years before the development of cognitive symptoms.8 Other hallmarks of AD are axonal degeneration and tangle pathology, which are reflected by increased CSF tau (total-tau, T-tau) and phosphorylated tau (P-tau), respectively.9 Reports on both autosomal-dominant AD8 and late-onset AD10 suggest that increased CSF T-tau and P-tau levels occur first after widespread amyloid plaque deposition, evidenced by reduced CSF Aβ42 and amyloid imaging. Currently, possibly aside from the Apolipoprotein E (APOE) ɛ4 variant,11, 12 there are no biomarkers that are known to predict the development of Aβ plaques in people without familial AD. Biomarkers predicting Aβ plaque formation in humans would be important both for determining individual risk of Aβ pathology and as in vivo evidence linking distinct molecular pathways to Aβ pathology in humans.
Experimental and genetic data have suggested that inflammation (especially microglial activity13, 14) and synaptic function15, 16 may be important for Aβ production, accumulation and/or toxicity, but there are little in vivo data supporting such pathways in humans. However, previous studies have suggested that some proteins in human CSF may reflect changes in such pathways.17, 18
To our knowledge, no previous study has attempted to identify protein biomarkers that predict longitudinal Aβ plaque formation in cognitively healthy people. Therefore, the main goals of this study were to investigate the following a priori hypotheses using proteins measured in normal controls in Alzheimer's Disease Neuroimaging Initiative (ADNI): (1) baseline levels of CSF proteins involved in Aβ metabolism, microglia activity, synaptic/neuronal function or other AD-related processes predict longitudinal CSF Aβ42 decrease in cognitively healthy people; (2) baseline levels of some of these proteins may predict CSF Aβ42 decrease even in subjects without Aβ plaque pathology at baseline (reflected by CSF Aβ42 levels below an AD-related cutoff4) and (3) These proteins are less predictive of P-tau and T-tau changes, according to a model in which P-tau and T-tau become abnormal downstream of Aβ pathology and associated biomarkers.19
Subjects and methods
Study design
This was a longitudinal study on the associations between CSF proteins at baseline and longitudinal changes in CSF Aβ42, T-tau and P-tau. Data used in the preparation of this article were obtained from the ADNI database (http://adni.loni.ucla.edu/). The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration, private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public–private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. The Principal Investigator of this initiative is Michael W Weiner, MD, VA Medical Center and University of California–San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the United States and Canada. The initial goal of ADNI was to recruit 800 subjects but ADNI has been followed by ADNI-GO and ADNI-2. To date, these three protocols have recruited over 1500 adults, aged 55–90 years, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI and people with early AD. The follow-up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO. Subjects originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org. Written consent was obtained from all subjects participating in the study according to the Declaration of Helsinki, and the study was approved by the institutional review board at each participating site.
Participants
Our study population was 46 ADNI-1 healthy control participants, from whom longitudinal CSF samples were taken three to four times and analyzed for Aβ42, T-tau and P-tau in a 4-year follow-up assay run, and in whom baseline samples had been analyzed using multiplex proteomics. Inclusion/exclusion criteria for ADNI-1 subjects are described in detail at www.adni-info.org. Briefly, all the control subjects included in ADNI-1 were between the ages of 55 and 90 years, had completed at least 6 years of education, were fluent in Spanish or English, were free of any significant neurologic disease and had CDR scores of 0. APOE genotypes were determined for all subjects. We also tested 16 ADNI-1 AD dementia patients, in whom longitudinal CSF samples (taken three to five times) had been analyzed for Aβ42, T-tau and P-tau, and in whom baseline samples had been analyzed using multiplex proteomics in the same assay runs as the samples from the healthy controls.
Procedures
The study procedures included lumbar punctures for CSF sampling at baseline and follow-up approximately every 12 months (the mean total follow-up 3.1 years, range 1.9–4.2). Procedures for CSF sampling, transport and storage have been described previously.4 Participants also underwent repeated medical evaluation and neuropsychological testing. For this study, we used total scores for mini mental state examination, Alzheimer's Disease Assessment Scale-Cognitive Behavior section (ADAS-Cog) and a modified 13-item version of ADAS-Cog (ADAS-MOD).20 For six subjects who had undergone 11C-Pittsburgh compound B (PiB)-PET scanning,21 we included these data to test the concordance between CSF Aβ42 measurements and the brain Aβ load as estimated by using PiB-PET.
CSF protein measurements
CSF Aβ42, T-tau and P-tau were measured at the ADNI Biomarker Core laboratory at the University of Pennsylvania Medical Center, using the multiplex xMAP Luminex platform (Luminex, Austin, TX, USA) with the INNOBIA AlzBio3 kit (Innogenetics, Ghent, Belgium).4
CSF multiplex proteomics was carried out using an xMAP multiplex kit developed by Rules Based Medicine (MyriadRBM). Details regarding the assay technology, validation and quality control can be found online (http://adni.loni.ucla.edu/). In brief, the kit included 159 analytes, selected for analytes believed to be relevant for a number of different diseases including cancer, autoimmune disorders and AD. Previous versions of the kit had been used to explore CSF or plasma proteins in AD.22, 23, 24 Seventy-six analytes were adequately quantifiable in the CSF samples in this study (the other analytes were mostly below the assay-detection limit or had other assay limitations as outlined in the assay documentation found online at http://adni.loni.ucla.edu/). For these, we did a systematic literature review to find previous evidence of associations with Aβ metabolism, microglial activity, synaptic function or other aspects of AD (See Supplementary Table 1 for results, including references). We identified 67 analytes of interest that were included in this study. Some analytes were used after log transformation as described in the assay documentation. Together with Aβ42, T-tau and P-tau, this resulted in 69 analytes available for each test described below (baseline Aβ42, T-tau or P-tau were excluded in tests of trajectories of Aβ42, T-tau and P-tau, respectively).
Statistical analyses
Potentially confounding effects by age, sex, education and APOE on explanatory proteins were evaluated by linear regression. Logistic regression was used to test whether any demographic parameter was associated with study drop-out.
Effects of explanatory proteins on longitudinal Aβ42, T-tau and P-tau were tested by using linear mixed-effects models, in which Aβ42, T-tau or P-tau was used as a response variable, and the time from the baseline visit in years, explanatory protein, the interaction between time and explanatory protein, age, sex, education and APOE ɛ4 status were included as fixed effects (rates of change of Aβ42, T-tau and P-tau were tested separately with each explanatory protein). All models included a random intercept and slope (except some models that would not converge with a random slope, and therefore only included a random intercept). After inspection of the correlation within subjects, a compound symmetry structure was assumed. We assessed the applicability of the linear mixed-effect model by evaluating (1) linearity of biomarker concentration over time within subjects, (2) the normality of the model residuals and (3) the difference between models with and without time as a random effect. Owing to the large number of proteins tested, we corrected for multiple comparisons using a false discovery rate correction.
Prognostic discrimination of explanatory proteins for longitudinal Aβ42 reduction was also tested by ROC statistics. For this, CSF Aβ42 change was calculated as the relative change over 4 years, estimated using individual intercepts and slopes from a mixed effect model, adjusted for age, sex, education and APOE. The cutoff defining Aβ42 reduction was determined by the 25th percentile, and the cutoff for Aβ42 stability by the 75th percentile. Dichotomous Aβ42 reduction status was then regressed on each significant protein separately, using logistic regression. Predicted probabilities from these models were then used to calculate each protein's ability to discriminate Aβ decliners from those with stable Aβ. This predictive accuracy was estimated as the area under the ROC curve (AUC). Each accuracy was cross-validated using a leave-one-out design. Confidence intervals (CIs) for cross-validated accuracies were calculated using variance estimates from 200 bootstrap resamples. To evaluate the proteins' collective ability to discriminate Aβ decliners from nondecliners, we used logistic regression with a least absolute shrinkage and selection operator penalty on the coefficients of the proteins. The least absolute shrinkage and selection operator penalty was used to simultaneously estimate the effects of a large number of correlated protein concentrations on Aβ reduction. Results were summarized with predictive accuracy (AUC) and 95% CIs estimated from 200 bootstrap resamples.
Significance was determined at P<0.05 after correction for multiple comparisons using a false discovery rate correction. All statistics were performed using R (v. 2.15.2, The R Foundation for Statistical Computing). The nlme package v.3.1-10525 was used for linear mixed effect models. The ROCR package v.1.0–226 was used to calculate AUC.
Results
Table 1 summarizes demographics and clinical characteristics. There were longitudinal reductions in the mini mental state examination (β=−0.18, s.e.=0.081, P=0.034) and increases in ADAS-MOD (β=0.47, s.e.=0.23, P=0.042) but no significant changes in ADAS-Cog (linear mixed effect models, adjusted for age, sex, education and APOE). Five subjects progressed to MCI during the study.
Table 1. Demographics of healthy control subjects.
| Characteristic | Baseline | Last follow-up |
|---|---|---|
| N (% male) | 46 (54%) | 46 (54%) |
| Age (year), mean (s.d.) | 77 (5) | 80 (5) |
| APOE ɛ4 allele carriers (%) | 24% | 24% |
| Education (year), mean (s.d.) | 16 (3) | 16 (3) |
| MMSE, mean (s.d.) | 29.4 (0·8) | 29.0 (1.5) |
| ADAS-Cog, mean (s.d.) | 6.1 (2.9) | 6.6 (4.1) |
| ADAS-MOD, mean (s.d.) | 9.3 (3.9) | 10.7 (5.8) |
| CSF Aβ42 (ng l−1), mean (s.d.) | 211 (57) | 195 (56) |
| CSF T-tau (ng l−1), mean (s.d.) | 73 (30) | 83 (30) |
| CSF P-tau (ng l−1), mean (s.d.) | 25 (11) | 36 (11) |
| N with three timepoints | 23 | |
| N with four timepoints | 23 |
Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Behavior section; CSF, cerebrospinal fluid; MMSE, mini mental state examination.
CSF Aβ42, T-tau and P-tau calculated using fitted data from linear mixed effect models (adjusted for age, sex, education and APOE) at baseline and 4-year follow-up.
In the six subjects in whom PiB-PET data of the brain Aβ were available, CSF Aβ42 and PiB-PET measurements were 100% concordant when using previously published cutoffs (Supplementary Figure 1), supporting the use of CSF Aβ42 to identify brain Aβ accumulation in this study.
Half the study population (n=23, 50%) had three longitudinal samples and the other half had four longitudinal samples over a maximum of 4 years of follow-up. There were no significant associations between the demographic parameters and lack of the 4th sample (age, P=0.51; sex, P=0.10; education, P=0.074; APOE ɛ4, P=0.53, tested by logistic regression).
Effects of age, sex, education and APOE on CSF proteins
Age, sex, education and APOE were potential confounders of the relationship between the investigated proteins and Aβ42, T-tau and P-tau. We tested for imbalance using linear regression with baseline proteins as dependent variables, and age, sex, education and APOE as independent variables. Higher age was associated with higher levels of monokine induced by gamma interferon (P<0.001), CD40 antigen (CD40, P=0.0025), β-2-microglobulin (B2M, P=0.026) and tumor necrosis factor receptor 2 (P=0.045); female sex with higher levels of follicle-stimulating hormone (P<0.001), T-tau (P=0.0081), P-tau (P=0.020) and leptin (P=0.0099), and lower levels of serum amyloid P-component (P=0.001), α-1-microglobulin (P=0.018), ApoD (P=0.024), immunoglobulin A (P=0.033), monocyte chemotactic protein 1 (P=0.040) and fibrinogen (P=0.042); longer education with higher levels of tissue inhibitor of metalloproteinases 1 (TIMP1, P<0.001), plasminogen activator inhibitor 1 (P=0.0049), tumor necrosis factor receptor 2 (P=0.0073), P-tau (P=0.013), macrophage colony-stimulating factor 1 (MCSF, P=0.017), interleukin (IL)-16 (P=0.018), von Willebrand factor (P=0.020), B2M (P=0.020), insulin-like growth factor-binding protein 2 (P=0.035) and Clusterin (CLU, P=0.035); APOE ɛ4 with lower levels of Aβ42 (P=0.0070), T-tau (P=0.0014), Osteopontin (P=0.0078), P-tau (P=0.0098) and chemokine CC-4 (P=0.032). On the basis of these results, we included age, sex, APOE and education as covariates in all subsequent analyses.
Baseline CSF proteins associated with baseline CSF Aβ42, T-tau and P-tau
Linear regression was used to test associations between individual baseline proteins and baseline Aβ42, T-tau and P-tau. No proteins were significantly associated with baseline Aβ42, but several proteins were significantly associated with baseline T-tau, including P-tau (P<0.001), Chromogranin A (CgA, P<0.001) and Axl receptor tyrosine kinase (AXL, P<0.001, Supplementary Table 2).
Proteins predicting longitudinal change in CSF Aβ42
The main goal of the study was to test the prediction by baseline proteins of longitudinal CSF Aβ42 (predictions of CSF T-tau and P-tau were also tested, presented below). See Figure 1 for plots of longitudinal CSF Aβ42, T-tau and P-tau. We tested the prediction by baseline proteins of longitudinal CSF Aβ42 in two ways. First, we used linear mixed effect models with longitudinal Aβ42 as a continuous variable, including all subjects. Second, we used ROC statistics as described in the Patients and methods section, dichotomizing the study population based on relative change in Aβ42 from baseline to 4-year follow-up (using estimated rates from the linear mixed effect model). To clearly separate the subjects compared in the ROC statistics, we only compared subjects with the most and the least Aβ42 change (1st and 4th quartiles of subjects ranked by relative Aβ42 change).
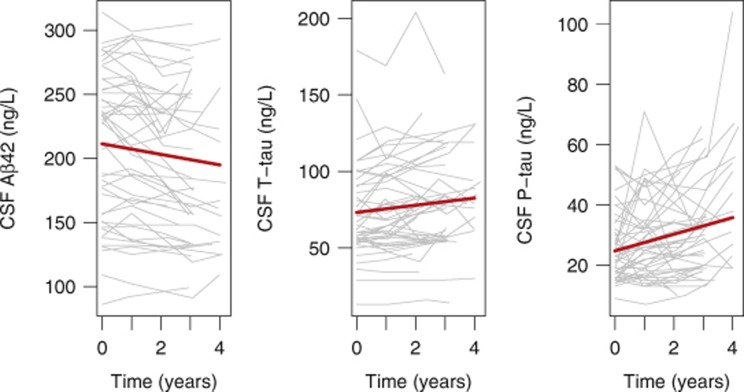
Figure 1.
Longitudinal measurements of CSF Aβ42, T-tau and P-tau. CSF Aβ42, T-tau and P-tau in cognitively healthy controls followed up to 4 years, with three to four samples each. Thick lines are regression curves calculated using linear mixed effect models, adjusted for age, sex, education and APOE. The changes over time (in years) were significant for Aβ42 (β=−4.11, s.e.=0.77, P<0.0001), T-tau (β=2.34, s.e.=0.51, P<0.0001) and P-tau (β=2.76, s.e.=0.49, P<0.0001).
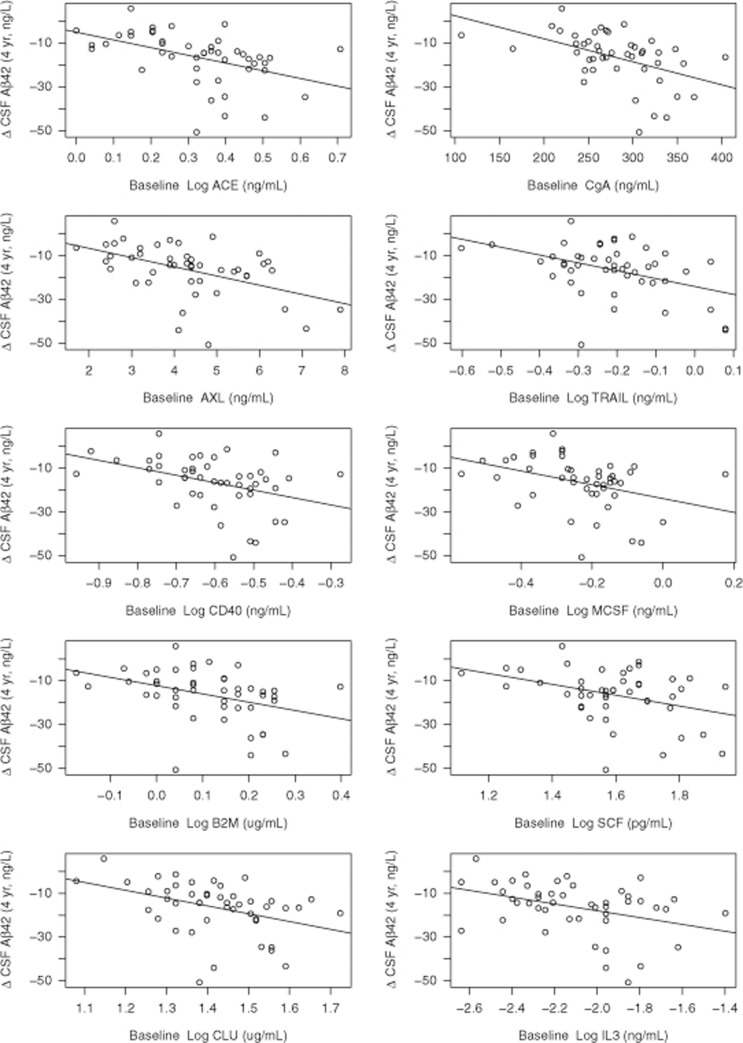
Using linear mixed effect models (with continuous Aβ42, including all subjects), 10 proteins had significant effects (See Table 2 and Figure 2 for the significant proteins, and Supplementary Table 3 for data on all tested proteins). The most significant proteins were angiotensin-converting enzyme (ACE), CgA, AXL, Log TNF-related apoptosis-inducing ligand receptor, CD40 and MCSF. For all these proteins, high baseline protein levels predicted reduced Aβ42 over time.
Table 2. Effects of CSF proteins on longitudinal CSF Aβ42 reduction in healthy controls.
|
Linear mixed effect models |
||||
|---|---|---|---|---|
| CSF biomarker | β | s.e. | P | Adjusted P |
| Log angiotensin-converting enzyme (ACE) | −3.09 | 0.81 | <0.001 | 0.009 |
| Chromogranin A (CgA) | −3.21 | 0.87 | <0.001 | 0.009 |
| AXL receptor tyrosine kinase (AXL) | −3.14 | 0.86 | <0.001 | 0.009 |
| Log TNF-related apoptosis-inducing ligand receptor (TRAIL) | −2.91 | 0.83 | 0.001 | 0.011 |
| Log CD40 antigen (CD40) | −3.14 | 0.91 | 0.001 | 0.012 |
| Log macrophage colony-stimulating factor 1 (MCSF) | −2.84 | 0.91 | 0.002 | 0.026 |
| Log beta-2-microglobulin (B2M) | −2.75 | 0.91 | 0.003 | 0.030 |
| Log stem cell factor (SCF) | −2.56 | 0.89 | 0.005 | 0.042 |
| Log clusterin (CLU) | −2.43 | 0.87 | 0.006 | 0.042 |
| Log interleukin-3 (IL-3) | −2.43 | 0.87 | 0.006 | 0.042 |
Linear mixed effect model data on β-coefficients, s.e. and P-values for the interaction terms between proteins and time (years from baseline). Adjusted P-values were corrected for multiple comparisons using a false discovery rate correction. Data were adjusted for age, sex, education and APOE. Protein levels were centered and standardized.
Figure 2.
Proteins significantly associated with CSF Aβ42 change in healthy controls. The graph includes the baseline proteins (x axes) that were significantly associated with CSF Aβ42 rates, as tested using linear mixed effect models. y axes show CSF Aβ42 reduction over 4 years (ng l−1), adjusted for age, sex, education and APOE. Each dot represents a study participant.
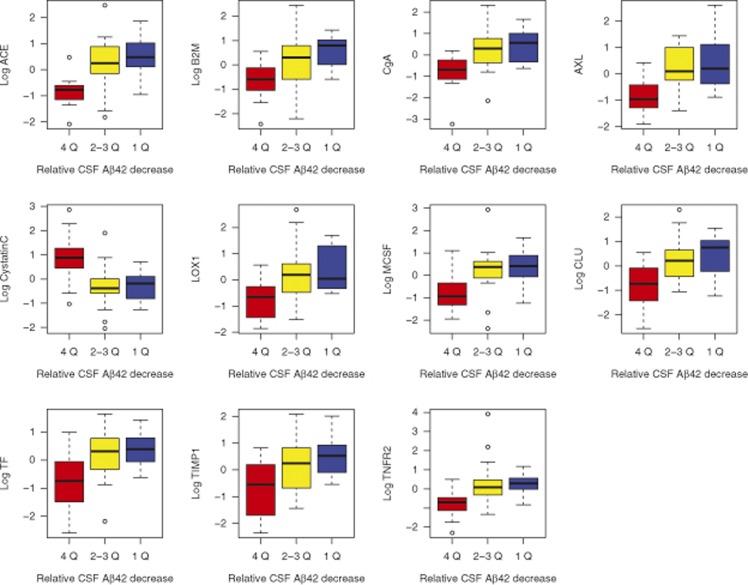
For the ROC statistics, we compared subjects showing the most Aβ42 reduction (1st quartile >11.5% reduction from baseline) with subjects showing the least reduction (4th quartile <4.0% reduction from baseline). Note that the 11.5% cutoff for Aβ42 reduction exceeds the within-run measurement variability of the xMAP AlzBio3 Aβ42 assay and thereby very likely reflects a biological change.27 By these definitions, 12 subjects had Aβ42 reduction (four APOE ɛ4+, six female subjects, mean age 80 years (range 73–88, mini mental state examination 29.3 (28–30), ADAS-Cog 5.7 (1.7–15), ADAS-MOD 8.9 (1.7–18)) and 12 subjects had stable Aβ42 (one APOE ɛ4+, six female subjects, aged 78 years (72–87), mini mental state examination 29.6 (20–30), ADAS-Cog 6.8 (2.3–10), ADAS-MOD 10 (4.0–15)). In the ROC analysis, 11 proteins significantly predicted Aβ42 reduction (Figure 3 and Supplementary Table 4). These proteins largely overlapped with the proteins identified by the linear mixed effect analysis described above. Highest AUC were seen for ACE (AUC=0.87 (95% CI 0.72–1.00)), B2M (AUC=0.83 (95% CI 0.67–0.98)) and CgA (AUC=0.83 (95% CI 0.67–0.98)). All identified significant proteins were higher at baseline in subjects with longitudinal Aβ42 reduction, except Cystatin C, which was lower in subjects with longitudinal Aβ42 reduction.
Figure 3.
Proteins and quartiles of CSF Aβ42 change in healthy controls. The graph includes the baseline proteins that significantly predicted longitudinal reduction in CSF Aβ42 in ROC analysis. For this analysis, subjects were ranked according to relative change in Aβ42 over 4 years, and subjects with most changes (1st quartile of subjects >11.5% reduction, red boxes) were compared with subjects with least changes (4th quartile of subjects <4.0% reduction, blue boxes). Subjects in the 2nd and 3rd quartiles (representing intermediate reductions) were not included in the ROC analysis but are shown for comparison in yellow boxes (for most proteins, subjects in the 2nd and 3rd quartiles had concentrations comparable to subjects in the 1st quartile). Baseline protein levels were centered and standardized.
Combination of biomarkers to predict CSF Aβ42 change
We used logistic regression with a least absolute shrinkage and selection operator penalty to test whether a combination of baseline proteins could be used to predict CSF Aβ42 change. When all proteins were included, it resulted in an 81% (95% CI 62–100%) cross-validated accuracy to discriminate between subjects with the most Aβ42 reduction (1st quartile >11.5% reduction from baseline) and subjects with the least reduction (4th quartile <4.0% reduction from baseline). Thus, we could not find evidence that combining biomarkers was superior to using the best individual biomarkers for the prediction of CSF Aβ42 change.
Proteins predicting longitudinal CSF Aβ42 change: effects of baseline Aβ42
The goal of this analysis was to test whether the predictive effect of baseline proteins differed between subjects with or without Aβ plaque pathology at baseline. For this, we dichotomized the subjects into those with normal baseline Aβ42 (Aβ42 >192 ng l−1, n=27, 59%, 12 female subjects, 1 APOE ɛ4+, mean age 77 years (range 72–88)) and reduced baseline Aβ42 (n=19, 41%, 9 female subjects, 10 APOE ɛ4+, mean age 79 years (range 63–93)). Aβ42 decreased with time in both these subgroups (time as years after first sample; normal baseline Aβ42, β=−4.9, s.e.=1.3, P<0.001; reduced baseline Aβ42, β=−3.2, s.e.=0.68, P<0.001).
In subjects with normal baseline Aβ42, several proteins were significantly associated with longitudinal Aβ42 reduction (co-varying for age, sex, education and APOE, and adjusting for multiple comparisons), including CgA (β=−5.69, s.e.=1.24, P=0.001), CD40 (β=−5.01, s.e.=1.46, P=0.036), AXL (β=−4.55, s.e.=1.38, P=0.036), ACE (β=−4.38, s.e.=1.36, P=0.036), Log TNF-related apoptosis-inducing ligand receptor (β=−4.17, s.e.=1.34, P=0.039) and IL-3 (β=−4.12, s.e.=1.36, P=0.042). In subjects with reduced baseline Aβ42, only TIMP1 (β=−2.2, s.e.=0.56, P=0.020) was associated with longitudinal Aβ42 reduction.
Proteins associated with longitudinal CSF T-tau and P-tau increase
No protein was significantly associated with the change in T-tau or P-tau (See Supplementary Tables 5 and 6).
Proteins associated with clinical outcome
Five of the 46 healthy control subjects progressed to MCI during the study follow-up. Although the differences were not statistically significant, these five subjects had lower baseline CSF Aβ42 (mean 175 (s.d. 43) versus 215 (58) ng l−1), and higher baseline CSF T-tau (85 (38) versus 72 (29) ng l−1) and P-tau (28 (16) versus 25 (11) ng l−1), than the 41 subjects who did not develop MCI. We compared protein levels between stable controls and developers by linear regression, adjusting for age, sex, APOE ɛ4 and education. Matrix metalloproteinase 2 (MMP2) was the only protein that was significantly different between developers and stable controls (reduced in developers, β=−1.64, s.e.=0.43, P=0.03 adjusted for multiple comparisons, protein levels centered and standardized).
Proteins associated with longitudinal CSF Aβ42, T-tau and P-tau changes in AD patients
To test whether CSF proteins were associated with longitudinal change in CSF Aβ42, T-tau or P-tau in subjects with advanced disease, we also examined data from 16 patients with clinical AD diagnosis (10 female patients, 13 APOE ɛ4+, mean age 75 (s.d. 6) years). As expected and as shown previously in the ADNI cohort,4 these subjects had reduced baseline CSF Aβ42 (mean 133 (s.d. 19) ng l−1), and elevated baseline CSF T-tau (131 (64) ng l−1) and CSF P-tau (42 (13) ng l−1). In contrast to what we found in the healthy controls, there were no significant changes over time in CSF Aβ42 (P=0.90) or T-tau (P=0.63), but there were significant longitudinal increases in P-tau (β=4.6, s.e.=1.23, P=0.0007) in the 16 AD patients (linear mixed effect models, co-varied for age, sex, education and APOE ɛ4). After correcting for multiple comparisons, no tested protein was significantly associated with rates of change for CSF Aβ42, T-tau or P-tau (data not shown).
Discussion
The major findings of this study were as follows: (1) there were significant longitudinal AD-like changes in CSF Aβ42, T-tau and P-tau—meaning Aβ42 fell and T-tau/P-tau rose—in this cohort of cognitively healthy elderly; (2) baseline levels of 10 CSF proteins, primarily related to the Aβ metabolism, microglial regulation or synaptic function, significantly predicted longitudinal reduction in Aβ42 when using linear mixed effect models (and 11 proteins when using ROC statistics); (3) several baseline proteins were associated with longitudinal Aβ42 changes in subjects with normal baseline Aβ42 but not in subjects with reduced baseline Aβ42 and (4) no baseline proteins predicted changes in T-tau or P-tau. The finding that the predictive effects of baseline proteins on the subsequent decline of Aβ42 were different in subjects with normal and reduced baseline CSF Aβ42 levels suggests that some proteins predict brain Aβ plaque accumulation at a very early stage of AD, prior to widespread plaque accumulation. The failure of baseline proteins to predict longitudinal changes of T-tau and P-tau suggests that these proteins are specifically predictive of Aβ pathology. Taken together, the evidence suggest that changes of CSF proteins primarily related to Aβ metabolism, microglial regulation or synaptic functions most likely reflect pathological alterations at very early stages of AD, and for some proteins even prior to widespread neuritic plaque formation.
The first major finding was that cognitively healthy elderly showed longitudinal changes in Aβ42, P-tau and T-tau, partly replicating a previous report using 3-year follow-up data from ADNI-1 (the same subjects in this study) showing that healthy controls manifested longitudinal decreases in Aβ42 and increases in P-tau.28 Cross-sectional studies have shown that cognitively healthy subjects (or MCI subjects) at risk for future development of AD dementia have altered Aβ42, P-tau and T-tau several years prior to symptoms, with reduced Aβ42 appearing first.8 A few previous studies have reported longitudinal measurements of Aβ42, P-tau and T-tau in cognitively healthy subjects.28, 29, 30, 31, 32, 33, 34 Most of these have found no or only small changes of CSF Aβ42, P-tau and T-tau levels in cognitively healthy subjects,30, 31, 32, 33, 34 but these have had fewer participants, shorter follow-up or fewer serial samples than the present study. Our finding supports the use of Aβ42, T-tau and P-tau to track the development of AD-like pathology prior to clinical symptoms.
Our second major finding was that 10 CSF proteins measured at baseline significantly predicted longitudinal decrease in CSF Aβ42 as determined using linear mixed effect models, and 11 proteins as determined using ROC analysis (these sets of proteins largely overlapped). There has been no published previous analysis demonstrating the predictive effect of some CSF proteins on longitudinal changes of CSF Aβ42, T-tau or P-tau. The finding that these CSF proteins predicted longitudinal decrease in CSF Aβ42 indicate that they may be used as very early biomarkers of Aβ pathology and thereby possibly also of AD.
Furthermore, the findings that these proteins predict the decrease in CSF Aβ42 provide in vivo evidence that biological pathways related to the Aβ metabolism, microglia activity and synaptic/neuronal function are involved in early Aβ pathology (see Supplementary Table 1 for references for the proteins described in this paragraph). Several of the identified proteins are associated with Aβ metabolism in different ways. For example, CLU and Cystatin C may bind Aβ peptides and influence their aggregation, ACE may degrade Aβ peptides, and Stem Cell Factor and IL-3 may protect against Aβ deposits and toxicity. Several of the proteins (including AXL, CD40, CgA, CLU, Cystatin C, MCSF and TIMP1) may modulate microglial activity or have their expression promoted by microglia, and some of the proteins (including ACE, B2M, CgA, IL-3 and TIMP1) have been described to be involved in synaptic or other neuronal functions. For example, CgA has been proposed as an indicator of presynaptic structures, and B2M and TIMP1 are involved in synaptic plasticity. Several of the identified proteins also have other associations with AD. For example, CLU is a major AD risk gene. Some of the proteins have been reported to have altered CSF levels in AD, with increased levels of MCSF and tumor necrosis factor receptor 2, and decreased levels of TIMP1, CgA and stem cell factor. Taken together, the evidence linking these proteins with Aβ, microglia and synaptic function, and other evidence of their association with AD, provides additional support for the view that these proteins are closely involved, and may reflect causal events, in the pathophysiological events resulting in AD pathology.
The third major finding was that several of the identified proteins (CgA, CD40, AXL, ACE, TNF-related apoptosis-inducing ligand receptor and IL-3) were associated with the future decrease in CSF Aβ42 only in subjects without any signs of Aβ pathology at baseline; however, only one tested protein (TIMP1) was associated with further Aβ42 accumulation in subjects in whom Aβ pathology was already present. This suggests that these proteins reflect biochemical processes occuring prior to widespread plaque deposition because widespread plaques are reflected by low CSF Aβ42.1, 7 Therefore, we interpret these results to suggest that some of the identified proteins reflect pathophysiological alternations that occur at the very earliest stages of AD. We also tested 16 patients with AD dementia, in whom we found no associations between baseline protein levels and change of CSF Aβ42, T-tau or P-tau. Although this result is difficult to interpret because of the small number of subjects, it may support the notion that the identified proteins mainly reflect brain processes involved in the early stages of AD.
The fourth finding of this study was that there were no significant associations between the baseline proteins and longitudinal T-tau/P-tau. This fits with the dynamic biomarker model, in which changes in Aβ42 (and thereby associated processes) are more likely to be detected in early stages of the disease than changes in T-tau and P-tau.19 One intriguing finding was that several proteins were associated with baseline T-tau levels. Among them were CgA, AXL and ACE, which themselves were strong predictors of longitudinal CSF Aβ42 reduction. One possible explanation for the association between baseline T-tau and these proteins is that they may share metabolic pathways unrelated to Aβ pathology. This view is supported by the fact that, although CgA, ACE and AXL were strong predictors of longitudinal CSF Aβ42 reduction, baseline T-tau did not predict longitudinal CSF Aβ42 reduction.
The main limitation of this study is the relatively low sample size, but this was balanced by the long follow-up time and multiple samples from each subject. Our results may be biased by the selection of proteins studied, included mainly because of known associations with Aβ metabolism, microglia activity or synaptic function. It is possible that another set of proteins would have shown stronger predictive effects on longitudinal change of T-tau and P-tau. It is not known whether these proteins may help identify patients at risk for future cognitive decline or AD. When comparing protein levels between the 41 cognitively stable controls and the 5 controls who progressed to MCI, the only protein that was differently expressed between the groups was MMP2, which was reduced in participants who developed MCI. This is in agreement with previous studies, showing reduced CSF MMP2 levels in AD.35 However, the small number of participants who developed MCI in this study makes the negative results for the other proteins difficult to interpret.
In sum, we found that baseline CSF proteins involved in the Aβ metabolism, microglial regulation and synaptic function predicted longitudinal change in CSF Aβ42 in cognitively healthy people. Some of the proteins were only predictive in people with normal CSF Aβ42, suggesting that they may be used as biomarkers predicting future development of Aβ pathology in persons without Aβ pathology at testing. No proteins predicted increases in CSF T-tau or P-tau, consistent with the view that these protein changes occur prior to widespread plaque accumulation and downstream axonal loss and tangle pathology. The results provide in vivo evidence for pathological mechanisms related to early Aβ42 accumulation, and especially point to a role for early alterations of the Aβ metabolism and microglia activity. These proteins may be useful tools to identify cognitively normal subjects at risk for future Aβ42 pathology, and subsequently AD.
Acknowledgments
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, Glaxo-SmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co, Medpace, Merck and Co, Novartis AG, Pfizer, F Hoffman-La Roche, Schering-Plough, Synarc, as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the US Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. The proteomics data set described in this paper was generated by the Biomarkers Consortium Project “Use of Targeted Multiplex Proteomic Strategies to Identify Novel CSF Biomarkers in AD“, as described on the ADNI website, adni.loni.ucla.edu. The Biomarker Consortium Project was funded by Alzheimer's Drug Discovery Foundation, Eisai, Lilly, Merck, Pfizer and Takeda. This research was also supported by NIH grants P30 AG010129 and K01 AG030514, the Swedish Research Council, Goteborgs Lakaresallskap, Svenska Lakaresallskapet, Sahlgrenska Universitetssjukhuset, Carl-Bertil Laurells fond and Klinisk Biokemi i Norden.
Author Contributions
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
JQT may accrue revenue in the future on patents submitted by the University of Pennsylvania, wherein he is the co-Inventor and he received revenue from the sale of Avid to Eli Lily as the co-inventor on imaging-related patents submitted by the University of Pennsylvania; and is the William Maul Measey-Truman G Schnabel, Jr, MD Professor of Geriatric Medicine and Gerontology. MW has been on scientific advisory boards for Pfizer and BOLT Inter-national; has been a consultant for Pfizer, Janssen, KLJ Associates, Easton Associates, Harvard University, inThought, INC Research, University of California, Los Angeles, Alzheimer's Drug Discovery Foundation and Sanofi-Aventis Groupe; has received funding for travel from Pfizer, AD PD meeting, Paul Sabatier University, Novartis, Tohoku University, MCI Group, France, Travel eDreams, Neuroscience School of Advanced Studies (NSAS), Danone Trading, BV, CTAD ANT Congres; serves as an associate editor of Alzheimer's & Dementia; has received honoraria from Pfizer, Tohoku University and Danone Trading, BV; has research support from Merck, Avid, DOD and VA; and has stock options in Synarc and Elan. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses. Arch Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- Engelborghs S, De Vreese K, Van De Casteele T, Vanderstichele H, Van Everbroeck B, Cras P, et al. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging. 2008;29:1143–1159. doi: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SR, Schneider JA, Bennett DA, Beach TG, Bedell BJ, Zehntner SP, et al. Correlation of amyloid PET ligand florbetapir F 18 binding with Abeta aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer Dis Assoc Disord. 2012;26:8–16. doi: 10.1097/WAD.0b013e31821300bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5–10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Tabatabaei S, Johansson P, Hansson O, Andreasson U, Mansson JE, et al. Cerebrospinal fluid microglial markers in Alzheimer's disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromolecular Med. 2011;13:151–159. doi: 10.1007/s12017-011-8147-9. [DOI] [PubMed] [Google Scholar]
- Willis M, Leitner I, Jellinger KA, Marksteiner J. Chromogranin peptides in brain diseases. J Neural Transm. 2011;118:727–735. doi: 10.1007/s00702-011-0648-z. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Laforet G, Devanarayan V. Alzheimer's Disease Neuroimaging Initiative. Derivation of a new ADAS-cog composite using tree-based multivariate analysis: prediction of conversion from mild cognitive impairment to Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:73–84. doi: 10.1097/WAD.0b013e3181f5b8d8. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, Shaw LM, et al. Novel CSF biomarkers for Alzheimer's disease and mild cognitive impairment. Acta Neuropathol. 2010;119:669–678. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro R, Kuhn M, Xiong C, Pickering EH, Liu J, Misko TP, et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer's disease diagnosis and prognosis. PLoS One. 2011;6:e18850. doi: 10.1371/journal.pone.0018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Holtzman DM, Fagan AM, Shaw LM, Perrin R, Arnold SE, et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79:897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D and the R Development Core Team. nlme: linear and nonlinear mixed effects models. R package version 3.1-110, 2013.
- Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21:3940–3941. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, et al. The Alzheimer's association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395, e386. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, et al. Longitudinal change of biomarkers in cognitive decline. Arch Neurol. 2011;68:1257–1266. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Taylor K, Teng E, Coppola G, Gylys K. Longitudinal change in CSF biomarkers in a presymptomatic carrier of an APP mutation. Neurology. 2011;76:2124–2125. doi: 10.1212/WNL.0b013e31821f4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MI, Scheffer PG, Koel-Simmelink MJ, Twaalfhoven H, Verwey NA, Veerhuis R, et al. Serial CSF sampling in Alzheimer's disease: specific versus non-specific markers. Neurobiol Aging. 2012;33:1591–1598. doi: 10.1016/j.neurobiolaging.2011.05.013. [DOI] [PubMed] [Google Scholar]
- De Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Blennow K, Zetterberg H, Stomrud E, Londos E, Andreasen N, et al. Longitudinal study of CSF biomarkers in patients with Alzheimer's disease. PLoS One. 2009;4:e6294. doi: 10.1371/journal.pone.0006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010;67:217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Pedersen M, Lind K, Svensson M, Rolstad S, Eckerstrom C, et al. Intra-individual stability of CSF biomarkers for Alzheimer's disease over two years. J Alzheimers Dis. 2007;12:255–260. doi: 10.3233/jad-2007-12307. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer's disease. Biomark Med. 2012;6:455–476. doi: 10.2217/bmm.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.